Abstract
Purpose
The objective of this study is to compare the chromosomal distribution of early miscarriages with or without embryonic poles.
Materials and methods
It was a retrospective study of 223 women who underwent dilation and curettage (D&C) between 1995 and 2013 for early miscarriages. The presence or absence of a fetal pole was evaluated by abdominal or transvaginal ultrasound. Cytogenetic tests of products of conception following culture were determined in both groups.
Results
Of the 223 early miscarriages, 143 had embryos and 80 did not. The abnormality rate differed significantly (61.5 % vs. 46.3 %, p < 0.05), with trisomy 18, 21 and 45X found only in miscarriages with embryos. There were no significant differences between groups in rates of triploidy, tetraploidy, mosaicism, structure and double abnormality. The female abortus rate was higher in miscarriages with or without embryonic poles, as well as in groups with normal and abnormal karyotypes.
Conclusions
Chromosome distribution differs in miscarriages with or without embryonic poles. The ultrasound findings might offer different direction to determine the causes of early miscarriages. The higher female abortus rate may be associated with early selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early miscarriage is the most common complication of pregnancy, occurring in an estimated 12–24 % of confirmed pregnancies, with the majority occurring in the first 12 gestational weeks [1]. More than half of these miscarriages are due to fetal chromosome anomalies, most frequently aneuploidy [2], but some may be due to fetal anomalies and/or maternal factors. Chromosomal analysis of the products of conception, including the number and arrangement of chromosomes, has been used to determine the reasons for miscarriages and recurrent ones [3]. The two methods of chromosomal analysis mostly used are the culture and semi-direct methods. Determining the cause of early pregnancy loss can help in relieving sadness and advising the couple about the possibility of successful pregnancy.
However, will the ultrasonography findings of early miscarriages indicate different reasons contributing to this results? It might provide different directions and thinking to the doctors and couples encountering this issue. The distribution and types of fetal chromosomes may differ in miscarriages with or without embryonic poles. Two previous studies [4, 5] found that the abnormality rates were similar, with autosomal trisomy being the most frequent chromosomal anomaly in both groups. Among the other chromosomal abnormalities were monosomy, triploidy, tetraploidy, structural abnormalities and double abnormalities, all of which showed similar rates in miscarriages with or without embryonic poles. These chromosomal abnormalities may be related to embryo formation and maturation. In addition, viable autosomal trisomies and X monosomies appear uncommon in anembryonic miscarriages [4].
The sex ratio of abortuses has been found to vary among studies [4, 5]. However, most of them believed that higher female abortus rate was related to maternal cells contamination from maternally derived cells or blood when extracting products of conception [6]. Although most studies have analyzed the sex ratio of abortuses with normal chromosomes, few have assessed the sex ratio of abortuses with chromosomal anomalies [4, 5]. The sex ratio(male/female) at birth worldwide is about 1.06. The higher number of males may be related to early in-utero selection or sex chromosome advantages. One study suggested that the maternal X-chromosome in XY embryos may result in more stable development [7]. An increasing trend in the sex ratio which indicated more male baby at birth has been noted in many countries [8], including Taiwan [9]. This study was therefore designed to determine the chromosome types of early miscarriages with or without embryonic poles and to compare the distribution of abnormalities and sex ratio in these two groups.
Materials and methods
In our hospital, a tertiary medical center, we routinely offered the option of cytogenetic tests for products of conception (POC) when explaining dilation and curettage (D&C) for miscarriages to find out the reasons. Therefore, data was collected retrospectively from women who underwent D&C for miscarriages between 1995 and 2013 and elected to learn the chromosomal results. All women underwent abdominal or transvaginal ultrasound to determine the presence of a fetal pole and cardiac activity. Transvaginal ultrasound would be performed to confirm any ambiguous findings on the absence of an embryo by abdominal ultrasound in our routine practice. Anembryonic miscarriages were defined as without a fetal pole for at least the first 7 weeks of gestation weeks. Miscarriages with a fetal pole but without cardiac activity were defined as embryonic. All women underwent D&C under abdominal ultrasound guidance. The POC was removed by forceps and placed in RPMI (Roswell Park Memorial Institute) culture medium right away. The sample was immediately transported to our laboratory. After receiving the sample, the appropriate villi were selected under a dissecting microscope with a fine needle, taking care not to select any blood, membrane or maternal decidua. The villi would be washed with RPMI culture medium twice thoroughly. The risk of maternal cell contamination was proved to be reduced in this way [10]. Then, the villi was dissociated with collagenase and trypsin. It would then be washed by Chang’s medium and cultured with this medium for 7–14 days with growth monitored daily and medium changed every other day. Karyotype analysis was performed on at least 20 metaphase cells, using the Wright technique for Giemsa-banding. If mosaicism was observed, more than two colonies would undergo analysis. We also documented patients’ age, gestational age and history of recurrent miscarriages. Products of early pregnancy not containing a Y chromosome were assumed to be female. Two abortuses classified as XY/XX were excluded from the comparison of sex ratio. Differences between groups were analyzed statistically using Student’s t-tests and chi-squared tests, with P values < 0.05 considered statistically significant. This study was approved by the Ethics Committee of Chang Gung Memorial Hospital. Approval from the Institutional Review Board was obtained for analysis of this series.
Results
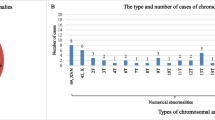
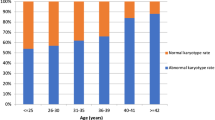
A total of 233 miscarriages between 1995 and 2013 were analyzed, of which 80 (35.9 %) was anembryonic (35.9 %). There was 10 failed cultures, making our cytogenetic success rate 95.7 %. The mean age of the 223 women was 32.4 years. Maternal and fetal chromosomal characteristics of miscarriages with or without embryonic poles are shown in Table 1. The abnormality rate was significantly higher in miscarriages with than without embryos (61.5 % vs. 46.3 %), but there were no significant differences between groups regarding maternal age, weeks of gestation, and history of previous miscarriages. Karyotype results are shown in Table 2. Trisomy was the most frequent chromosomal abnormality in both groups. Monosomy (45X) was present only in miscarriages with embryos. The rates of triploidy, tetraploidy, mosaicism, structure and double abnormality were similar in the two groups. Table 3 shows the detailed classification of chromosomal abnormalities. Trisomy 16 was the most common. Of the viable trisomy, trisomy 18 and 21 were found only in miscarriages with embryos, but trisomy 13 was present in both groups. The sex contribution of these two groups was shown in Fig. 1. A comparison of sex distribution showed female predominance in every group (Table 4).
Discussion
The chromosome abnormality rate was high in early miscarriages with or without embryonic poles. Moreover, in agreement with previous results, most early miscarriages were related to chromosome anomalies [2]. Aneuploidy contributes greatly to early pregnancy loss. Interestingly, in contrast to results showing that abnormality rates were similar in miscarriages with or without embryonic poles [4, 5], we found that the abnormality rate was higher in embryonic than in anembryonic miscarriages. It provided direction when encountering these two types of miscarriages. It was known that women with first miscarriage would not raise the rate of chromosome anomaly in the next pregnancy [3]. The first miscarriage was always sporadic. Therefore, in current practice, detailed investigation of the reasons might not be necessary for the first miscarriage. But cytogenetic tests might be offered first if the couples would like to find out the cause. In the area where cytogenetic tests were not available, maternal factors such as infection, drug use, chronic disease, endocrine disease, uterine factors or immunological factors must also be taken into consideration in anembryonic miscarriages. We found that this information was important for pregnant women who experienced pregnancy loss in our clinical practice and that it was useful for genetic counseling and prenatal care in future pregnancies. And for women with recurrent miscarriages of chromosome anomaly, we could provide further pre-implantation genetic diagnosis in the following conceptions. Although the history of receiving ART (assisted reproductive technology) not being documented in our study, Qin et al. reviewed 1896 cases and declared that ART treatment wouldn’t increase the risk for chromosomal abnormalities in the first trimester miscarriages [11].
To analyze chromosome types, we found that 45X was the most common single abnormality in early pregnancy loss. It was only found in embryonic pregnancies, in agreement with previous results [4, 5, 12]. Monosomy and even mosaicism were found to induce early miscarriages [13]. Trisomy was the most common subtype, with trisomy 16 being the most frequent trisomy in both embryonic and anembryonic miscarriages, in agreement with previous findings [4, 5]. We also found that chromosome types affected embryo formation. Another difference between miscarriages with or without embryonic poles was observed among the viable chromosome trisomies. We observed trisomy 18 and 21 only in embryonic miscarriages, whereas trisomy 13 was present in both types. It indicated that trisomy 13 might affect earlier embryo development. Besides, women with a trisomy 13 fetus were prone to have an abnormal placenta such as small placenta volume, reduced placental vascularization, partial molar appearance of the placenta, placental mesenchymal dysplasia and preeclampsia [14]. The poor placenta support might cause earlier embryo shrinkage. Another study reported similar results, except that the rates of trisomy 18 and trisomy 21 were higher [4]. In analyzing miscarriages occurring after week 12 of gestation in our data, we found that many of these had trisomy 18 or trisomy 21 showing that trisomy 18 and 21 might affect later organ developement. Non-viable trisomies were also observed among our miscarriages, including trisomies of chromosomes 2, 3, 6, 7, 8, 9, 14, 15, 16, 20 and 22. This result was partially compatible with a series using transcervical embryoscopy prior to evacuation to confirm embryo morphology for cytogenetic tests [15]. We also found that the rates of polyploidy, mosaicism, structural abnormalities and double abnormalities were similar in the groups of miscarriages with and without embryos.
We found a significantly higher female than male abortus rate in miscarriages with both normal and abnormal chromosomes, and in those that were embryonic and anembryonic. Although previous studies reported similar results, those findings were believed due to contamination with maternal cells. For example, one study used single-nucleotide polymorphism (SNP) microarray technology to analyze specimens from 1222 POC, finding that over half of the miscarriages with normal (46,XX) chromosomes were due to maternal cell contamination. They also found that the contamination rate varied in frequency by centers from <5 to 50 %. Nevertheless, even after discounting these samples, the female abortus rate was slightly higher [6]. Another study analyzed X and Y chromosomes in maternal and villous DNA extracted from microdissected, formalin-fixed, and paraffin-embedded archival tissues, using polymerase chain reaction assays and confirming by fluorescence in situ hybridization [16]. They also found inaccuracies in cytogenetic analysis of early miscarriages, most likely due to maternal cell contamination. However, we used a standard culture method with villi washed twice before culture. This step was believed to reduce maternal cell contamination [10]. Tatyana et al. analyzed cytogenetic data from 21 studies and divided them into two groups with high contamination risk (long-term cultures or extraembryonic tissues) and low contamination risk (direct preparation method, short-term in situ cultures). The frequency of chromosomal anomalies was 63.4 % for studies with low risk and 46.5 % for with high risk ones [17]. Our data showed high detection rate for abnormal chromosome (56.1 %) with few 46XX mosaicism found. It might explain the quality of our laboratory and low risk of maternal cell contamination.
Our finding, that the rate of female abortuses was higher in miscarriages with both normal and abnormal chromosomes, may suggest a relative weakness in female embryo formation and development. Faster male embryos development than female ones were discovered in some animal studies [18, 19]. A cytogenetic examination of 342 spontaneous abortions divided into three groups according to the severity of embryonic developmental disturbance, and in which the likelihood of maternal cell contamination was low following analysis of embryonic and parental DNA, suggested, based on the male-to-female ratios, that the expression of genes from the maternal X-chromosome in XY embryos may enhance more stable development during early embryogenesis [7] because that one of the two copies of X chromosome on female was inactivated randomly during embryogenesis (X-chromosome inactivation). Determination of sex ratios of early miscarriages based on gestational age found a predominance of male abortuses before 10 weeks but a female predominance at higher gestational ages [20]. Government statistics in Taiwan have shown a male-to-female ratio at birth of about 1.07 to 1.08. Even after excluding cultural effects, the male-to-female ratio was greater than 1.0 [9]. Skewed sex ratios have also been observed in other countries [8], suggesting that the predominance of female abortuses may be associated with early selection in utero or during embryo development. However, our study lacked information on maternal environment, paternal age, stressful events and other factors that might affect sex ratio [21–23].
Our study was limited by the small numbers of anembryonic miscarriages, which may have affected the abnormality rate. Moreover, chromosomes of early spontaneous miscarriages are difficult to analyze, and ultrasound evaluation of miscarriages may be subjective, altering the ratio of embryonic to anembryonic miscarriages. However, in our center, only well-trained and experienced technicians performed ultrasound examinations, with combined abdominal and transvaginal ultrasound performed to confirm any ambiguous findings on the presence or absence of an embryo. We also needed extra experiments to prove the reliability of 46XX not highly influenced by maternal cell contamination.
More accurate and sophisticated techniques are needed for villous cytogenetic testing. A previous study found that the sensitivity of cytogenetic testing can be increased by draining blood from products of conception, followed by rinsing thoroughly with saline, selecting a proper sample with forceps and scissors and washing it prior to chromosome analysis [10]. A semi-direct method has also been used to reduce the likelihood of culture failure and contamination with maternal cells contamination [4, 17]. This technique is considered a rapid, comprehensive, cost-effective and reliable method of obtaining more precise chromosome results [24]. The future development of easier and more sensitive techniques may improve the quality and reliability of chromosome analysis and provide better information for couples who experience miscarriages.
In conclusion, we observed differences in chromosome distribution between miscarriages with or without embryonic poles. All groups of abortuses, with or without embryonic poles and with and without abnormalities, showed a female predominance.
References
Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ. 2013;346:f3676.
Zhang HK, Luo FW, Geng Q, et al. [Analysis of fetal chromosomal karyotype and etiology in 252 cases of early spontaneous abortion]. Zhonghua yi xue yi chuan xue za zhi = Zhonghua yixue yichuanxue zazhi =. Chin J Med Genet. 2011;28:575–8.
van den Berg, van Maarle, van Wely, et al. Genetics of early miscarriage. Biochim Biophys Acta. 2012;1822(12):1951–9.
Munoz M, Arigita M, Bennasar M, et al. Chromosomal anomaly spectrum in early pregnancy loss in relation to presence or absence of an embryonic pole. Fertil Steril. 2010;94:2564–8.
Lathi RB, Mark SD, Westphal LM, et al. Cytogenetic testing of anembryonic pregnancies compared to embryonic missed abortions. J Assist Reprod Genet. 2007;24:521–4.
Lathi RB, Gustin SL, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178–82.
Evdokimova VN, Nikitina TV, Lebedev IN, et al. Sex ratio in early embryonal mortality in man. Ontogenez. 2000;31:251–7.
Grech V. Secular trends and latitude gradients in sex ratio at birth in Asia during the past 60 years. Pediatr Int: Off J Jpn Pediatr Soc. 2013;55:219–22.
Lee IW, Lai YC, Kuo PL, et al. Human sex ratio at amniocentesis and at birth in Taiwan. Taiwan J Obstet Gynecol. 2012;51:572–5.
Lathi RB, Milki AA. Tissue sampling technique affects accuracy of karyotype from missed abortions. J Assist Reprod Genet. 2002;19:536–8.
Qin JZ, Pang LH, Li MQ, et al. Risk of chromosomal abnormalities in early spontaneous abortion after assisted reproductive technology: a meta-analysis. PLoS One. 2013;8(10):e75953.
Minelli E, Buchi C, Granata P, et al. Cytogenetic findings in echographically defined blighted ovum abortions. Ann Genet. 1993;36:107–10.
Hsu TY, Liou JD, Copel JA, et al. Prenatal detection of two different monosomic cell lines by chorionic villus sampling. Prenat Diagn. 1996;16:169–72.
Chen C-P. Placental abnormalities and preeclampsia in trisomy 13 pregnancies. Taiwan J Obstet Gynecol. 2009;48(1):3–8.
Philipp T, Kalousek DK. Generalized abnormal embryonic development in missed abortion: embryoscopic and cytogenetic findings. Am J Med Genet. 2002;111:43–7.
Bell KA, Van Deerlin PG, Haddad BR, et al. Cytogenetic diagnosis of “normal 46, XX” karyotypes in spontaneous abortions frequently may be misleading. Fertil Steril. 1999;71:334–41.
Tatyana V, Igor N, Natalia N, et al. A mathematical model for evaluation of maternal cell contamination in cultured cells from spontaneous abortions: significance for cytogenetic analysis of prenatal selection factor. Fertil Steril. 2005;83:964–72.
Avery B, Madison V, Greve T. Sex and development in bovine in-vitro fertilized embryos. Theriogenology. 1991;35:953–63.
Nedambale TL, Dinnyes A, Yang X, et al. Bovine blastocyst development in vitro: timing, sex, and viability following vitrification. Biol Reprod. 2004;71:1671–6.
Hoshi N, Hanatani K, Kishida T, et al. Chromosomal analysis in 894 induced abortuses from women of advanced maternal age in relation to gestational weeks and fetal sex ratio. J Obstet Gynaecol Res. 1997;23:1–7.
Matsuo K, Ushioda N, Udoff LC. Parental aging synergistically decreases offspring sex ratio. J Obstet Gynaecol Res. 2009;35:164–8.
Chan BC, Lao TT. Effect of parity and advanced maternal age on obstetric outcome. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2008;102:237–41.
Mizuno R. The male/female ratio of fetal deaths and births in Japan. Lancet. 2000;356:738–9.
Morales C, Sanchez A, Bruguera J, et al. Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am J Med Genet A. 2008;146A:66–70.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule Trisomy 18, 21 and monosomy X were found in miscarriages with embryos. The female abortus rate was higher in miscarriages with or without embryos.
Rights and permissions
About this article
Cite this article
Cheng, HH., Ou, CY., Tsai, CC. et al. Chromosome distribution of early miscarriages with present or absent embryos: female predominance. J Assist Reprod Genet 31, 1059–1064 (2014). https://doi.org/10.1007/s10815-014-0261-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0261-9