Abstract
Purpose
Aim of this prospective observational study was to analyze fertility status of Hodgkin lymphoma (HL) patients treated with different types of chemotherapy while receiving GnRH analogues to preserve ovarian function.
Methods
Fertility status was assessed among 108 females in reproductive age treated by curative chemotherapy for freshly diagnosed HL between 2005 and 2010 in university-based tertiary fertility and oncology center. All patients received GnRH analogues during chemotherapy to preserve their ovarian function. Their reproductive functions were assessed by follicle-stimulating hormone (FSH) measurement and pregnancy achievement. Ovarian function was determined separately in three groups with increasing gonadotoxicity of chemotherapy.
Results
One year following the treatment, normal ovarian function was found in 89 (82.4 %) of patients. Two years after chemotherapy, 98 (90.7 %) of patients retained their ovarian function, and 23 (21.3 %) achieved clinical pregnancy during the follow-up period. Average FSH after chemotherapy was 11.6 ± 17.9 IU/l 1 year after the treatment resp. 9.0 ± 13.8 at the 2 years interval. There were significantly more patients with chemotherapy induced diminished ovarian reserve (chDOR) among the group receiving escalated BEACOPP chemotherapy in comparison with the other types of treatment (58.1 % vs. 87.9 % resp. 95.5 %).
Conclusion
The rate of chDOR is significantly higher after EB poly-chemotherapy and there is no tendency for improvement in time. The 2 + 2 chemotherapy with GnRH-a required for more advanced HL retained ovarian function significantly better after 2 years. Another important advantage of GnRH-a co-treatment is the excellent control of patient’s menstrual cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern treatment of Hodgkin lymphoma (HL) based mainly on chemotherapy is highly effective with a 5-year progression free survival (PFS) of 87 % [1]. Intensive modern regiments such as escalated BEACOPP, including bleomycin, vincristine, procarbazine, prednisone, and increased doses of etoposide, doxorubicin, and cyclophosphamide are applied during advanced-stage of the disease. However, these modern therapies are associated with ovarian follicle pool damage commonly represented by amenorrhea, irregular menstrual cycle, or infertility among pre-menopausal women. This condition is usually described as chemotherapy induced diminished ovarian reserve (chDOR). The chDOR is characterized by a low number of eggs in a woman’s ovaries and/or impaired development of the existing eggs. Clinically chDOR is determined by secondary amenorrhea and persisting high levels of gonadotropins [2]. Complete depletion of ovarian follicles may also lead to premature ovarian failure (POF), which is defined as loss of ovarian function before the age of 40. The risk of chDOR mainly depends on patient age, type of chemotherapy, and its overall cumulative dose [3, 4]. According to the most recent Cochrane review of available evidence, administration of gonadotropin-releasing hormone analogues (GnRH-a) during chemotherapy may have a protective effect on ovarian toxicity [5]. Moreover, the systematic review and meta-analysis of randomized trials evaluating the efficacy of GnRH-a in the prevention of chDOR showed significant reduction of the chDOR risk in young cancer patients [6]. The mechanism of action of GnRH is based on suppression of the gonadotropin levels and decrease of utero-ovarian perfusion. However, detailed mechanisms underlying the protective effects of GnRH-a are not completely understood and they are probably more complex than just the interruption of the gonadotropic axis [7].
The aim of our prospective observational study was to analyze fertility status of HL patients treated with different types of chemotherapy and receiving GnRH analogues to preserve their ovarian function.
Material and methods
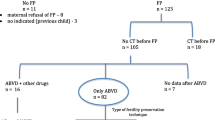
Study analysis was comprised of 108 reproductive age (18–40 year old) female patients with newly diagnosed HL during a period of 5 years from January 2005 to January 2010 – see study timeline CONSORT diagram on Fig. 1. All subjects had regular menstrual cycles. None of the women included and monitored in the study was on hormonal replacement therapy or hormonal contraception. Patients treated for HL relapses were excluded from the follow-up. Another study inclusion criterion was normal hormonal function of the ovaries assessed by follicle-stimulating hormone (FSH) levels in peripheral blood taken on the 1st–5th day of the menstrual cycle. Women with FSH values over 15 IU/l were excluded from the study. Study was carried out on a Caucasian Czech (central European) population.
All women were divided into three groups according to the Ann Arbor(AA) and German Hodgkin Study Group (GHSG) classification system [8, 9]. Each group of patients received different chemotherapy regimens with increasing gonadal toxicity. Patients with HL stage IA,IB, IIA or IIB without GHSG risk factors were classified to group A and received two cycles of adriamycin, bleomycin, vinblastine and dacarbazine chemotherapy (ABVD). Chemotherapy group B included patients with AA stage IA,IB, IIA or IIB with one or more GHSG risk factors. These women received two cycles of ABVD together with two cycles of bleomycin, vincristine, procarbazine, prednisone, etoposide, doxorubicin, and cyclophosphamide (BEACOPP) chemotherapy (regimen 2 + 2). Patients with advanced HL (AA stages III and IV or stage IIB with extranodal disease or large mediastinal mass as risk factor) were added to chemotherapy group C and received 6–8 cycles of BEACOPP chemotherapy (escalated BEACOPP, EB). Throughout the course of oncology treatment, patients were administered monthly triptorelin (Diphereline SR 3 mg, Ibsen) i.m. injections simultaneously with their chemotherapy, in order to inhibit the hormonal functions of the ovaries. The first injection of GnRH-a was timed to the 1st–5th day of patient menstrual cycle, and chemotherapy began at least 7 days later to overcome the gonadotrophins flare-up effect.
Patients age and previous pregnancy history was recorded in all three patient subgroups. The women’s reproductive functions were furthermore assessed two times after oncology treatment - in the period of 1 and 2 years after the cancer treatment. Major ovarian function outcome parameter was FSH levels from peripheral blood taken the same way as before the chemotherapy - on the 1st–5th day of the menstrual cycle (the sampling was not timed if menstruation was absent). This so called “basal FSH” measurement (assessed in early follicular phase) has a high inter-cycle variability, but for decades it was the best commonly available test for measuring ovarian reserve [3]. Many studies have now demonstrated that anti-Müllerian hormone (AMH) has higher sensitivity and less inter-cycle variability and nowadays along with basal antral follicle count (AFC) is considered as best currently available measure of ovarian reserve [10]. However, this new laboratory marker was not widely available during the study follow-up period. The reproductive status of women with FSH levels over 15 IU/l was considered as chemotherapy induced diminished ovarian reserve (chDOR). The cut-off value was established based on European Society of Human Reproduction and Embryology (ESHRE) definition of ovarian factor of infertility [11], laboratory definition of diminished ovarian reserve [12], and our experience [13]. The second outcome parameter observed was achievement of clinical pregnancy during the study follow-up period. Clinical pregnancy was defined according to WHO/ICMART definition as ultrasound visualization of a gestational sac in the uterus.
The recorded data were statistically analyzed and compared between the subgroups according to the type of chemotherapy. Continuous data were described by the mean (SD) and median (5th–95th percentile). Categorical data were described by N (%). The statistical significance of differences in continuous parameters among groups of patients was tested using ANOVA; maximum likelihood chi-square test was adopted for the computation of statistical significance of relationship between categorical parameters. The p-values < 0.05 have been considered as statistically significant in all analyses. Statistical analysis was computed using SPSS 22 (IBM Corporation, 2013).
Study was approved by the Regional Ethical Review Board of Brno University Hospital. Written informed consent with the study enrollment was collected from all participants.
Results
The analysis included a total of 108 patients divided into three subgroups A, B, and C, according to cytotoxicity of their chemotherapeutic regimen. The characteristics of the patient group in total and within the three subgroups are summarized in Table 1. The mean age of patients was 26.5 ± 5.8 years (median of 27.0 years). Majority of the patients were nuliparas (61.1 %) with average parity of 0.59 ± 0.81. Patient’s average basal FSH level before treatment was 3.3 ± 1.5 IU/l (median of 3.3 IU/l). There was no significant difference among the subgroups with respect to age, parity, and FSH levels before the treatment.
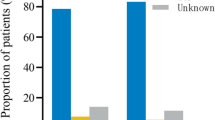
Ovarian function characteristics of patients during the period of 1 and 2 years after cancer treatment are summarized in Table 2. After 1 year of surveillance, the mean FSH level was 11.6 ± 17.9 IU/l. Fertility was preserved in 89 patients (82.4 %) with average FSH of 7.6 ± 11.8 IU/l. Nineteen patients (17.6 %) experienced chDOR with average levels of FSH 50.6 ± 21.1 IU/l. At the 2 year follow-up interval, the mean FSH dropped to 9.0 ± 13.8 IU/l. The chDOR was diagnosed in 10 patients only (9.3 %) with average FSH levels of 46.0 ± 21.5 IU/l. Normal ovarian reserve measures was found in 98 women (90.7 %), and their average basal FSH also dropped to 5.2 ± 3.1 IU/l.
Detailed data about ovarian function and pregnancy achievement divided according to the increasing gonadotoxicity of chemotherapy are described in Table 3. Among patients receiving less gonadotoxic chemotherapy (group A), there were 2 cases of chDOR (4.5 %) after 1 year, and no case of chDOR at the 2 year follow-up. Fifteen patients (34.1 %) achieved clinical pregnancy. In the more aggressive chemotherapy group B, four cases of chDOR (12.1 %) were diagnosed after 1 year. Two of them recovered by the 2 year interval (6.1 % of chDOR), and seven clinical pregnancies (21.2 %) occurred. The analysis of the most gonadotoxic chemotherapy group C confirmed 13 cases of chDOR (41.9 %) at the 1 year follow-up and almost half that number (8 cases, 25.8 %) after 2 years. There was only one case of pregnancy (3.1 %) in this group of patients.
Interesting results were obtained when fertility status and pregnancy occurrence were compared between specific chemotherapy groups with increasing gonadotoxicity – see Table 4. After chi-square test comparative analysis, the greatest statistically significant difference recorded was between chemotherapy group A and C (p < 0.001) in both 1 and 2 year follow-up time points. Less statistically significant differences in fertility preservation and pregnancy rates were also observed between groups B and C (p < 0.05). On the other hand, no statistically significant difference was noted between chemotherapy groups A and B.
Discussion
HL treatment has rapidly improved over the past decade with 5-year disease free survival of 65–81 % depending on the stage of disease [14]. Intensive therapy for advanced stages of HL such as EB is more effective; however this is accomplished at the cost of serious long term side effects including secondary leukemia, chDOR, or heart and lung damage [15]. For women of reproductive age, fertility impairment or fertility loss are important issues. Ovarian damage is mainly caused by alkylating agents, which form the backbone of the BEACOPP regimen [16].
In our study, optimum fertility preservation results were observed in patients receiving shorter and less intensive chemotherapy - i.e. two series of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) or a combination of two cycles of ABVD and two cycles of BEACOPP. After these chemotherapy regimens (A and B), the number of patients with chDOR decreased over time. After 1 year, only 2 patients (4.5 %) had chDOR, and there were no chDOR patients after 2 years of following regimen A. With regimen B patients, 12.5 % had chDOR after 1 year and 6.1 % experienced chDOR after 2 years. The rate of chDOR was significantly higher after EB poly-chemotherapy (regimen C) though there was considerable tendency for improvement in time. These patients are in the greatest risk of chDOR and also other means of fertility preservation as oocyte/embryo or ovarian tissue cryopreservation should be offered to them prior initiation of chemotherapy, if possible. Our observations are consistent with previous studies that also indicated limited or no protection of the ovarian follicle pool with GnRH-a in young women treated with EB chemotherapy – regimen C in our study [13, 17].
The statistical analysis of fertility status and pregnancy occurrence between different chemotherapy groups (A, B and C) indicated no difference in fertility status between two cycles of ABVD (or ABVD like) chemotherapy (regimen A) and 2x BEACOPP + 2x ABVD chemotherapy (regimen B). The acquired pregnancy rates could also be affected depending on, when patients start trying to conceive after cancer treatment. The more advanced is the disease; patients may more hesitate to start or delay childbearing. During study period all followed patients did not take contraceptives, on the other and they were advised not to utilize any technique of assisted reproduction like ovarian stimulation or in vitro fertilization. However, our observations may lead to a recommendation that 2 + 2 chemotherapy (regimen B) required for more advanced Hodgkin disease retained ovarian function significantly better within a 2 year observation time. Good fertility preservation can be achieved especially in patients undergoing GnRH analogues co-treatment.
Previously published randomized studies with GnRH-a during HL chemotherapy evaluated the rate of chDOR after non-standardized chemotherapy regimen [16, 18]. Our study confirmed significant differences in fertility outcomes among different chemotherapy groups and thus suggested optimum chemotherapy regarding fertility preservation in HL patients. Also, the 2 year follow-up time was exceptionally long when compared to previously published studies with an average follow-up of 6 months [19]. In a majority of studies, patient fertility outcome is usually assessed by presence or absence of menstrual cycle. Our study evaluated fertility outcomes much more precisely by FSH blood analysis and direct pregnancy diagnostics. This profound analysis was only possible with close cooperation between oncology and reproductive medicine specialists. The interdisciplinary cooperation worked very well in one tertiary referral medical center, but extension to a multicenter trial was not successful in routine clinical practice.
As with the number of patients with chDOR, significant differences were also observed in the number of pregnant women after cancer treatment. During a 2 year follow-up time, 15 pregnancies (34.1 %) occurred in the group of patients receiving ABVD chemotherapy. On the other hand, there was only one pregnancy case among patients treated with escalated BEACOPP. The number of achieved pregnancies was directly related to the number of ovarian follicles that were not damaged by cancer treatment. There is very little relevant information regarding pregnancy occurrence after HL treatment [5, 20]. There were two pregnancies reported in a Giuseppe randomized trial in the group of patients not receiving GnRH-a during their HL treatment [16]. Our study is the first one reporting pregnancy rates after different regimes of HL chemotherapy.
All patients in our trial received GnRH-a to protect their ovarian function. Opinions on the effectiveness of these drugs in ovarian protection are controversial. Published randomized controlled trials (RCTs) have recognized that GnRH-a administration was effective in protecting menstruation and ovulation after chemotherapy [5, 6]. The majority of analyzed publications are based on single-centre studies and small numbers of participants. Presently, there is no evidence that GnRH-a administration increases pregnancy rates after chemotherapy. Factors which mostly affect the level of ovarian injury include patient’s age and type of chemotherapy regime. Previously published studies report a high percentage of women with chDOR after treatment of HL, if no GnRH-a protection is received. In our previous studies, we recorded 71.0 % cases of chDOR 1 year after the end of chemotherapy [13]. Dann et al. confirmed a 40.0 % chDOR rate in 4 years follow-up time [21]. The average rates of chDOR after cyclophosphamide based chemotherapy was 40.0 % for women under 40 years of age [22]. When GnRH-a are used for ovarian protection, the number of chDOR patients can be significantly lower. The data presented above indicated only 9.3 % of patients with chDOR 2 years after the chemotherapy termination. Ovarian reserve before and after chemotherapy was measured by basal FSH measurement. Due to known inter-cycle variability of this ovarian reserve measurement, the presented chDOR results may have been over/under estimated. If chDOR was measured with now available more sensitive assays like AMH, it may show in fact significantly even more diminished ovarian reserve after less gonadotoxic chemotherapy. On the contrary, the real reproductive lifespan may be longer as indicated even after most gonadotoxic chemotherapy. Further observational studies are ongoing, also in our center, to confirm the degree of agreement between basal FSH and AMH.
Our study substantiated significantly better fertility outcomes among patients receiving less gonadotoxic chemotherapy. However, since all study patients received GnRH-a, its protective effect cannot be objectively evaluated. When our study was planned, the protective effect of GnRH-a had been previously reported with many studies and was generally accepted by most of our clinical oncologists [23]. For ethical reasons, we did not design our prospective study as a randomized trial with a control group not receiving GnRH analogues, although large and well-designed randomized trials should be conducted to clarify the effects of GnRH-a in preventing chemotherapy-induced chDOR. Furthermore, studies should have at least a 2 year follow-up and should address the effects on pregnancy rates, as previously noted.
Another important advantage of GnRH analogues co-treatment during chemotherapy is the excellent control of a patient’s menstrual cycle. Long term amenorrhea induced by GnRH-a significantly reduces the occurrence of irregular uterine bleeding [24]. According to our experience, these patients require considerably fewer blood derivatives and hematopoietic growth factors, which can substantially reduce overall treatment costs. Therefore, consideration of GnRH analogues in combination with chemotherapy may become one of the pivotal tools to preserve future fertility of reproductive age women, who must deal with cancer.
Conclusion
Significantly better fertility outcomes were found among patients receiving less gonadotoxic chemotherapy. The rate of chDOR is significantly higher after EB poly-chemotherapy and there is no tendency for improvement in time. The 2 + 2 chemotherapy regimen with GnRH-a required for more advanced HL disease retained ovarian function significantly better within a 2 year observation time. The GnRH-a used for fertility protection in our study may play positive role in preventing chemotherapy-induced chDOR. Another important advantage of GnRH-a co-treatment is the excellent control of patient’s menstrual cycle.
References
Engert A, Diehl V, Franklin J, Lohri A, Dörken B, Ludwig W-D, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–54.
Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005;11(1):69–89.
Huser M, Smardová L, Ventruba P, Mayer J. Impact of oncological treatment on human reproduction. Klin Onkol. 2010;23(3):165–70.
Huser M, Zakova J, Smardova L, Crha I, Janku P, Hudecek R, et al. Combination of fertility preservation strategies in young women with recently diagnosed cancer. Eur J Gynaecol Oncol. 2012;33(1):42–50.
Chen H, Li J, Cui T, Hu L. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2011;11:CD008018.
Del Mastro L, Ceppi M, Poggio F, Bighin C, Peccatori F, Demeestere I, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–83.
Huser M, Jurankova E, Crha I, Ventruba P, Hudecek R, Zakova J, et al. Fertility preservation strategies in women undergoing chemotherapy for haematological malignancy. Eur Clin Obstet Gynaecol. 2006;2(2):77–81.
Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31(11):1860–1.
Klimm B, Diehl V, Pfistner B, Engert A. Current treatment strategies of the German Hodgkin Study Group (GHSG). Eur J Haematol Suppl. 2005;66:125–34.
Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701.
Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2012;98(2):302–7.
Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113(6):1355–63.
Huser M, Crha I, Ventruba P, Hudecek R, Zakova J, Smardova L, et al. Prevention of ovarian function damage by a GnRH analogue during chemotherapy in Hodgkin lymphoma patients. Hum Reprod. 2008;23(4):863–8.
Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32(3):163–8.
Diefenbach C, Steidl C. New strategies in Hodgkin lymphoma: better risk profiling and novel treatments. Clin Cancer Res. 2013;19(11):2797–803.
Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD). Hematol Amst Neth. 2007;12(2):141–7.
Behringer K, Wildt L, Mueller H, Mattle V, Ganitis P, van den Hoonaard B, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21(10):2052–60.
Gilani MM, Hasanzadeh M, Ghaemmaghami F, Ramazanzadeh F. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia Pac J Clin Oncol. 2007;3(2):79–83.
Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91(3):694–7.
Harel S, Ferme C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma. Haematologica. 2011;96(11):1692–9.
Dann EJ, Blumenfeld Z, Bar-Shalom R, Avivi I, Ben-Shachar M, Goor O, et al. A 10-year experience with treatment of high and standard risk Hodgkin disease: six cycles of tailored BEACOPP, with interim scintigraphy, are effective and female fertility is preserved. Am J Hematol. 2012;87(1):32–6.
Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–29.
Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14(6):543–52.
Zakova J, Sedlackova M, Polak S, Dumkova J, Ventruba P, Crha I. Methods for preserving fertility in young women suffering from cancer: some aspects of ovarian tissue cryopreservation. Bratisl Lek Listy. 2012;113(3):192–4.
Acknowledgments
This scientific work was supported by the Czech Republic Ministry of Health-project FNBr 65269705 and IGA NT11124.
Conflict of interest
The authors declare that they have no conflict of interest.
Author’s roles
M.H. and L.S. designed the study and drafted the manuscript. P.J., I.C. and J.Z. recruited and followed the participants. P.S. and J.J. collated and analyzed the data. J.M. and P.V provided clinical and intellectual oversight. All authors critically reviewed the manuscript and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
The rate of chemotherapy induced diminished ovarian reserve is significantly higher after escalated BEACOPP chemotherapy with no tendency for improvement in time.
Rights and permissions
About this article
Cite this article
Huser, M., Smardova, L., Janku, P. et al. Fertility status of Hodgkin lymphoma patients treated with chemotherapy and adjuvant gonadotropin-releasing hormone analogues. J Assist Reprod Genet 32, 1187–1193 (2015). https://doi.org/10.1007/s10815-015-0452-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0452-z