Abstract
Purpose
To investigate whether in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), influence the embryo’s development and its quality using the mouse as a model.
Methods
Assisted fertilization was performed using ICSI and IVF. Fluorescent beads were adhered to the fertilization cone or place of previous sperm injection in the natural mated (NM), IVF and ICSI embryos, respectively. Embryo examination was carried out at the two-cell and blastocyst stage to determine the position of fluorescent bead. Protein expression was detected by fluorescence immunocytochemical staining and confocal microscopic imaging of blastocysts.
Results
IVF and ICSI embryos developed at rates comparable to NM group. Embryos show similar expression patterns of two transcription factors, Oct4 and Cdx2. The most preferred place for spermatozoa attachment was the equatorial site of the egg, whether fertilization occurred in vitro or under natural conditions. We also link the sperm entry position (SEP) to embryo morphology and the number of cells at the blastocyst stage, with no influence of the method of fertilization.
Conclusions
IVF and ICSI, do not compromise in vitro pre-implantation development. Additional data, related to sperm entry, could offer further criteria to predict embryos that will implant successfully. Based on embryo morphology, developmental rate and protein expression level of key transcription factors, our results support the view that ART techniques, such as IVF and ICSI, do not perturb embryonic development or quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, over 30 years after the first US child was born from in vitro fertilization (IVF), assisted reproduction has become one of the most commonly used techniques to deal with the issue of human infertility [21]. The number of babies born with the aid of assisted reproductive technology (ART) grows every year and currently accounts for approximately 1–4 % of all births in the United States and Europe [2, 8]. The majorities of these newborns are healthy and develop well. However, some studies report poorer birth outcomes and a higher risk of certain types of birth defects for infants born as a result of assisted fertilization than for infants conceived naturally [8, 24, 31].
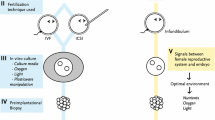
Progress in the field of assisted reproduction and micromanipulation has been remarkable since the first report of a successful delivery from IVF in 1978 [49]. IVF is a complex process and involves multiple steps resulting in the fertilization of oocytes in the laboratory. Development of micromanipulation techniques enabled the microinjection of a single spermatozoon directly into the cytoplasm, a procedure called intracytoplasmic sperm injection (ICSI), which can help to overcome low rates or failure of fertilization in cases of male factor infertility [34]. Both IVF and ICSI require several manipulations including collection, observation and selection of gamete, egg fertilization, embryo culture and embryo transfer followed by embryo assessment. Although most of these procedures are considered be safe, manipulations of embryos in animals may influence fetal growth and viability [1, 28] suggesting that many of these effects can be triggered by epigenetic changes and frequently affected imprinted genes.
ART techniques represented by IVF and ICSI are intended to overcome natural barriers to fertilization. For instance, the ICSI procedure bypasses the sperm binding to and penetration of the zona pellucida and sperm fusion with the oolemma, allowing fertilization by spermatozoa with poor motility or abnormal morphology. The ICSI process also introduces additional sperm components into the egg cytoplasm. Despite documented efficiency and success in infertility treatment using ART, there is increasing evidence of congenital malformations and chromosome aberrations in children born after ICSI and IVF [14, 15, 20, 29]. A study that assessed the risk of nuclear spindle damage following ICSI suggests that this technique might interfere with regular chromosome segregation at the second meiotic division of the oocytes [27]. It is possible that ICSI affects an important event(s) that occurs in the narrow time-window between fertilization and pronucleus formation, thus affecting the long-term cascade of gene expression and embryonic development [12, 18, 25, 32]. Therefore, it is important to follow an early embryo during development and evaluate events that could affect its quality as a result of assisted fertilization. In order to investigate whether ART does compromise in vitro pre-implantation development, we compared three methods of fertilization, natural mating (NM), IVF, and ICSI, in relation to spermatozoa attachment, embryo developmental competence, morphology, and quality, and expression patterns of two key transcription factors, Oct4 and Cdx2.
Materials and methods
Oocytes, zygotes and semen collection
Eggs were collected from CD-1 (4–6 weeks) females superovulated with 10 IU pregnant mare serum gonadotropin (PMSG, Sigma). This was followed by 10 IU human chorionic gonadotropin (hCG, Sigma) 48 h later. Then metaphase II-arrested oocytes were recovered 14 h after hCG. Some females after hormonal superovulation were mated with males in order to collect in vivo fertilized zygotes (NM) 17 h later. Both eggs and zygotes were collected into phosphate-buffered saline (PBS) containing 200 IU ml−1 hyaluronidase, dispersed and transferred to M2 medium (Sigma). The contents of caudae epididymides from mature CD-1 males were released into 1 ml of equilibrated fertilization medium (HTF) to allow capacitation.
Assisted fertilization and culture
-
1)
ICSI was performed as follows: A mouse oocyte was held by a holding pipette on the left where the polar body was located, at the six o’clock position. A single sperm was aspirated tail-first into a blunt-ended injection pipette and placed next to the oocyte at the three o’clock position. The injection needle was then used to penetrate through the zona pellucida and into the oocyte, and the sperm head was placed at the tip of the needle. Once a PIEZO pulse was applied, the relaxation of the oolemma indicated successful penetration of the needle into the ooplasm followed by the injection of the sperm head into the ooplasm. The needle was withdrawn slowly without damaging the oolemma.
-
2)
IVF was performed as follows: Metaphase II oocytes with attached follicular cells were placed, about 25–30 per drop, into 200 μm droplets of the HTF medium under paraffin oil. After 1.5 h of incubation, the sperm suspension was added to eggs at final concentration of approximately 106 spermatozoa/ml. Five-to-six hours post-insemination, sperm was removed and eggs were examined for the appearance of pronuclei and the extrusion of the second PB.
Culture was performed in drops of equilibrated KSOM-AA medium (Specialty Media) supplemented with amino acids and 4 mg/ml BSA under mineral oil in an atmosphere of 5 % CO2 in air at 37 °C.
Labeling technique
In NM embryos, the fertilization cone (FC), which forms above male chromatin and indicates the sperm entry position, was marked by a small bead as previously described [36]. In order to mark the sperm entry site in IVF and ICSI embryos, a new labeling technique was developed. Fluorescent (FITC labeled) beads (3 μm diameter, Polysciences) were placed in FHM medium containing 350 mg/ml phytohaemagglutinin for 30 min and then transferred to the chamber containing eggs in FHM + BSA. Individual beads were mounted on the tip of a beveled, sharpened micropipette for the IVF fertilized egg or injection needle for ICSI fertilized egg, and then were introduced through the zona pellucida where beads were placed in contact with the membrane. Once the bead had adhered to the FC or place of previous sperm injection, the micropipette was withdrawn. Timing of the FC formation for NM and IVF embryos was 17 h after mating and 2.5 h after mixing eggs and sperm, respectively. Labeled eggs were transferred into 35 μl drops of KSOM-AA media (Specialty Media) and then cultured under mineral oil with 5 % CO2 in air at 37 °C.
Embryo examination
All embryos were observed under an inverted (Nikon) microscope using Hoffman’s optics at the two-cell and blastocyst stage to determine the position of SEP marker. Three equal sectors on the surface of 2-cell embryos were defined: central, middle and lateral. At the blastocyst stage the SEP marker was scored as lying within any of the following three areas: (i) the embryonic (Em) part; (ii) the abembryonic (Ab) part; or (iii) the medial part, which was defined as an equatorial zone of four bead diameters in breadth.
Sperm entry marker distribution
Since the position of the incoming sperm has been linked to the embryonic-abembryonic axis formation in the blastocyst [36–39], we investigated this relationship in eggs where sperm entered the cytoplasm in different ways. We used the SEP provided by NM, IVF, or ICSI as a point of reference during preimplantation embryo development. To follow this entry site, each embryo was labeled and then scored according to the SEP at the two-cell and blastocyst stage [36, 37].
Fluorescence immunocytochemical staining of blastocysts
After removing zonae pellucida by acidic Tyrode’s solution, zona-free embryos were fixed in 2 % paraformaldehyde (PFA) for 30 min at room temperature (RT), permeabilized and blocked for 1 h. Blastocysts were incubated with the following antibodies overnight at 4 °C: mouse monoclonal Oct4, 4 ug/ml (Sigma) and rabbit polyclonal Cdx2, 1:100 (Sigma). A secondary antibody conjugated with an appropriate fluorochrome was applied for detection of the primary antibody for 2 h at RT in dark: 1:200 Alexa Fluor 488- and 594-conjugated goat anti-mouse and goat anti-rabbit antibodies (Sigma), and 5 ug/ml Hoechst 33342 (Molecular Probes).
Confocal microscopic imaging of blastocysts
Following immunocytochemical staining, each blastocyst was transferred to a MaTek glass bottom dish and placed in separate drop of PBS under mineral oil. High-resolution laser scanning confocal microscopy was performed using a Zeiss LSM 510 META confocal unit (Zeiss, Inc) equipped with a ‘Plan-Apochromat’ 40× DIC oil-immersion objective. Serial optical sections were collected (512 × 512 pixel size, z-step 3 μm). Captured images were processed using the LSM Image browser and three-dimensional reconstruction of the obtained blastocysts was performed to demonstrate spatial localization of the protein.
Analysis of the spatial localization of Oct4 and Cdx2 in embryos
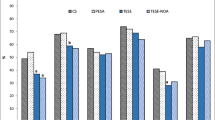
In order to detect and localize Oct4 and Cdx2 protein in embryos derived from different methods of fertilization all embryos underwent immunocytochemistry and confocal microscopy after they reached the blastocyst stage. Oct4- and Cdx2-positive cells represented ICM and TE respectively. Cells were visualized and counted under the confocal microscope at different focal planes across the blastocyst (Fig. 1a).
Spatial distribution of Oct4 and Cdx2 protein in blastocysts derived from naturally and assisted fertilized mouse embryos. a Confocal analysis of blastocysts developed from eggs fertilized by different methods. Following immunostaining, cells of each blastocyst were visualized and counted under confocal microscope at different focal planes across the embryo from the top to the bottom. Sections were taken every 3 μm. Oct4 and Cdx2 positive cells served as ICM and TE markers respectively. Single cross sections show an example of NM, IVF and ICSI blastocyst. Bar = 20 μm. b Cell number comparison between blastocysts derived from embryos fertilized by different methods. In NM, IVF and ICSI embryos there were no differences between groups in total number of cells and number of Oct4 and Cdx2 positive cells (P > 0.05; student’s t-Test). Values are mean ± s.d. Error bars represent s.d
Statistical analysis
We used Chi-squared and paired Student’s t test to compare the differences between groups. Data are presented as the mean ± standard deviation. A P value < 0.05 was considered statistically significant.
Results
In order to investigate whether ART does compromise in vitro pre-implantation development, we compared the developmental competence of the generated embryos using three methods of fertilization, NM, IVF, and ICSI. Following fertilization and in vitro embryo culture, we determined that fertilization, cleavage, and blastocyst rates in the IVF and ICSI group did not differ from NM embryos (P = 0.555; df = 2, Table 1). These results demonstrate that assisted reproduction techniques do not compromise in vitro pre-implantation embryonic development in mice.
To find out whether fertilization techniques influence the distribution and expression of some key transcription factors responsible for the developmental program of embryogenesis, we have analyzed the spatial localization of Oct4 and Cdx2 in embryos derived from different methods of fertilization. Of the 30 blastocysts derived from NM, IVF and ICSI, the total number of cells and the ratio of Oct4- to Cdx2- positive cells were similar in all analyzed groups (Fig. 1b). Our results indicate that assisted fertilization does not influence the expression pattern of these two transcription factors or the quality of the resulting blastocysts.
Our results showed no difference in sperm entry marker distribution regardless of the method of fertilization. In most NM, IVF and ICSI embryos, the first cleavage plane (65 %, 67 %, and 71 % respectively) as well as the boundary between the embryonic and abembryonic part of the blastocyst (71 %, 63 %, and 70 % respectively) were related to the position of the incoming sperm (Fig. 2a). In contrast, a marker placed randomly around the egg did not show similar correlation during the first cleavage or at the blastocyst stage (Supplementary Fig. 1). These results are consistent with previous studies in ICSI-derived monkey embryos [38] and mouse embryos fertilized in vivo [19, 36, 39]. Most importantly, the presented data suggest that the relationship established between an egg and a sperm in early embryo development is not altered by the method of spermatozoon introduction.
Sperm entry point localization and spatial distribution of Oct4 and Cdx2 protein in blastocysts derived from NM, IVF and ICSI embryos. a The SEP was traced in NM, IVF and ICSI embryos by using small fluorescent bead (black arrow). The bead position was scored within three defined zones at the two-cell and blastocyst stage [36]. There was no difference in SEP distribution between the fertilization methods. In the majority of embryos, the sperm entry marker was located at the central zone of two-cell stage and at the embryonic-abembryonic boundary (Em-Ab) in the blastocyst, showing a significantly different distribution from expectation (*P < 0.001; chi-square test). Bar = 20 μm. b Number of cells in blastocysts derived from SEP labeled embryos with respect to the method of fertilization. The total number of cells, as well number of cells in ICM and TE, demonstrated by Oct4 and Cdx2 protein localization respectively, was similar between each treatment group (P > 0.05; student’s t-Test). Values are mean ± s.d. Error bars represent s.d. c Number of cells in blastocysts derived from SEP labeled embryos with respect to the method of fertilization and SEP distribution. Statistically significant differences were found within each treatment group in number of cells: * significant difference within NM SEP group between Central and Middle/Lateral embryos in cell number and difference in number of Oct4 and Cdx2 positive cells P < 0.0005, ∆ significant difference within IVF SEP group between Central and Middle/Lateral embryos in cell number and difference in number of Oct4 and Cdx2 positive cells P < 0.0005, † significant difference within ICSI SEP group between Central and Middle/Lateral embryos in cell number and difference in number of Oct4 positive cells P < 0.05; no significant difference in Cdx2 positive cells within the ICSI SEP group (P = 0.14; student’s t-Test). Values are mean ± s.d. Error bars represent s.d; d Number of cells in blastocysts derived from SEP labeled embryos with respect to SEP distribution. Results showed significant variations between Central and Medial/Lateral group in total number of cells; as well Oct4 and Cdx2 positive cells for all treatments (P < 0.0001; student’s t-Test). Values are mean ± s.d. Error bars represent s.d
To determine whether spatial localization of Oct4 and Cdx2 differs between blastocysts derived from assisted and natural fertilization in relation to spermatozoa entry position, we performed immunocytochemistry and confocal scanning of NM, IVF and ICSI embryos previously labeled at the SEP. The average number of ICM and TE cells was similar in blastocysts generated by different fertilization methods (Fig. 2b, Supplementary Table 1). However, significant differences in the expression pattern of Oct4 and Cdx2 were found in blastocysts in relation to the SEP at the two-cell stage (Fig. 2c). Embryos that did not cleave at the two-cell stage according to the SEP (Middle/Lateral) formed small blastocysts with a lower number of cells (19 cells on average from all treatments, Fig. 2d) and a much higher ratio of Cdx2 to Oct4 positive cells (Supplementary Table 2) when compared to embryos that divided along the SEP during first cleavage division (Central) (34 cells on average from all treatments, Fig. 2d). Confocal analysis and ICM/TE cell counting revealed that blastocyst morphology differs depending on the site of sperm entry. However, the method in which the sperm is introduced into the ovum does not affect early differentiation, with no difference in expression pattern of the two key transcription factors at the blastocyst stage.
Throughout the process of fertilization, either under natural conditions or by assisted reproduction, the decision regarding the site of sperm entry is the primary difference between fertilization groups. In NM mouse eggs there is a reduced tendency to be penetrated by sperm near the animal pole, which is marked by the second polar body [13], whereas the most preferred place for sperm attachment is the equatorial region of the egg [19, 36]. Our observations showed that both NM and IVF oocytes are most often approached by sperm at the equatorial site. Resulting embryos displayed the fertilization cone (FC), a structure formed soon after the sperm-egg fusion, approximately 90° away from the extruding second polar body (PB2) at similar percentages (69 % NM and 76 % IVF; Fig. 3a). These data showed no difference in sperm entry preference whether fertilization occurred in natural conditions or in culture in vitro. Taking into account the spermatozoon’s preference to enter the egg at the equatorial region during in vivo and in vitro fertilization, we speculated whether there was any correlation with its later position during early embryo development. We found no link between FC position at the fertilized egg and the marker of SEP at the two-cell stage in either IVF or NM embryos (Fig. 3b). This observation suggests that the first cleavage division plane is not dependent on the specific site of sperm entry on the egg surface. In contrast to NM and IVF, during ICSI, the site of sperm injection is arbitrarily decided by the manipulator, and the injection is typically performed away from the meiotic spindle [3]. The first polar body (PB1), regularly used as a marker of chromosomes in the metaphase II oocytes, was observed at various positions and often apart from the meiotic spindle (Fig. 3c).
Egg morphology before and after fertilization in natural and assisted conditions. a Fertilization cone (FC) position detected soon after sperm-egg fusion in NM and IVF embryos. The structure was located in three different positions (animal, equatorial or vegetal) in similar frequency for both analyzed groups (observed value of chi-square is non-significant for df = 2). The data show preference for spermatozoon to enter the egg at the equatorial region under both in vivo and in vitro conditions (P < 0.001, df = 1; chi-square test). Bar = 20 μm. b Relationship between FC position in the fertilized egg and the SEP distribution in the two-cell stage embryo for both NM and IVF groups combined. There is no significant correlation between FC position and SEP location (P > 0.05, df = 2; chi-square test). c The spindle position in metaphase II mouse oocytes. The angle between the spindle and the 1st polar body (PB1) was observed as approximately 45°, 90° or 180°. The egg spindle was observed at various positions in relation to the PB1 (observed value of chi-square is non-significant for df = 2). Bar 20 μm
Discussion
Our observations show that, in mice, assisted reproduction techniques do not compromise in vitro embryonic developmental competence when compared to natural conception. Fertilization, cleavage, and blastocyst rates in the IVF and ICSI groups did not differ from NM embryos. Whether fertilization occurred in vitro or under natural conditions, the most preferred place for spermatozoa attachment was the equatorial site of the egg. Taking into account the spermatozoon’s preference to enter the oocyte at the specific region, we could not link its position on the egg and the marker of SEP at the two-cell stage in IVF and NM embryos. This observation suggests that the first cleavage division plane is not dependent on the specific site of sperm entry on the egg surface. Moreover, our results showed no differences in the sperm entry marker distribution regardless of the method of fertilization. Following NM, IVF, and ICSI, resulting embryos developed and differentiated into ICM and TE cells at comparable rates with similar expression patterns of Oct4 and Cdx2. However, when embryos were analyzed with respect to the SEP position at the two-cell stage, we could detect differences in morphology and number of cells when they reached the blastocyst stage (Central versus Middle/Lateral group; Fig. 4). These results suggest that the position of incoming sperm might affect blastocyst formation without being influenced by fertilization method.
Morphology of NM, IVF and ICSI embryos according to sperm entry point (SEP) distribution. The majority of embryos, in which the first cleavage division correlated to the SEP (Central), presented good morphology with a clearly visible inner cell mass (a, d, g). In contrast, embryos in which the first cleavage occurred with no respect to the SEP (Middle/Lateral) developed into poor quality blastocysts with low cell number and possibly reduced embryonic viability (b, c, e, f, h, i). Bar = 20 μm
A transitory protrusion, the FC, forms above male chromatin and indicates the sperm entry position, but is not detectable after the sperm head has decondensed and formed the pronucleus. In mouse, in vivo fertilized eggs can be approached by sperm at any position but typically spermatozoa enters the egg approximately 90° away from the second polar body at the equatorial zone [19, 36; Fig 3a]. Here, we observed a similar tendency for the sperm-egg fusion position during in vitro fertilization. It remains unclear what the biological function of the FC is during fertilization and whether the actual site of sperm entry is simply random or dictated by egg-sperm interactions. During ICSI, in contrast, the site of sperm injection is arbitrarily decided by the manipulator. Further, in natural fertilization and conventional IVF, the fertilizing sperm is selected through the biological process of sperm-oocyte interaction, especially sperm-ZP binding [23, 50]. However, for ICSI, this selection is performed manually by the embryologist based on motility and morphology of the sperm. Several studies have used zona pellucidia (ZP)-bound sperm for ICSI to enhance embryo quality, implantation and clinical pregnancy rates [5, 26, 33].
Some studies suggest that the ICSI technique might disturb regular chromosome segregation at the second meiotic division of the oocytes [27]. Typically, the sperm injection during ICSI is performed away from the PB1, regularly used as a marker of chromosomes in the metaphase II oocytes [3]. However, our observations showed that the meiotic spindle is not, as had been assumed, always adjacent to the polar body, but located at various positions and often apart from the PB1 (Fig. 3c). Similar displacement in human and hamster oocytes has also been reported [41, 48].
In human ART, concerns remain about whether fertilization techniques influence the distribution and expression of key transcription factors responsible for the developmental program of embryogenesis [7, 22, 51]. One of them, the POU-family transcription factor Oct4, is expressed throughout early embryonic development and becomes progressively restricted first to the entire inner cell mass (ICM) and then to the epiblast (EPI) at the blastocyst stage [35, 46]. The reverse expression pattern is observed in a second transcription factor, caudal-related homeobox 2 (Cdx2), whose expression is restricted to the trophectoderm (TE) by the blastocyst stage [4]. Targeted deletion of Oct4 in mice leads to lethality at implantation, and blastocysts fail to generate an ICM [30]. Cdx2 knock-out embryos fail to implant [9]. The expression profiles of these genes in human embryos are similar to those seen in murine embryos [22]. Although, changes in global gene expression and development were reported in in vitro cultured embryos from zygote to the blastocyst stage [42, 43] as well in IVF and ICSI derived embryos [17] we recorded no differences in spatial localization of those two transcription factors, Oct4 and Cdx2, in blastocysts, regardless of the method of fertilization. However, we noticed differences in embryo morphology and expression pattern of Oct4 and Cdx2 at the blastocyst stage in relation to the position of fertilizing sperm (Fig 4).
Observation of assisted fertilized eggs during the first cleavage division using a live imaging system [10, 52] would allow identification of low quality Middle/Lateral blastocysts, which develop from approximately 30 % of all two-cell embryos (Fig. 3a [38]). This would permit selection of embryos at the two cell stage with the best viability (Central group) and in turn increase developmental success rate. In human reproductive centers, the clinical decision of which embryo to recommend for transfer, cryopreservation or rejection is based on a scoring system that includes an embryo image evaluation, early cleavage and developmental rates or other embryo selection criteria [6, 11, 44, 47]. Blastocyst quality is usually measured based on blastocoel expansion, development of ICM, development of TE, and state of the zona pellucida [16]. In agreement with expectations, blastocysts with higher morphology scores have consistently more cells and a highly defined inner cell mass [45]. Moreover, ICSI-derived embryos with a low morphological grade are more likely to display H3K9 demethylation than their IVF counterparts [40]. Additional information related to the observation of the SEP could offer further possibilities to the evaluation system for selecting those embryos with the highest potential. Based on embryo morphology, developmental rate and protein expression level of key transcription factors, our results support the view that ART techniques, such as IVF and ICSI, do not perturb embryonic development or quality.
References
Amor DJ, Halliday J. A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum Reprod. 2008;23(12):2826–34.
Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod. 2008;23:756–71.
Avery S, Blayney M. Effect of the position of the meiotic spindle on the outcome of intracytoplasmic sperm injection. Hum Fertil (Camb). 2003;6:19–22.
Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–27.
Black M, Liu DY, Bourne H, Baker HW. Comparison of outcomes of conventional intracytoplasmic sperm injection (ICSI) and ICSI using sperm bound to the zona pellucida of immature oocytes. Fertil Steril. 2010;93:672–4.
Brezinova J, Oborna I, Svobodova M, Fingerova H. Evaluation of day one embryo quality and IVF outcome – a comparison of two scoring systems. Reprod Biol Endocrinol. 2009;3:7–9.
Bridges PJ, Jeoung M, Kim H, Kim JH, Lee DR, Ko C, Baker DJ. Methodology matters: IVF versus ICSI and embryonic gene expression. Reprod Biomed. 2011. Online May 8:Epub ahead of print
Center for Disease Control and Prevention (2008) “ART National Report”, November
Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx-2 mutant mice. Nature. 1997;386:84–7.
Cruz M, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25(4):371–81.
Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update. 2003;9(3):251–62.
Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, et al. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101:1595–600.
Evans JP, Foster JA, McAvey BA, Gerton GL, Kopf GS, Schultz RM. Effects of perturbation of cell polarity on molecular markers of sperm-egg binding sites on mouse eggs. Biol Reprod. 2000;62:76–84.
Foresta C, Garolla A, Bartoloni L, Bettella A, Ferlin A. Genetic abnormalities among severely oligospermic men who are candidates for intracytoplasmic sperm injection. J Clin Endocrinol Metab. 2005;90:152–6.
Fortunato A, Tosti E. The impact of in vitro fertilization on health of the children: an update. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):125–9.
Gardner DK, Schoolcraft WB. Towards reproductive certainty: infertility and genetics beyond. In: Jansen R, Mortimer D, editors. In vitro culture of human blastocysts. Carnforth: Parthenon Press; 1999. p. 378–88.
Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson AT, Rinaudo PF. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction. 2007;134(1):63–72.
Giritharan G, Li MW, De Sebastiano F, Esteban FJ, Horcajadas JA, Lloyd KC, et al. Effect of ICSI on gene expression and development of mouse preimplantation embryos. Hum Reprod. 2010;25(12):3012–24.
Gray D, Plusa B, Piotrowska K, Na J, Tom B, Glover DM, et al. First cleavage of the mouse embryo responds to egg geometry that reflects the position of sperm entry. Curr Biol. 2004;9:397–405.
Hvidtjørn D, Grove J, Schendel D, Vaeth M, Ernst E, Nielsen L, et al. ‘Vanishing embryo syndrome’ in IVF/ICSI. Hum Reprod. 2005;20:2550–1.
Jones Jr HW, Jones GS, Andrews MC, Acosta AA, Bundren C, Garcia J, et al. The program for in vitro fertilization at Norfolk. Fertil Steril. 1982;38:14–21.
Kimber SJ, Sneddon SF, Bloor DJ, El-Bareg AM, Hawkhead JA, Metcalfe AD, et al. Expression of genes involved in early cell fate decisions in human embryos and their regulation by growth factors. Reproduction. 2008;135:635–47.
Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121(8):2397–405.
Klemetti R, Gissler M, Sevón T, Koivurova S, Ritvanen A, Hemminki E. Children born after assisted fertilization have an increased rate of major congenital anomalies. Fertil Steril. 2005;84:1300–7.
Kohda T, Ogonuki N, Inoue K, Furuse T, Kaneda H, Suzuki T, et al. Intracytoplasmic sperm injection induces transcriptome perturbation without any transgenerational effect. Biochem Biophys Res Commun. 2011;410(2):282–8.
Liu F, Qiu Y, Zou Y, Deng ZH, Liu DY. Use of zona pellucida-bound sperm for intracytoplasmic sperm injection produces higher embryo quality and implantation than conventional intracytoplasmic sperm injection. Fertil Steril. 2011;95(2):815–8.
Macas E, Imthurn B, Rosselli M, Keller PJ. The chromosomal complements of multipronuclear human zygotes resulting from intracytoplasmic sperm injection. Hum Reprod. 1996;11:2496–501.
Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131(15):3727–35.
Martin-Du Pan RC, Sakkas D, Stalberg A, Bianchi PG, de Boccard G, Campana A. Treatment of male sterility using intra-oocytic sperm injection: critical evaluation. Schweiz Med Wochenschr. 1995;125:1483–8.
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct-4. Cell. 1998;95:379–91.
Olson CK, Keppler-Noreuil KM, Romitti PA, Budelier WT, Ryan G, Sparks AE, et al. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril. 2005;84:1308–15.
Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300(534–544):51.
Paes Almeida Ferreira de Braga D, Iaconelli Jr A, Sávio C, de Figueira R, Madaschi C, Semião-Francisco L, et al. Outcome of ICSI using zona pellucida-bound spermatozoa and conventionally selected spermatozoa. Reprod Biomed Online. 2009;19:802–7.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Induction of acrosome reaction in human spermatozoa used for subzonal insemination. Hum Reprod. 1992;7:248–54.
Palmieri SL, Peter W, Hess H, Scholer HR. Oct-4 transcription factor is differently expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–67.
Piotrowska K, Zernicka-Goetz M. Role for sperm in spatial patterning of the early mouse embryo. Nature. 2001;409:517–21.
Piotrowska K, Zernicka-Goetz M. Early patterning of the mouse embryo–contributions of sperm and egg. Development. 2002;129(24):5803–13.
Piotrowska-Nitsche K, Yang SH, Banta H, Chan AWS. Assisted fertilization and embryonic axis formation in higher primates. Reprod Biomed Online. 2009;18:382–90.
Plusa B, Piotrowska K, Zernicka-Goetz M. The first cleavage plane of the mouse zygote passes close by the sperm entry point defined by several labelling techniques. Genesis. 2002;32:193–8.
Qiao J, Chen Y, Yan LY, Yan J, Liu P, Sun QY. Changes in histone methylation during human oocyte maturation and IVF- or ICSI-derived embryo development. Fertil Steril. 2010;93(5):1628–36.
Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–93.
Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128(3):301–11.
Rinaudo P, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4 Suppl):1252–65.
Ruiz de Assin R, Clavero A, Gonzalvo MC, Ramírez JP, Zamora S, Fernández A, et al. Comparison of methods to determine the assigned value in an external quality control programme for embryo evaluation. Reprod Biomed Online. 2009;19:824–9.
Santos F, Hyslop L, Stojkovic P, Leary C, Murdoch A, Reik W, et al. Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum Reprod. 2010;25(9):2387–95.
Scholer HR, Hatzopoulous AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germ-line specific expression of an Oct factor. EMBO J. 1989;8:2543–50.
Scott L. The biological basis of non-invasive strategies for selection of human oocytes and embryos. Hum Reprod Update. 2003;9:237–49.
Silva CP, Kommineni K, Oldenbourg R, Keefe DL. The first polar body does not predict accurately the location of the metaphase II meiotic spindle in mammalian oocytes. Fertil Steril. 1999;71:719–21.
Steptoe P, Edwards RG. Birth after the reimplantation of a Human Embryo. The Lancet. 1978;312(8085):366.
Tesarik J, Rolet F, Brami C, Sedbon E, Thorel J, Tibi C, et al. Spermatid injection into human oocytes. II. Clinical application in the treatment of infertility due to non-obstructive azoospermia. Hum Reprod. 1996;11(4):780–3.
Turan N, Katari S, Gerson LF, Chalian R, Foster MW, Gaughan JP, et al. Inter- and intra-individual variation in allele-specific DNA methylation and gene expression in children conceived using assisted reproductive technology. PLoS Genet. 2010;6(7):e1001033.
Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–21.
Acknowledgments
The authors thank K. Larkin, S. Frankenberg, V. Horner and A. Long for comments on the manuscript. We also thank the veterinary staff and the animal resources at the Yerkes National Primate Research Center (YNPRC). All animal procedures were approved by the IACUC and the Biosafety Committee at Emory University. YNPRC is supported by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research and Infrastructure Program (ORIP)/OD P51OD11132. This study is supported in part by Emory University Research Fund and grant awarded by the ORIP/NIH (RR018827) to AWSC.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Capsule
IVF and ICSI, do not compromise in vitro pre-implantation development. Additional data, related to sperm entry, could offer further criteria to predict embryos that will implant successfully.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1061 kb)
Rights and permissions
About this article
Cite this article
Piotrowska-Nitsche, K., Chan, A.W.S. Effect of sperm entry on blastocyst development after in vitro fertilization and intracytoplasmic sperm injection — mouse model. J Assist Reprod Genet 30, 81–89 (2013). https://doi.org/10.1007/s10815-012-9896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9896-6