Abstract
Purpose: To relate pronuclear patterns (PN) and zygote cytoplasmic appearance and embryo morphology.
Methods: The usefulness of PN classification described by Tesarik et al. 1999 (patterns p0-5) and Scott et al. 2000 (Z1-4), for embryo selection is assessed.
Results: Sinchrony on polarization and number of nucleolar precursor bodies (NPB) were associated with good quality embryos (p0 60.9% and p3 67.3%, and Z1 62.5% and Z2 64.7%; p<0.01). Pattern 4 zygotes were associated with small number of NPB developed into multinucleated embryos (14.3%) and poor quality embryos (61.9%). No significant differences were found in the pregnancy rate between transfer of at least one good prognosis PN pattern and transfer of poor prognosis PN patterns, although 75% of the transfers included at least one embryo derived from a pattern 0 zygote, and 55% included embryos from categories Z1 or Z2.
Conclusions: Sequential assessment involving the evaluation of oocyte quality, the classification of PN patterns and embryo morphology allows a more accurate evaluation of embryos to be selected for transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several strategies have been proposed for the selection of embryos for uterine transfer in human assisted reproduction. The possibility of choosing adequate embryos with high implantation potential will allow the reduction in the number of embryos transferred. This will lead to a decrease in the percentage of multiple pregnancies, one of the major complications of IVF treatments. The scoring criteria of embryo selection are based on serial morphological observations conducted on day 1 (during the assessment of fertilization), on days 2 and 3 (based on cleavage and blastomere fragmentation), on day 5, or on combinations of these criteria. Extending the culture of embryos to the blastocyst stage may also be a good option to select high quality embryos [1]. Unfortunately, not all cases are good candidates and benefit from extended culture.
Pronuclear morphology assessment has been extensively described as a method to score zygotes. Also, some countries are only allowed to freeze zygotes and the selection of embryos at this stage is thus necessary [2, 3]. More recently, increasing interest in pronuclear morphology has resulted in reports that describe different PN patterns and associate them with embryo morphological parameters [4, 5].
Zygote morphology has been associated with subsequent embryo development as far as fragmentation, arrested cleavage, chromosome dotation and multinucleation is concerned [6]. Survival after cryopreservation has been related to morphological characteristics of cytoplasm maturity, nucleolar aspect and relative location of PN inside the zygote [7]. The aim of this study is to evaluate the usefulness of pronuclear patterns as described by Tesarik's and Scott's classifications as predictors of embryo morphology and implantation potential.
Material and methods
Patients
A total of 64 couples participated in this prospective study. A total of 569 fertilized oocytes from 8 conventional IVF, 48 ICSI cycles and 8 cycles where the oocytes were shared between the two methods (ICSI/IVF) were evaluated.
The mean age of the female patients was 33.1 years (±2.93). All women older than 39 years were excluded from the study. Patients had to fulfill the following selection criteria: no previous history of poor ovarian response or basal serum FSH levels ≤10 U/l, regular menstrual cycles, absence of polycystic ovarian syndrome or severe (grade II-IV) endometriosis as cause of infertility, no more than 3 previous failed IVF cycles. The patients included in the study must have between 8 and 20 mature oocytes collected at oocyte retrieval.
Ovarian stimulation
Ovarian stimulation was performed following a protocol that used gonadotrophin-releasing hormone agonist (GnRHa) in association with gonadotrophins. Ovarian response was monitored after day 6 by daily evaluation of serum oestradiol concentrations and transvaginal follicle measurements. Human chorionic gonadotrophin (HCG) 10000 IU was administered 36 h before oocyte retrieval, which was carried out by ultrasound-guided puncture.
Zygote scoring
Between 14 to 23 h after insemination (±1.65) the zygotes were placed in individual 50-μl drops of medium (Vitrolife Ref. G1-10091) under oil and scored using an inverted microscope with Nomarski differential contrast optics at a magnification of ×400. Time of examination was minimized in order to preserve temperature and pH medium, which may affect subsequent embryo development.
Two different classification systems were used to describe the polarization and number of nucleolar precursor bodies (NPBs) into the pronucleus (PN).
According to Tesarik and Greco [4] a pronucleus is considered to be polarized when all NPBs were present in the pronuclear hemisphere whose pole is the point of contact with the other PN. The zygotes are divided into six different categories depending on the number and distribution of NPBs (Fig. 1). In pattern 0 (p 0) zygotes, the NPBs in both PN are polarized if the number of NPBs is between 3 and 7, and they are non-polarized if the number of NPBs is equal or greater than 7. Pattern 1 zygotes have a large difference (>3) between the number of NPBs in each pronuclei. Pattern 2 zygotes are characterized by a small number (<7) of NPBs without polarization in at least one PN. Pattern 3 zygotes present polarization and a large number (>7) of NPBs in at least one PN. Pattern 4 zygotes have a very small number of NPBs in at least one PN. Pattern 5 zygotes present one polarized PN and one non-polarized PN. When the zygote can be assigned to more than one pattern, p 4 has the highest priority, followed by p1, p2, p3 and p5. Pronuclei are considered asymmetrical when the size difference between them is larger than 4 μm. This parameter was analyzed independently.
When looking at the relative position of the pronuclei, zygotes can be classified in adjacent (only one contact point between pronuclear membranes), adherent (when pronuclear membranes are in close contact), and separate (no contact of pronuclear membranes).
The second classification was the Z-scoring system described by Scott et al. in 2000 [5], which divided the zygotes into 4 patterns (Z1, Z2, Z3, Z4) (Fig. 2). Patterns Z1, Z2 and Z3 zygotes show symmetry of the PN and PN membranes in contact, which is not the case in Z4 zygotes. In Z1 zygotes both PNs have between 3 and 7 NPBs, the difference between PNs in the number of NPBs is not greater than one, and both PNs are polarized. Z2 zygotes have the same characteristics as Z1 zygotes, but both PNs are non-polarized. Z3 zygotes have alterations in the number of NPB and/or one polarized PN together with one non-polarized PN.
Apart from PN morphology, zygotes were evaluated for cytoplasmic appearance through the presence of vacuoles, cytoplasmic halos, darkness and presence of cytoplasmic granules.
Embryo scoring
Embryos were evaluated 40–44 h (day 2) after insemination, and embryo selection was exclusively based on embryo morphology. Embryos at day 2 were scored following morphological parameters such as the number of cells, their symmetry, the percentage of cytoplasmic fragmentation and the multinucleation. Grade 1 embryos have at least 4 equal-sized, non-multinucleated blastomeres with no fragments. Grade 2 embryos have between 2–4 non-multinucleated blastomeres, blastomeres with different sizes, or 15–25% of fragments, and grade 3 embryos have fewer than 2 blastomeres, multinucleated blastomeres, ≥30% fragments, or arrested embryos. Grade 1 and 2 embryos were combined in a single good prognosis group, whereas grade 3 zygotes were considered the poor prognosis group.
Statistical analysis
Qualitative characters were compared using the χ2-test or Fisher's test, whereas quantitative variables were analyzed using Student's T-test [2] or ANOVA [3]; with a significance level of p<0.05.
This study establishes the relationship between PN patterns with zygote cytoplasmic appearance, embryo morphology and implantation potential. The usefulness of both PN classifications for embryo selection is assessed.
Results
Patients were previously selected for the study in order to evaluate the correlation between position of NPBs in the PNs and both embryo quality and the rate of pregnancy and implantation without the interference of other factors. Neither age nor baseline levels of serum oestradiol showed any significant correlation with the patterns allocated to the zygotes according to the two PN classification systems.
Pronuclei were scored at an average of 19.1 h (±1.65) after insemination or ICSI. No differences were found with respect to distribution of the pronuclear patterns in relation with the timing of observation (≤18 h, >18 h).
Distribution of zygotes according to Tesarik's 1999 classification is showed on Table 1. Due to the small number of pattern 5 zygotes found, these were excluded from the statistical analysis. Distribution of zygotes according to Scott's 2000 classification is showed on Table 2.
Symmetry and relative position of the pronuclei are considered in Tesarik's classification only. Symmetry was found in 92.5% of pattern 0 zygotes, 86.1% of pattern 1 zygotes, 87.6% of pattern 2 zygotes, 89.3% of pattern 3 zygotes, and 90.9% of pattern 4 zygotes. No statistically significant differences were found.
More adherent pronuclei were found in the 0 pattern (23.5%), with statistical differences between patterns (p<0.01) (Table 3). The relevance of separate position was not analyzed due to the small number of cases.
There is no relationship between the morphological aspect of the zygotes and the PN patterns in the two PN classification systems. No significant differences between the patterns were found (Table 4). Embryo quality showed a significant correlation with the presence of intracytoplasmatic granules, but not with halo or vacuoles (Table 5).
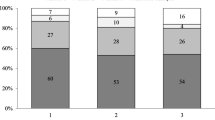
The percentages of zygotes that developed into different morphological categories of embryos on day 2 are shown in Figs. 3 and 4, according to the two classification systems. Patterns 0 and 3 were associated with good quality embryos at a higher frequency (p0: 60.9% and p3: 67.3%; p<0.01). A more accurate examination of each category of embryo quality included the assessment of the cleavage, the symmetry of blastomeres and multinucleation. Interestingly, pattern 4 zygotes were associated with a high percentage of zygotes developing into multinucleated embryos (14.3%) and poor quality embryos with various poor prognosis parameters (61.9%) (Table 6).
Patterns Z1 and Z2 were associated with good quality embryos at a higher frequency (Z1: 62.5% and Z2: 64.7%). Since the number of zygotes assigned to Z3 is high and correspond to different morphological patterns, we divided Z3 in two subgroups: Z3a which correponds to the classical Z3, and Z3b where only was determinant the number of NPBs [3,7]. We divided Z3 group in order to evaluate the importance of the number of NPBs in front of the polarization of NPBs; no statistically significant differences in embryo quality were found when comparing these subgroups. A high percentage of Z4 zygotes developed into poor quality embryos (51.7%). Z3 zygotes were also associated with poor quality embryos (47.1%), but an independent analysis of this association showed that the incidence of poor quality embryos was similar to that found in groups Z1(37.3%) and Z2 (35.3%). (Table 6).
No significant differences were found in the pregnancy rate between transfer of at least one good prognosis PN pattern (Tesarik's p0 or Scott's Z1 and Z2) and transfer of poor prognosis PN patterns (62.2% at least 1 p0 vs. 46.6% none p0; and 66.7% at least 1 Z1/Z2 vs. 48.2% none Z1/Z2). It must be taken into account that 75% of the transfers included at least one embryo derived from a pattern 0 zygote (Tesarik classification), and 55% of them included embryos from categories Z1 or Z2 (Scott classification). The ongoing pregnancy rate per transfer was 48.4% and the miscarriage rate per transfer 4.7%. The global implantation rate was 32.4%. The implantation rate was 35% for embryos from transfers of at least one pattern 0 embryo and 39.7% for embryos from transfers of Z1/Z2 embryos. The number of homologous transfers where all the replaced embryos were from the same PN pattern was too small to allow a more detailed statistical analysis.
Discussion
The selection criteria used in patients enrolled in the present study were designed in order to avoid the effects of clinical variables, such as age of the patients [2] and response to the stimulation protocol [8].
The distribution of PN patterns in our data favors zygotes from patterns p0, p1 and p2. Tesarik and Greco [4] found statistical differences within all patterns. Pattern 0 is the most common, followed by p 2 and p 5 and then patterns 1, 4 and 3. This distribution differs from ours (p<0.01) maybe because of the patient's selection criteria used. Another possibility to explain the differences in pattern distribution is that some zygotes can be included in two patterns at the same time [9]. Although pattern 0 includes polarized and non-polarized PNs, the number of NPBs seems to be related to the size and the position of NPBs [4, 10]. It has been suggested that patterns 1–5 are irregularities of normality [4].
Scott et al. [5] showed a very different distribution of Z patterns than that the one found in our study. While the percentage of Z1 and Z4 is similar (22.7% compared to that of Scott 36.3%and 5.1% vs. 5.2%), our data shows a clear difference between Z2 (6% vs. 31.7%) in favor to Z3 (66.2% vs. 26.8%). This statistically different distribution (p<0.01) can be related to the previous selection of patients involved in our study, or to the low number of zygotes analyzed in comparison to the study presented by Scott [5]. Scott uses more strict criteria than Tesarik with respect to the number of NPBs and the difference in the number of NPBs observed between the two pronuclei; this may result in a larger Z3 group. However, in the last report [11] less stringent criteria are described and Z3 pattern is subdivided in order to allow a more accurate classification of the different zygotes.
This study correlates the different morphological patterns of zygotes described in the literature with embryo development in order to determine their predictive value. The basic procedure used in the routine laboratory methodology consisted in evaluating the zygotes by a single observation, establishing the pronuclear pattern using the classification systems described by Tesarik and Scott, and then monitoring the development of embryos. In an IVF laboratory, fertilization check is routinely conducted at 16–20 h after insemination, despite the fact that the two pronuclei appear 6 h after ICSI in 60% of the cases [12]. The asynchrony in the appearance of the pronuclei recommends a second observation when the presence of two polar bodies in the first observation is noted. Nagy et al. [12] used serial observations of the zygote to describe the movement of the NPBs from non-polarization to polarization. Payne et al. [10] described pronuclear appearance and migration to the centre of the zygote, mostly accompanied by cytoplasmic waves.
Only one single observation is performed in most of the cases in our study and 50.4% of the zygotes show both PN with polarized NPB. This fact can be attributed to the fact that the observation was done late but no differences in polarization where found in early observations when compared to late ones (data not shown).
The evaluation of zygote considering PN morphology and cytoplasmic morphology at the same time has been related to embryo quality in previous reports [8]; but lasts classifications based only on PN morphology presented relationships with further embryo development [4, 5]. The simple application of these classifications optimize the time of zygote observation. The relevance of the PN position and NPB distribution has been previously described. The presence of NPB in the PN is related to the synthesis of DNA. The association between the chromatin and NPB depends on the cellular cycle phase of the zygote [13] which takes place at the late stages of the zygote when the G1 phase is ending. The appearance of pronuclei coincides with the beginning of the S phase [14]. Different length of S and G2 phase reflects the asynchrony in first cell cycle between embryos [15]. The asynchrony of phase S is not related with PN size but with the grade of DNA synthesis [16]. Despite that in NPB there is no DNA replication, RNA synthesis exists before the beginning of phase S. Tesarik et al. [17] suggests that the presence of chromatin in NPB is temporal and occurs in two steps: at the end of the first step where the chromatin is synthetically active the chromatin is present in the assembly of NPB. Chromatin appears to be retracted from the resulting structures which subsequently remain quiescent during 2 cell cycles. The re-entry of chromatin into the NPBs marks the end of NPB quiescence and the beginning of the second step of the nucleologenesis when nucleoli assume the characteristic ultrastuctural organization and start the synthesis and processing of ribosomal RNA.
Eighty-three percent of PNs were in an adjacent relative position. It has been reported that contact of their membranes is followed by chromatin polarization, which is a previous important step in the formation of the axis for embryo cleavage [18]. It seems that the most relevant factor to predict embryo morphology is the synchrony in PN appearance [10, 19] as well as in NPB distribution [4, 5, 7].
Pronuclei appear following extrusion of the second polar body at the end of oocyte meiosis. At the time of pronuclear formation, cytoplasmic movements of energy-producing organelles from the cell periphery towards the centre take place [10]. The appearance of a dark halo of energy-producing mitochondria around the PN [20] has been considered of good prognostic value as a part of a complex scoring system [8, 21] and associated with the implantation ability of embryos. In the literature, the incidence of halo appearance in zygotes ranges from 67.5% to 88.7% [21, 22]. Any halo presence has been related to blastocyst quality [23].
In our study we find no correlation between the presence of halo and embryo quality on day 2. Oocyte maturity and quality is reflected by a homogenous cytoplasm without vacuoles and granules [24]. Our findings suggest that a large number of dark granules in zygotes are associated with poor quality embryos. The negative correlation between granules and embryo quality is not confirmed in other reports [25]. Our data show a statistically significant correlation between the presence of granules and the occurrence of poor embryo quality. Van Blerkom et al. 1992 [26] defined these dark granules as regions of intracellular necrosis that arise during the latter stages of maturation, particularly during the time of the first polar body extrusion. In this work the author describes a similar frequency of aneuploidies in oocytes with granules in comparison with oocytes with normal appearance. For Alikani et al. [27] the most commonly encountered cytoplasmic anomaly was partial intracellular necrosis, evidenced by the presence of several small scattered dense bodies (presumably chromatin) or pyknotic nuclei and the majority of such oocytes were normally fertilized.
More than half (55.8 %) of the good quality embryos corresponded to zygotes with patterns 0 and 3 (60.9% of p0 develop into good quality embryos and 67.3% of p3) although no differences are observed in the probability of pattern 0, 1, 2 and 3 zygotes to develop into good quality embryos. Pattern 4 zygotes have a significantly decreased probability to become a good quality embryo (p<0.001). Tesarik and Greco [4] reported that 45% of pattern 1 and 2 zygotes show arrested development. It has to be taken into account that the data on embryo development obtained on day 3 were difficult to correlate with our data obtained on day 2. In our data, embryos coming from p0 (60.8% vs. 37.3%, p<0.001), p1 (55.4% vs. 14.3%, p<0.001), p2 (52.5%vs 27.8%, p<0.001) and p3 (67.3% vs. 26.7%, p<0.001) developed into good quality embryos in a higher percentage than Tesarik et al. [28]. However, there are no statistical differences from embryos coming from p4. Multinucleation is present in the same proportion in embryos for each PN pattern both in our data and in Tesarik's. We find poor prognosis embryos from pattern 4 zygotes, with a higher percentage of blastomere multi-nucleation compared to the data from Tesarik and Greco [4] and Sadowy et al. [6]. It would seem that the presence of a very low number of NPBs (corresponding to p4) in any PN is a poor prognosis factor for embryo development. Balaban et al. [29] reported that a higher percentage of pattern 0 zygotes in Tesarik's system reached the blastocyst stage when compared to zygotes with double PN abnormality. The relationship between the pronuclear morphology and embryo development and chromosome constitution have also been noted [30] confirming the good prognosis of pattern 0 from Tesarik's classification.
Our data suggest that zygotes from groups Z1 and Z2 develop into good quality embryos as previously reported. Group Z3 is a neutral predictor, although this is probably related to the heterogeneity of the group. In our results from group Z4, the association between asymmetric and/or separate PNs and poor prognosis embryos was confirmed [5]. Pronuclear morphology studies have found that differences of more than 4 μm in the diameter of PNs are associated with aneuploidy [6]. The analysis of chromosomal status of embryos from different patterns suggests a lower incidence of chromosomal abnormalities in patients aged <37 years with zygotes with similar PN size [31]. A high risk of chromosomally abnormal embryos and low rate of blastocyst formation was observed in zygotes with asymmetry or asynchrony [30, 32]. The poor quality of embryos from Z4 zygotes in our data is confirmed by Scott et al. [5] through a high rate of development arrest and low rate of blastocyst formation.
Both Tesarik and Scott claim that a successful implantation is mostly associated with embryos from pattern 0 zygotes, or embryos developing from group Z1/Z2 zygotes. No data on implantation rate related to PN pattern are available in our study as the number of homologous transfers is too low to allow the analysis. Interestingly, choosing embryos for transfer based solely on embryo quality in our patients results in the selection of 75% embryos from pattern 0 zygotes and 55% of embryos from groups Z1/Z2. The analysis of implantation of embryos developed from the same zygote pattern and its correlation with embryo quality has been reported on day 3 [4] and on blastocyst stage [5]. These data are useful in the assessment of good quality embryos with high implantation potential.
The usefulness of the two classifications is limited although the application of classifications based solely on the PN morphology simplifies and minimizes the time of observation. First of all, Tesarik's classification is not unique; some PN can be included into various patterns of irregularities at the same time. This fact presents a reasonable doubt about the prognosis of the future zygote development. On the other hand, Scott's classification is not discriminatory enough; the wideness of Z3 group supposes irregularities of normality without an accurate evaluation of the subsequent embryo development.
The challenge for ART clinics is to transfer fewer embryos in order to minimize the risk of multiple pregnancies and births, while still maintaining the greatest chance of pregnancy for their patients [33]. An optimized method for embryo selection is required to achieve this goal while not decreasing the pregnancy and implantation rates.
A sequential embryo assessment method [34] can be used as a valid indicator of good subsequent embryo development and implantation prognosis, especially if the patient has several morphologically similar embryos available on the day of the transfer.
The assessment of morphological parameters that can be predictive of embryo quality can be performed at very early stages and proceed through oocytes, zygotes, early cleavage embryos and blastocysts. It has also been described that follicular vascularization and oxygen concentration can be related to oocyte quality and chromosomal/spindle normality [35]. Sequential assessment would involve the evaluation of oocyte quality, the classification in PN patterns, embryo morphology and cleavage rate analysis and the ability to reach the blastocyst stage. All these criteria, although always subjective, have been correlated to embryo quality, chromosomal normality and implantation potential, and will allow a more accurate evaluation of embryo quality, resulting in an optimized method to select embryos for transfer.
References
Gardner DK, Lane M. Towards a single embryo transfer. Reprod BioMed Online 2003;6:470–81.
Ludwig M, Schopper B, Al-Hasani S, Dietrich K. Clinical use of a pronuclear stage score following intracytoplasmatic sperm injection: impact on pregnancy rates under the conditions of the German embryo law. Hum Reprod 2000;15:325–29.
Zollner U, Zollner K-P, Hartl G, Dietl J, Steck T. The use of a detailed zygote score after IVF/ICSI to obtain good quality blastocysts: the German experience. Hum Reprod 2002;17:1327–33.
Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod 1999;14:1318–23.
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod 2000;15:2394–403.
Sadowy S, Tomkin G, Munné S, Ferrara-Congedo T, Cohen J. Impaired development of zygotes with uneven pronuclear size. Zygote 1998;6:137–41.
Wright G, Wiker S, Elsner C, Kort H, Massey J, Mitchell D, Toledo A, Cohen J. Observations on the morphology in human zygotes and implications for cryopreservation. Hum Reprod 1990;5:109–15.
Scott L, Smith S. The successful of pronuclear embryo transfers the day following oocyte retrieval. Hum Reprod 1998;13:1003–13.
Wittemer C, Bettahar-Lebugle K, Ohl J, Rongières C, Nisand I, Gerlinger P. Zygote evaluation: and efficient tool for embryo selection. Hum Reprod 2000;15:2591–7.
Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse cinematography. Hum Reprod 1997;12:532–41.
Scott, L. Pronuclear scoring as a predictor of embryo development. Reprod BioMedOnline 2003;6:470–81.
Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A. Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Hum Reprod 1994;9:1743–8.
Tesarik J, Kopecny V. Developmental control of the human male pronucleus by ooplasmic factors. Hum Reprod 1989;4:962–8.
Tesarik J, Kopecny V. Development of human male pronucleous: ultrastructure and timing. Gamete Res 1989;24:135–49.
Capmany G, Taylor A, Braude PR, Bolton VN. The timing of pronuclear formation, DNA synthesis and cleavage in the human 1-cell embryo. Molec Hum Reprod 1996;2:299–306.
Balakier H, MacLusky NJ, Casper RF. Characterization of the first cell cycle in human zygotes. implications for cryopreservation. Fertil Steril 1993;59:359–65.
Tesarik J, Kopecny V. Assembly of the nucleolar precursor bodies in human male pronuclei is correlated with an early RNA synthetic activity. Experimental Cell Research 1990;191:153–56.
Edwards RG, Beard K. Oocyte polarity and cell determination in early mammalian embryos. Molec Hum Reprod 1997;3:863–905.
Staessen C, Janssenswillen C, Devroey P, Van Steirteghem AC. Cytogenetic and morphological observations of single pronucleated human oocytes after in-vitro fertilization. Hum Reprod 1993;8:221–3.
Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP contents and competence. Hum Reprod 2000;15:2621–33.
Salumets, A, Hydén-Granskog, C, Suikkari, A-M, Tiitinen, A, Tuuri, T. The predective value of pronuclear morphology of zygotes in the assessment of human embryo quality. Hum Reprod 2001;16:2177–81.
Demirel LC, Evirgen O, Aydos K, Ünlü C. The impact of the source of spermatozoa used for ICSI on pronuclear morphology. Hum Reprod 2001;16:2327–32.
Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Wiesinger R, Puchner M, Tews G. Presence, but not type or degree of extension, of a cytoplasmic halo has a significant influence on preimplantation development and implantation behavior. Hum Reprod 2003;18:2406–12.
Plachot M, Crozet N. Fertilization abnormalities in human in vitro fertilization. Hum Reprod 1992;7(Suppl 1):89–94.
Ebner T, Yaman C, Moser M. Prognostic value of first polar body morphology on fertilization rate and embryo quality in intracytoplasmic sperm injection. Hum Reprod 2000;15:427–30.
Van Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod 1992;3:379–90.
Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote 1995;3:283–88.
Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-Bacrie P, Dumont-Hassan. Embryos with high implantation potential after intracytoplasmic sperm injection can be recognized by a simple, non-invasive examination of pronuclear morphology. Hum Reprod 2000;15:1396–99.
Balaban B, Urman B, Isiklar A, Alatas C, Aksoy S, Mercan R, Mumcu A, Nuhoglu A. The effect of pronuclear morphology on embryo quality parameters and blastocyst transfer outcome. Hum Reprod 2001;16:2357–61.
Balaban B. Pronuclear morphology predicts embryo development and chromosome constitution. Reprod BioMed Online 2004;8(6):695–700.
Gmiz P, Rubio C, de los Santos MJ, Mercader A, Simón C, Remohí J, Pellicer A. The effect of pronuclear morphology on early development and chromosomal abnormalities in cleavage-stage embryos. Hum Reprod 2003;18:2413–9.
Kahraman S, Kumtepe Y, Sertyel S, Dönmez E, Benkhalifa M, Findikli N, Vanderzwalmen P. Pronuclear morphology scoring and chromosomal status of embryos in severe male infertility. Hum Reprod 2002;17:3193–3200.
Tur R, Barri PN, Coroleu B, Buxaderas R, Parera N, Balasch J. Use of a prediction model for a high-order multiple implantation after ovarian stimulation with gonadotrophins. Fertil Steril 2005;83(1):116–21.
Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Hum Reprod 2003;18:1307–12.
Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 1997;12:1047–55.
Acknowledgements
This work was carried out under the auspices of the Catedra de Investigación de Obstetricia y Ginecología del Institut Universitari Dexeus
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arroyo, G., Veiga, A., Santaló, J. et al. Developmental prognosis for zygotes based on pronuclear pattern: Usefulness of pronuclear scoring. J Assist Reprod Genet 24, 173–181 (2007). https://doi.org/10.1007/s10815-006-9099-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-006-9099-0