Abstract
Effect of various macroalgae substitution for Sargassum thunbergii in diet on growth, body composition, and survival of sea cucumber (Apostichopus japonicus) subjected to air and low salinity exposures was determined. Nine hundred juvenile sea cucumber were distributed into 18 tanks (50 sea cucumber per tank). Six experimental diets were prepared. Sargassum thunbergii was included in the control (Con) diet. The macroalgae Sargassum horneri, Undaria pinnatifida, Saccharina japonica, Ulva australis, and combined U. pinnatifida and S. japonica were included to replace S. thunbergii in the Con diet, referred to as the SH, UA, UP, SJ, and combined diets, respectively. All diets were assigned to triplicate groups of sea cucumber. Sea cucumbers were fed daily for 8 weeks. After the 8-week feeding trial, sea cucumbers were subjected to 30-h air and 12-h low salinity at 10 psu exposures. Weight gain and specific growth rate of sea cucumber fed the Con, SH, and UA diets were significantly greater than those of sea cucumber fed the UP, SJ, and combined diets. The chemical composition of the whole sea cucumber, except for moisture content, was not affected by the experimental diets. No difference in survival of sea cucumber fed all experimental diets was observed at the end of 4-day post observation period after the 30-h air and 12-h low salinity exposures. In conclusion, S. thunbergii can be substituted with either S. horneri or U. australis in sea cucumber feed without retarding growth and stress resistance against air and low salinity exposures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea cucumber (Apostichopus japonicus) exhibiting cryptic nocturnal behavior (Chang et al. 2004; Guancang et al. 2011) is a commercially important aquaculture species in China, Republic of Korea, Japan, and the Russian Federation (Yuan et al. 2006; Gu et al. 2010; Seo and Lee 2011; Oh et al. 2014; Shahabuddin and Yoshimatsu 2015; Ru et al. 2019). In the Republic of Korea, the annual aquaculture production of sea cucumber reached 93 tonnes in 2019 (KOSIS 2020). Dried sea cucumber has been used as an ideal tonic food with high protein and low fat (Chen 2003). Production of sea cucumber has become a commercially lucrative area and needs to be extended to satisfy the high demand for the increasing human population (Ru et al. 2019), which can be largely supplied by aquaculture (Shahabuddin et al. 2017).
Sea cucumbers are known to feed on sedimentary organic litter like other holothurians in the wild (Yuan et al. 2006). In the aquaculture industry, formulated diets mainly composed of macroalgae powder and sea mud are commonly applied to improve the production of sea cucumber (Chang et al. 2003). The brown macroalga, Sargassum thunbergii, is one of the most common feeds for sea cucumber farming in the Republic of Korea, China, and Japan (Sui 1989; Battaglene et al. 1999). However, the wild resource of S. thunbergii has dramatically decreased due to its high demand resulting from the expanded sea cucumber farming, and it has eventually resulted in an increased price of this macroalga (Yuan 2005).
As S. thunbergii is not being produced commercially, it is difficult for local suppliers or collectors to satisfy an increasingly high demand for this macroalga for the expanded sea cucumber farming in Asia. Therefore, development of formulated feed is highly needed to achieve stable production of sea cucumber. Sea cucumber fed diets substituting 50% of S. thunbergii with different macroalgae (Undaria pinnatifida and Saccharina japonica) and plant ingredients (Brassica oleracea var. capitata and rice straw powder) or diets substituting 25% of S. thunbergii with other plant ingredients (fermented soybean meal and distiller dried grain powder) achieved superior SGR to the control diet containing S. thunbergii at 40% (Seo et al. 2011). Wu et al. (2015) evaluated dietary substitution effect of corn (Zea mays) leaf for S. thunbergii on growth of sea cucumber when 25, 50, 75, and 100% of S. thunbergii were substituted with corn leaf in sea cucumber feed composed of 30% S. thunbergii and 70% sea mud and recommended the combined use of 18.8% S. thunbergii and 11.2% corn leaf in sea cucumber feed for the best growth performance. Searching for an alternative source for S. thunbergii is highly needed to reduce the overharvesting pressure of S. thunbergii in the wild and achieve stable and effective production of sea cucumber.
Macroalgae, such as U. pinnatifida, S. japonica, U. australis, and S. horneri, are commonly found in the shallow coastal areas of Republic of Korea. As these macroalgae are rich in proteins, soluble dietary fibers, minerals, vitamins, phytochemicals, antioxidants, and polyunsaturated fatty acids containing low caloric value, and defensive and storage compounds (Fleurence 1999; Colombo et al. 2006; Mohamed et al. 2012; Wan et al. 2019; Gao et al. 2020), they can be considered as feed ingredients in aquafeeds, especially U. pinnatifida and S. japonica, some of the most common feeds adopted by the farmers to raise abalone and sea urchins (Yone et al. 1986; Agatsuma et al. 2002; Qi et al. 2010; Cho et al. 2011). Anisuzzaman et al. (2017) described that dietary inclusion of macroalgae (Ulva lactuca or S. japonica) and microalgae (Nannochloropsis oculata) at 15% improved growth of sea cucumber compared to the control diet containing the same amount of wheat flour. Xia et al. (2012a) also reported that sea cucumber fed diets containing different seaweed (S. thunbergii, S. polycystum, Zostera marina, U. lactuca, S. japonica, and boiled S. japonica) powders and sea mud powders at the ratio of 3:7, respectively greatly affected growth, ingestion rate, digestibility, and ammonia–nitrogen production of sea cucumber, and suggested that U. lactuca, Z. marina, and fresh S. japonica would be the optimum seaweeds for the commercial culture of sea cucumber. However, knowledge on a suitable and sustainable substitute for S. thunbergii is still insufficient to expand the commercial culture of sea cucumber.
Abiotic factors, such as air exposure and salinity change, are the most important ecological factors affecting growth and metabolism of aquatic animals (Vidolin et al. 2002; Hou et al. 2019). Therefore, these abiotic stressors resulted in severe economic and resource losses, and strongly limit the expansion of sea cucumber industry (Huo et al. 2018). Salinity change mainly caused by water exchange, evaporation, and precipitation (Wang et al. 2014) can lead to osmotic stress and severe mortality of sea cucumber (Meng et al. 2011). Sea cucumber is also frequently subjected to air exposure (desiccation) stressor during various farm activities and transportation without water (Hou et al. 2019). Minimizing the undesirable effect of air exposure during transportation of mollusk is critical for successful aquaculture since it affects survival and growth (Malham et al. 2003; Morash and Alter 2016). Balanced nutrition in formulated feed can help to improve the metabolic and physiological status of aquatic animals and strengthen their resistant capacity against various abiotic stressors, such as air exposure and salinity change stressors (Liu et al. 2015; Lee et al. 2016; Ansary et al. 2019a, b).
In this study, therefore, dietary substitution effect of various macroalgae (S. horneri, U. australis, U. pinnatifida, S. japonica, and the combined U. pinnatifida and S. japonica) for S. thunbergii on growth, body composition, and stress resistance of sea cucumber subjected to air and low salinity exposures was evaluated.
Materials and methods
Rearing condition of the experimental animals
Juvenile sea cucumbers purchased from a private hatchery (Namhae-gun, Gyeongsangnam-do, Korea) were moved and acclimated to the experimental conditions for a couple of weeks before starting the feeding trial. During the acclimation period, sea cucumbers were daily fed with a commercial diet (the mixture of Sargassum spp. and sea mud) at 3–5% biomass.
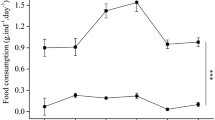
A total of 900 juvenile (initial weight of 1.46 g) sea cucumbers were randomly disseminated into indoor 18 300-L round-shaped tanks (50 juveniles per tank) with flow-through system. Sand-filtered seawater temperature fluctuated from 8.7 to 15.5 °C (mean ± SD; 12.0 ± 0.16 °C) and flow rate of tank was 3 L min−1. Continuous aeration was provided into each tank and a photoperiod of 10 h: 14 h (light: dark) cycle was adapted based on Guancang et al. (2011)’s study, in which the optimal photoperiod for rearing sea cucumber was 6–15 h light per day. The diets were mixed well with seawater in a 50-mL tube and fed to sea cucumber once a day (14:00) to satiation (ca. 2–5% of total biomass) with a little leftover for 8 weeks. Aeration and water supply stopped for 3 h after the designated daily feed supply. Aeration was supplied at 17:00 again, but sand-filtered seawater was again supplied at the morning (09:00) in the following day until the next designated feed supply. To maintain the water quality, the bottom of the tank was cleaned and dead sea cucumbers were removed daily.
Preparation of the experimental diets
Six experimental diets were prepared (Table 1). The control (Con) diet contained 40% defatted soybean meal and 10% brown fish meal as the protein source. Thirty five percent Sargassum thunbergii was included in the Con diet. Sargassum thunbergii in the Con diet was replaced with an equal amount of S. horneri, U. australis, U. pinnatifida, S. japonica, and the combined U. pinnatifida and S. japonica (= 1:1, dry weight basis) powder, referred to as the SH, UA, UP, SJ, and combined diets, respectively. Fresh clean seaweeds were collected in the wild, air-dried at 40 °C for 48 h, and then ground to powders. All diets were assigned to triplicate groups of sea cucumber. All feed materials were sieved with 250 µm mesh sieve (Samwoo Industry Co., Korea) and mixed well. All experimental diets satisfied the protein and lipid requirements of sea cucumber (Seo and Lee 2011). All diets were stored in a freezer (− 15 °C) and used in small amounts whenever necessary.
Sample collection and measurements
All surviving sea cucumbers from each tank were counted and collectively weighted to measure weight gain at the end of the 8-week feeding trial. Specific growth rate (SGR) (% day−1) was calculated as follows: SGR = (ln final weight − ln initial weight) × 100 / day of feeding.
Analyses of the chemical composition of the experimental diets and animals
Ten sea cucumbers from each tank were randomly selected and sacrificed for the chemical analysis. The chemical composition of the experimental diets and sea cucumber was determined according to the standard AOAC (1990) methods. Crude protein (N × 6.25) was measured by the Kjeldahl method (Buchi B-324/435/412, Auto Kjeldahl System, Switzerland), crude lipid content was analyzed using an ether-extraction method, moisture content was calculated by oven drying at 105 °C for 24 h, and ash content was estimated by a muffle furnace at 550 °C for 4 h.
Monitoring survival of sea cucumber subjected to air exposure stressor
After the 8-week feeding trial, 15 sea cucumbers were randomly chosen from each tank and the rest of the sea cucumbers were removed. Seawater was completely drained out from all experimental tanks and exposed to air for 30 h. Then all tanks were refilled with sand-filtered seawater and other rearing conditions were maintained as same as the 8-week feeding trial conditions except for feeding. Sea cucumber was starved and survival was monitored for the next 4 days after the 30-h air exposure. Dead sea cucumbers were removed every 3 h for the first 2 days and every 6 h for the remaining 2 days. Sufficient oxygen was supplied to seawater in tanks during the 4-day post observation period.
Monitoring survival of sea cucumber subjected to low salinity stressor
Fifteen sea cucumbers were randomly selected from each tank at the end of the 8-week feeding trial to evaluate resistance against low salinity change stressor. Eighteen round-shaped plastic bottom–screened containers (30 cm in diameter and 8.5 cm in height) were placed in one 3-t rectangular water tank adjusted at 10 psu salinity by mixing tap water with sand-filtered seawater (32 psu). Salinity was measured by using YSI 6-Series Multi Parameter (YSI, USA). Sea cucumbers were exposed to 10 psu saline water for 12 h, which was then drained out and then refilled with sand-filtered seawater to monitor survival of sea cucumber for the next 4-day post observation period. Other rearing conditions were maintained as same as the experimental conditions except for feeding. Sea cucumber was starved and survival was monitored for the next 4 days after the 12-h low salinity exposure. Dead sea cucumbers were removed every 3 h for the first 2 days and every 6 h for the remaining 2 days. Sufficient oxygen was supplied to water in a tank during the 4-day post observation period.
Statistical analysis
Statistical analysis was accomplished by the software SAS version 9.3 (SAS Institute, USA). Percentage data were subjected to arcsine-transformation prior to analysis. One-way ANOVA and Duncan’s multiple range test (Duncan 1955) were used to compare the means of treatment. Survival of sea cucumber during the 4-day post observation period after the 30-h air and 12-h low salinity exposure stressors was analyzed by using Kaplan–Meier survival curve, log-rank, and Wilcoxon tests.
Results
Survival and growth of sea cucumber at the end of the 8-week feeding trial
Survival of sea cucumber (≥ 98%) was not significantly (p < 0.3) affected by the experimental diets (Table 2). Significantly greater weight gain and SGR (p < 0.004 and p < 0.003, respectively) were obtained in sea cucumber fed the Con, SH, and UA diets than those of sea cucumber fed the UP, SJ, and combined diets. However, there was no significant (p > 0.05) difference in weight gain and SGR of sea cucumber fed the Con, SH, and UA diets.
Whole body proximate composition of sea cucumber
Moisture content of sea cucumber fed the UP diet was significantly (p < 0.0001) higher than that of sea cucumber fed the Con, SH, UA, and SJ diets, but not significantly (p > 0.05) different from that of sea cucumber fed the combined diet (Table 3). Crude protein content of sea cucumber ranged from 3.4 to 3.5%, crude lipid ranged from 0.3 to 0.4%, and ash ranged from 3.4 to 3.5%. These parameters were not significantly (p > 0.05) affected by the experimental diets.
Survival of sea cucumber exposed to the 30-h air exposure stressor
Sea cucumbers fed all experimental diets were all alive during the 30-h air exposure after the 8-week feeding trial (Fig. 1). Mortality was first observed in sea cucumbers fed the Con, SH, SJ, and combined diets at 9 h after the 30-h air exposure. However, survival of sea cucumber was not significantly (p > 0.8 for log-rank test) affected by the experimental diets at the end of the 4-day post observation period.
Survival of sea cucumber subjected to the 12-h low salinity stressor
Mortality of sea cucumbers fed the combined diet started at 6 h after the 12-h low salinity stressor. However, survival of sea cucumber was not significantly (p > 0.4 for log-rank test) affected by the experimental diets at the end of the 4-day post observation period (Fig. 2).
Discussion
Relatively high SGR values (1.63–1.70%/day) of sea cucumbers obtained in this study compared to − 0.04–0.54 (Seo and Lee 2011), 0.15–1.0 (Seo et al. 2011), 0.29–1.47 (Song et al. 2017), 0.39–0.59 (Wu et al. 2015), 0.48–0.80 (Xia et al. 2012a), 0.83–1.16 (Li et al. 2021), and 0.96–1.42 % day−1 (Wen et al. 2016) reported in the same species of sea cucumber in other studies indicated that growth of sea cucumber was well achieved in this study. No difference in weight gain and SGR of sea cucumber fed the Con, SH, and UA diets indicated that S. thunbergii could be replaced with either S. horneri or U. australis in formulated feed without lowering the growth performance of sea cucumber.
Since S. horneri and U. australis are the main macroalgae causing golden and green tides, respectively, in Republic of Korea (Hawang et al., 2016; Kim et al. 2017), their availability as feed ingredients in aquafeed seems to be high over S. thunbergii. In addition, substitutability of those fouling macroalgae (S. horneri and U. australis) and their combination for U. pinnatifida in abalone (H. discus) feed was also well proved (Ansary et al. 2019a, b, c). This is another study to suggest substitutability of those fouling macroalgae for S. thunbergii in formulated sea cucumber feed. Similarly, Song et al. (2017) showed that green tide–causing macroalgae (Chaetomorpha linum) could be used as a replacer for S. thunbergii in sea cucumber feed because of its high protein and low cellulose content. Gao et al. (2011a) also suggested that red algae (Gracilaria lemaneiformis) would be more preferable feed for sea cucumber rather than S. thunbergii because the administration of G. lemaneiformis achieved superior SGR to S. thunbergii with or without a mixture of benthic matter.
Inferior weight gain and SGR of sea cucumber fed the UP, SJ, and combined diets to the Con diet in this study indicated that U. pinnatifida and S. japonica seemed to be less suitable than S. thunbergii in formulated sea cucumber feed. Unlike this study, however, Seo et al. (2011) reported that sea cucumber fed formulated diets substituting 50% of S. thunbergii with U. pinnatifida, S. japonica, and plant source (Brassica oleracea var. capitata and rice straw) achieved greater SGR compared to the control diet containing S. thunbergii at 40% without any substitution in the 10-week feeding trial. This difference could have resulted from differences in substitution ratio of S. thunbergii with macroalgae in diets (complete (100%) substitution of S. thunbergii with macroalgae (U. pinnatifida and S. japonica) in this study vs. 50% of substitution of S. thunbergii with macroalgae (U. pinnatifida or S. japonica) (Seo et al. 2011)). Similarly, combined macroalgae produced superior growth performance of abalone to a single macroalga (Naidoo et al. 2006; Robertson-Andersson et al. 2011; Viera et al. 2011). Nevertheless, the combined diet did not improve the growth of sea cucumber in this study because both UP and SJ diets produced inferior growth to the Con diet, and their combined effect had no desirable effect on growth.
Administration of a nutrition-balanced feed is one of the critical factors affecting the growth and development of all living animals. Generally speaking, sea mud is the main part of the diet for sea cucumber, which contains low nutrients (Zhao et al. 2012). This could be another reason for superior SGR of sea cucumber fed with nutrition-balanced diets to a diet containing sea mud powder (Xia et al. 2012a, b; Wu et al. 2015; Anisuzzaman et al. 2017; Song et al. 2017; Li et al. 2021). Substitutability of S. thunbergii with other macroalgae (either common feed for abalone (U. pinnatifida and S. japonica) or fouling macroalgae (S. horneri and U. australis)) seemed to be also closely related to with or without supplementation of sea mud powder in sea cucumber feed.
The chemical composition of the whole body sea cucumber, except for the moisture content, was not affected by the experimental diets in this study. Similarly, the whole body composition of the same species of sea cucumber was unaffected by diets substituting S. thunbergii with corn leaf (Wu et al. 2015) or substituting fish meal with red alga (Pyropia) spheroplasts (Shahabuddin et al. 2015). Unlike this study, however, the chemical composition of whole body of the same species of sea cucumber was affected by diets containing different feed ingredients (Sargassum muticum, G. lemaneiformis, U. lactuca, and benthic matter collected from an outdoor pond) (Wen et al. 2016) or substituting S. thunbergii with various plant ingredients (Seo et al. 2011). The chemical composition of sea cucumber was similar to that in this study, but varied seasonally (Gao et al. 2011b).
A nutrition-balanced feed may act as an immune regulator to lower the impact of various abiotic stressors in aquatic animals (Liu et al. 2015; Lee et al. 2016; Ansary et al. 2019a, b). No difference in survival of sea cucumber fed all experimental diets in the 4-day post observation periods after the 30-h air and 12-h low salinity exposures in this study indicated that dietary substitution of S. thunbergii with other macroalgae (S. horneri, U. australis, U. pinnatifida, S. japonica, and combined U. pinnatifida and S. japonica) did not deteriorate survival of sea cucumber. Similarly, Hou et al. (2019) reported that less than 6 h of air exposure did not cause irreparable damage to sea cucumber in handling and shipping when four groups of sea cucumber were resubmerged in aerated seawater for 24 h after 0 (control group), 1, 3, and 6 h of air exposure. Meng et al. (2011) showed that no mortality was observed during salinity decrease, but 40–50% mortality occurred when salinity was maintained at low levels when sea cucumbers were reared at 30 psu, acclimated at either 20 psu or 25 psu at a rate of 2.5 psu every 6 h, maintained at designated salinity for 4 days, and then returned at 30 psu for 4 days, and suggested that low salinity can cause mortality of sea cucumber partially due to the rapid drop of osmotic pressure in the coelomic fluid during hypo-osmotic stress after heavy rainfall in summer. Unlike this study, however, commercial diet supplemented with U. lactuca and grape seed extract improved productivity and lowered mortality of greenlip abalone (H. laevigata) during high summer water temperatures in Southern Australia (Lange et al. 2014).
Therefore, we can suggest that macroalgae (S. horneri and U. australis) can be effectively used as a substitute for S. thunbergii in sea cucumber feed without impairing growth performance and survival of sea cucumber against the 30-h air and 12-h low salinity exposures.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agatsuma Y, Yamada Y, Taniguchi K (2002) Dietary effect of the boiled stipe of brown alga Undaria pinnatifida on the growth and gonadal enhancement of the sea urchin Strongylocentrotus nudus. Fish Sci 68:1274–1281
Anisuzzaman M, Jeong U, Jin F, Choi J, Kabery K, Lee D, Yu HS, Kang S (2017) Effects of different algae in diet on growth and interleukin (IL)-10 production of juvenile sea cucumber Apostichopus japonicus. Fish Aquatic Sci 20:24
Ansary MWR, Baek SI, Jeong HS, Lee KW, Cho SH, Kim HS, Jwa MS (2019a) Substitution effect of the combined fouling macroalgae, Ulva australis and Sargassum horneri for Undaria pinnatifida in formulated diets on growth and body composition of juvenile abalone (Haliotis discus, Reeve 1846) subjected to air exposure stressor. J Appl Phycol 31:3245–3254
Ansary MWR, Jeong HS, Lee KW, Kim PY, Kim J, Yun A, Cho SH (2019b) Dietary substitution effect of Ulva australis for Undaria pinnatifida on growth, body composition and air exposure stress of juvenile abalone, Haliotis discus (Reeve 1846). J Appl Phycol 31:1467–1474
Ansary MWR, Jeong HS, Lee KW, Kim HS, Kim J, Yun A, Cho SH, Kim PY, Kim T (2019c) The effect of substituting Undaria pinnatifida in formulated feeds with Sargassum horneri on growth and body composition of juvenile abalone (Haliotis discus, Reeve 1846). J Appl Phycol 31:2125–2132
AOAC (1990) Official methods of analysis (15th edn). Association of Official Analytical Chemists, Arlington, VA, USA, 1298 pp.
Battaglene SC, Seymour EJ, Ramofafia C (1999) Survival and growth of cultured juvenile sea cucumbers Holothuria scabra. Aquaculture 178:293–322
Chang ZY, Yi JL, Mu KQ (2003) Factors of influence on growth and survival of Apostichopus japonicus. Mod Fish Inf 18:24–26
Chang Y Q, Ding J, Song J, Yang W (2004) Biological research and aquaculture of sea cucumber and sea urchin. Ocean Press, Beijing. China. pp 3–89. (In Chinese)
Chen J (2003) Overview of sea cucumber farming and sea ranching practices in China. SPC Beche-De-Mer Information Bulletin 18:18–23
Cho SH, Cho YJ, Choi CY (2011) Effect of feeding regime on compensatory growth of juvenile abalone, Haliotis discus hannai, fed on the dry sea tangle, Laminaria japonica. J World Aquacult Soc 42:122–126
Colombo ML, Rise P, Giavarini F, De Angelis L, Galli C, Bolis CL (2006) Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods Hum Nutr 61:64–69
Dantong L, Yaqing C, Zhenhai W, Wei C, Junya W, Guodong C (2009) Analysis of nutritive composition of body wall in wild sea cucumber Apstichopus japonicus Selenka at Zhangzi Island in spring and autumn. Fish Sci 28:365–369
Duncan CB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Gao Q, Wang Y, Dong S, Sun Z, Wang F (2011a) Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea): evidence from carbon stable isotope. Aquaculture 319:272–276
Gao F, Qiang X, Hongsheng Y (2011b) Seasonal biochemical changes in composition of body wall tissues of sea cucumber Apostichopus japonicus. Chin J Ocean Limnol 29:252–260
Gao X, Qu H, Shan S, Song C, Baranenko D, Li Y, Lu W (2020) A novel polysaccharide isolated from Ulva pertusa: structure and physicochemical property. Carbohydr Polym 233:115849
Gu M, Ma H, Mai K, Zhang W, Ai Q, Wang X, Bai N (2010) Immune response of sea cucumber Apostichopus japonicus coelomocytes to several immunostimulants in vitro. Aquaculture 306:49–56
Guancang D, Shuanglin D, Xiangli T, Fang W (2011) Effects of photoperiod on daily activity rhythm of juvenile sea cucumber, Apostichopus japonicus (Selenka). Chin J Oceanol Limnol 29:1015–1022
Hawang EK, Lee SJ, Ha DS, Park CS (2016) Sargassum golden tides in the Shinnan-gun and Jeju Island, Korea. Kor J Fish Aquat Sci 49:689–693
Hou S, Jin Z, Jiang W, Chi L, Xia B, Chen J (2019) Physiological and immunological responses of sea cucumber Apostichopus japonicus during desiccation and subsequent resubmersion. PeerJ 7:e7427
Huo D, Sun L, Ru X, Zhang L, Lin C, Liu S, Xin X, Yang H (2018) Impact of hypoxia stress on the physiological responses of sea cucumber Apostichopus japonicus: respiration, digestion, immunity and oxidative damage. PeerJ 6:e4651
Kim J, Kwak HS, Kim BG (2017) Effects of various physical and chemical factors on the death of trouble seaweed Ulva australis. Weed Turfgrass Sci 6:222–234
KOSIS (2020) Korean Statistical Information Service. Daejeon Korea
Lange B, Currie KL, Howarth G, Stone DAJ (2014) Grape seed extract and dried macroalgae, Ulva lactuca Linnaeus, improve survival of greenlip abalone, Haliotis laevigata Donovan, a thigh water temperature. Aquaculture 433:348–360
Lee KW, Kim HS, Yun A, Choi DG, Jang BI, Kim HJ, Cho SH, Joo Y, Kim B, Min B (2016) Effect of the formulated diets on performance and resistance of juvenile abalone [Haliotis discus, (Reeve 1846)] subjected to various stress conditions. J Shellfish Res 35:481–491
Li X, Wang W, Liu G, Jiang X, Li H, Ji L (2021) Effects of dietary peptide intake on growth, energy budget, body composition and non-specific immunity of the sea cucumber Apostichopus japonicus (Selenka). Aquac Nutr 27:287–296
Liu J, Mai K, Xu W, Zhang Y, Zhou H, Ai Q (2015) Effect of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture 446:48–56
Malham SK, Lacoste A, Gélébart F, Cueff A, Poulet SA (2003) Evidence for a direct link between stress and immunity in the mollusc Haliotis tuberculata. J Exp Zool A 295:136–144
Meng X, Dong Y, Dong S, Yu S, Zhou X (2011) Mortality of the sea cucumber, Apostichopus japonicus Selenka, exposed to acute salinity decrease and related physiological responses: osmoregulation and heat shock protein expression. Aquaculture 316:88–92
Mohamed S, Hashim SN, Rahman HA (2012) Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci and Technol 23:83–96
Morash AJ, Alter K (2016) Effects of environmental and farm stress on abalone physiology: perspectives for abalone aquaculture in the face of global climate change. Rev Aquac 8:342–368
Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A comparison of various seaweed-based diets and formulated feed on growth rate of abalone in a land-based aquaculture system. J Appl Phycol 18:437–443
Oh M, Kwon I, Kim T (2014) Biological performance evaluation of tubular subsurface cage system for sea cucumber, Apostichopus japonicus, grow-out by in-situ tests. J Korean Soc Fish Ocean Technol 50:202–213
Qi Z, Liu H, Li B, Mao Y, Jiang Z, Zhang J, Fang J (2010) Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus hannai Ino. Aquaculture 300:189–193
Robertson-Andersson DV, Maneveldt GW, Naidoo K (2011) Effects of wild and farm-grown macroalgae on the growth of juvenile South African abalone Haliotis midae Linnaeus. Afr J Aquat Sci 36:331–337
Ru X, Zhang L, Li X, Liu S, Yang H (2019) Development strategies for the sea cucumber industry in China. J Oceanol Limnol 37:300–312
Seo J, Lee S (2011) Optimum dietary protein and lipid levels for growth of juvenile sea cucumber Apostichopus japonicus. Aquac Nutr 17:e56–e61
Seo J, Shin I, Lee S (2011) Effect of dietary inclusion of various plant ingredients as an alternative for Sargassum thunbergii on growth and body composition of juvenile sea cucumber Apostichopus japonicus. Aquac Nutr 17:549–556
Shahabuddin AM, Khan MND, Mikami K, Araki T, Yoshimatsu T (2017) Dietary supplementation of red alga Pyropia spheroplasts on growth, feed utilization and body composition of sea cucumber, Apostichopus japonicus (Selenka). Aquac Res 48:5363–5372
Shahabuddin AM, Yoshimatsu T (2015) Present status and future prospect of Japanese sea cucumber: impacts of climate change. Pathumthuni, Thailand: Regional Forum on Climate Change (RFCC), Asian Institute Technology.
Slater MJ, Jeffs AG, Carton AG (2009) The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber, Australostichopus mollis. Aquaculture 292:219–224
Song X, Xu Q, Zhou Y, Lin C, Yang H (2017) Growth, feed utilization and energy budgets of the sea cucumber Apostichopus japonicus with different containing the green tide macroalgae Chaetomorpha linum and the sea grass Zostera marina. Aquaculture 470:157–163
Sui X (1989) The main factors influencing the larval development and survival rate of the sea cucumber Apostichopus japonicus. Haiyang Yu Huzhao 20:314–321
Vidolin D, Santos-Gouvea IA, Freire CA (2002) Osmotic stability of the coelomic fluids of a sea-cucumber (Holothuria grisea) and a starfish (Asterina stellifera) (Echinodermata) exposed to the air during low tide: a field study. Acta Biol Par Curitiba 31:113–121
Viera MP, Courtois de Vicose G, Gomez-Pinchetti JL, Bilbao A, Fernandez-Palacios H, Izquierdo MS (2011) Comparative performance of juvenile abalone (Haliotis tuberculata coccinea Reeve) fed enriched vs non-enriched macroalgae: effect on growth and body composition. Aquaculture 319:423–429
Wan AH, Davies SJ, Soler-Vila A, Fitzgerald R, Johnson MP (2019) Macroalgae as a sustainable aquafeed ingredient. Rev Aquac 11:458–492
Wang Q, Yu S, Qin C, Dong S, Dong Y (2014) Combined effects of acute thermal and hypo-osmotic stresses on osmolality and hsp70, hsp90 and sod expression in the sea cucumber Apostichopus japonicus Selenka. Aquac Int 22:1149–1161
Wen B, Gao Q, Dong S, Hou Y, Yu H, Li W (2016) Effects of different feed ingredients on growth, fatty acid profiles, lipid peroxidation and aminotransferases activities of sea cucumber Apostichopus japonicus (Selenka). Aquaculture 454:176–183
Wu B, Xia S, Rahman MM, Rajkumar M, Fu Z, Tan J, Yang A (2015) Substituting seaweed with corn leaf in diet of sea cucumber (Apostichopus japonicus): effects on growth, feed conversion ratio and feed digestibility. Aquaculture 444:88–92
Xia S, Yang H, Li Y, Liu S, Zhou Y, Zhang L (2012a) Effects of different seaweed diets on growth, digestibility, and ammonia-nitrogen production of the sea cucumber Apostichopus japonicus (Selenka). Aquaculture 338–341:304–308
Xia S, Zhao P, Chen K, Li Y, Liu S, Zhang L, Yang H (2012b) Feeding preferences of the sea cucumber Apostichopus japonicus (Selenka) on various seaweed diets. Aquaculture 344–349:205–209
Yone Y, Furuichi M, Urano K (1986) Effects of dietary wakame Undaria pinnatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream. Nippon Suisan Gakkaishi 52:1465–1468
Yuan CY (2005) Current status and development of feed in sea cucumber. Fish Sci 24:54–56
Yuan X, Yang H, Zhou Y, Mao Y, Zhang T, Liu Y (2006) The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 256:457–467
Zhao H, Yang H, Zhao H, Liu S, Wang T (2012) Differences in MITF gene expression and histology between albino and normal sea cucumbers (Apostichopus japonicus Selenka). Chin J Oceanol Limnol 30:80–91
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2020R1A2C1009903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J., Cho, S.H. Macroalgal substitution effect in diet on growth, body composition, and stress resistance of juvenile sea cucumber (Apostichopus japonicus) subjected to air and low salinity exposures. J Appl Phycol 34, 1123–1130 (2022). https://doi.org/10.1007/s10811-022-02689-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02689-z