Abstract
The effect of substituting the combined macroalgae Ulva australis and Sargassum horneri for Undaria pinnatifida in formulated diets on growth and body composition of abalone subjected to air exposure stressor was investigated. A total of 1260 juvenile abalone were distributed into 21 cages. Six formulated diets were prepared. The control (CUS0) diet contained 20% U. pinnatifida. Twenty, 40, 60, 80, and 100% of U. pinnatifida were substituted with an equal amount of the combined U. australis and S. horneri, referred to as the CUS20, CUS40, CUS60, CUS80, and CUS100 diets, respectively. Finally, dry U. pinnatifida was prepared to compare the growth performance of abalone. Abalone were fed with one of the experimental diets once a day for 16 weeks and then subjected to air stressor for 24 h. The cumulative mortality of abalone was monitored for the following 4 days after 24 h of air exposure. Abalone fed all formulated diets attained higher survival, weight gain, and specific growth rate (SGR) than U. pinnatifida. Abalone fed the CUS100 diet achieved greatest weight gain and SGR, followed by the CUS80 and CUS60 diets. The greatest shell growth and heaviest soft-body weight were obtained in abalone fed the CUS100 diet. Proximate composition of the soft body of abalone, except for moisture content, was not affected by the experimental diets. The cumulative mortality of abalone fed the U. pinnatifida was higher than that of abalone fed all formulated diets at 84 h until the end of the 4-day post observation. The lowest cumulative mortality was obtained in abalone fed the CUS80 diet at the end of the 4-day post observation. Therefore, U. pinnatifida could be completely replaced with the combined U. australis and S. horneri in abalone (H. discus) feed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abalone (Haliotis spp.) are one of the most commercially important mollusks for aquaculture in Eastern Asia including Korea, Japan, and China and their annual aquaculture production reached 16,027 t in Korea in 2017 (KOSIS 2018). Wando-gun and Jeju Special Self-Governing Provinces are the main production regions of abalone in Korea, dominated by the two important species, Haliotis discus hannai Ino 1952 and H. discus Reeve 1846 (Park and Kim 2013). Abalone farmers in a number of countries such as Korea, China, South Africa and Chile, are still using macroalgae as the feed for abalone (Bansemer et al. 2016). Macroalgae (Undaria pinnatifida, (Harvey) Suringar and Saccharina japonica (Areschoug) C.E.Lane, C.Mayes, Druehl, and G.W. Saunders) are highly preferred by the abalone farmers in Korea due to their convenience and easy management of water quality of farms, but one of the most expensive (> US$4 kg−1) components in abalone feed (Hernández et al. 2009; O’Mahoney et al. 2014; Jang et al. 2018). An international market price of macroalgae is expected to increase due to the global expansion of abalone farming and high demand for human consumption (Jang et al. 2018).
Macroalgae (U. pinnatifida and S. japonica) are only exclusively harvestable during the winter season in wild and farmers commonly use either dry or salted macroalgae for the rest of the seasons in Korea (Lee et al. 2018). In addition, low protein (amino acids) and lipid (fatty acids) contents of these macroalgae are not ample to satisfy dietary requirements for optimal growth of abalone (Uki et al. 1986; Mai et al. 1995a, b). For these reasons, abalone farmers as well as researchers are interested to determine the suitability of alternative source for these macroalgae or develop a formulated diet for a sustainable expansion of abalone culture. In addition, low growth rate (Park and Kim 2013) and mortality due to the air stressors resulting from various farm activities such as larval movement to grow-out tanks, size grading, cleaning, and transporting (Baldwin et al. 1992; Lee et al. 2016) have posed a serious threat to the sustainable development of the abalone industry. Therefore, the use of an economically and environmentally sustainable feed source with high nutritional value is a current issue for abalone culture.
Administration of a combination of macroalgae has produced improved growth performance of abalone over a single macroalgae (Naidoo et al. 2006; Robertson-Andersson et al. 2011; Viera et al. 2011). O’Mahoney et al. (2014) also showed that the growth rate of abalone (H. discus hannai) fed a diet substituting fish meal with mixed macroalgae (Ulva lactuca, Laminaria digitata and Palmaria palmata) was comparable with that of abalone fed a diet containing fish meal and highlighted the potential use of the mixed macroalgae as fish meal replacement in abalone feed. Since macroalgae are rich in fibers, protein, vitamins, minerals, lipid, and fatty acids (Yaich et al. 2011), the preference of macroalgae by abalone varies depending on the abalone species, nutritional level of macroalgae, various environmental factors, growth stages, and recognition of taste (Fleming 1995; Lee and Kim 2013). Kemp et al. (2015) assumed that the formulated feed protein becomes more available for abalone in the presence of macroalgae. Still, limited information is available on an inclusion effect of the combined macroalgae in formulated diet on growth performance of abalone.
An unexpected massive algal bloom of fouling macroalgae, such as Ulva australis Kjellman (Kim et al. 2017) and Sargassum horneri (Turner) C. Agardh (Hwang et al. 2016), has been frequently reported in the coastal region of Korea, referred to as the “green tide” and “golden tide,” respectively, in recent years. It has become one of the most serious aquatic disasters worldwide resulting in profound and deleterious effects on aquatic ecosystems, aquaculture, tourism, and public health by creating hypoxia and generation of pungent hydrogen sulfide into the atmosphere (Kim 2006; Jiang et al. 2009; Anderson et al. 2012; Su et al. 2018). Since these fouling macroalgae are good sources of nutrition and have the potential as an ingredient in abalone feed (Ansary et al. 2019a, b), their alternative uses for macroalgae in formulated abalone feed can be one of the best solutions to minimize their undesirable and harmful effects on the surroundings. Protein-enriched U. lactuca has also been shown to improve the growth rate of abalone (H. discus hannai and H. tuberculata) (Shpigel et al. 1999) and H. midae (Naidoo et al. 2006). Seasonal variability the affects nutrient content in macroalgae, whereas the nutrient content in formulated diet remains constant throughout the year around and can be adjusted and manipulated according to the growth rate of abalone (Daume et al. 2007; Stone et al. 2013).
Therefore, the dietary substitution effect of the combined macroalgae (U. australis and S. horneri) for U. pinnatifida on growth and body composition of juvenile abalone (H. discus) subjected to air exposure stressor was investigated in this study and water stability of the diets was compared.
Materials and methods
Collection of macroalgae
Free-floating macroalgae, Ulva australis and Sargassum horneri, were collected separately from the coast of Jeju Island and then dried in an electrical drying oven at 40 °C for 48 h.
Preparation of the experimental area and rearing of abalone
Juvenile abalone were purchased from a private hatchery, transferred to Daegun abalone farm (Jeju Island, Jeju Special Self-Governing Province, Jeju, Korea), and acclimated to the experimental conditions for 2 weeks. During the acclimatization periods, abalone were daily fed with dry U. pinnatifida at a ratio of 2–2.5% of total biomass. A total of 1260 abalone averaging 6.0 g were distributed into 21, 100-L net cages (50 cm × 50 cm × 40 cm; length × width × height) (60 abalone per net cage), which were set up into 3 of 3.6-t concrete flow-through raceway tanks (water volume 3.4 t; 7 net cages per raceway). Sand-filtered seawater was supplied throughout the feeding trial at temperature ranging from 18 to 18.6 °C (mean ± SD, 18.2 ± 0.01 °C) and the flow rate was 108 L min−1 raceway−1.
The photoperiod followed natural condition and proper aeration was maintained in each raceway. Abalone were fed with one of the experimental diets once a day (17:00 h) at a satiation level (ca. 1.5–2.5% of total biomass) with a little leftover for 16 weeks. The bottom of the raceways was cleaned every other day and the dead abalone were removed daily. At the end of the 16-week feeding trial, all surviving abalone from each net cage were harvested, collectively weighed, and raised for further analysis.
Preparation of the experimental diets
The experimental design was completely randomized. Six formulated diets and dry U. pinnatifida were prepared in triplicate (Table 1). Twenty-five percent anchovy meal, 5% casein, and 4% soybean meal were included as the protein sources in the control (CUS0) diet. Twenty-two percent wheat flour and 0.5% squid liver and soybean oils were used as the carbohydrate and lipid sources, respectively, in the CUS0 diet. Twenty percent U. pinnatifida was also included in the CUS0 diet. Twenty, 40, 60, 80, and 100% of the combined macroalgae (U. australis and S. horneri = 1:1) were included in the formulated diets replacing equal amounts of U. pinnatifida, referred to as the CUS20, CUS40, CUS60, CUS80, and CUS100 diets, respectively. Dietary nutrient requirements of abalone were satisfied (Mai et al. 1995a, b; Fleming et al. 1996). Finally, the dry U. pinnatifida was prepared to compare the effect of formulated diets on the performance of abalone.
A 20% sodium alginate was added to each diet. All prepared feed ingredients were mixed mechanically and water was added at a ratio of 1:1. An electronic mixer was used to make a fine paste from each of the diets and then shaped into 0.15-cm-thick sheets. Those sheets were then cut into 1- cm2 flakes manually and dipped into an aqueous solution of 5% CaCl2 for 1 min and the excess solution was drained naturally. Finally, the flakes were dried naturally for 48 h and stored at − 20 °C until use.
Sample collection and measurement of abalone growth
Thirty abalone from each net cage were randomly sampled and frozen at − 20 °C for the biological measurement and chemical analysis of the soft body of abalone after the 16-week feeding trial. At first, all samples were slightly thawed followed by separation of the shell and soft-body tissue. The specific growth rate (SGR) (% body weight gain/day) was calculated according to Britz (1996): SGR = [(ln(Wf) − ln(Wi))/days of feeding] × 100, where ln(Wf) = natural log of the final mean weight of abalone and ln(Wi) = natural log of the initial mean weight of abalone. A digital caliper was used to measure the shell length and width in mm. The ratio of soft-body weight to total body weight (the soft-body weight + the weight of the excised shell) was calculated to determine a condition index for abalone.
Proximate analysis of the soft body of abalone and experimental diets
All the separated soft-body tissue of abalone from each replicate cage was homogenized for the chemical analysis according to AOAC (1990). Moisture content was analyzed by drying at 105 °C for 24 h. The Kjeldahl method (Auto Kjeldahl System, Buchi B-324/435/412, Switzerland) was used to determine the crude protein content. Crude lipid content was measured by an ether-extraction method and ash content was determined using a muffle furnace at 550 °C for 4 h.
Calculation of cumulative mortality of abalone subjected to air stressor
After collectively measuring the weight of abalone, they were fed with the same diets as feeding trial for a week to minimize stress. Then, abalone were exposed to air stressor according to Lee et al. (2016) and Ansary et al. (2019a). Twenty abalone remained in each net cage and the rest of the abalone were removed. Twenty abalone were then subjected to air exposure stressor by draining out all seawater from the net cages. After 24 h of air exposure, net cages were again filled with sand-filtered seawater and their mortality was monitored for the next 4 days. Dead abalone were removed every 4 h during the 4-day observation period.
Water stability of the experimental diets
Ten gram of each formulated diet and U. pinnatifida in 63 laboratory dishes was placed in 7 separate 50-L plastic rectangular containers (51 cm × 36 cm × 30 cm) without abalone in triplicate (9 laboratory dishes/container) at a flow rate of 1.4 L container−1 min−1 and sampled at 12, 24, and 48 h. Dry matter content in the experimental diets was assessed using the same procedure as the chemical composition of the experimental diets. Water stability of dry matter content in the experimental diets was expressed as the percentage of difference between the final dry content and the initial dry content based on Mai et al. (1995a).
Statistical analysis
Significant differences among the treatments were determined by one-way ANOVA and Duncan’s multiple range test (Duncan 1955) using SAS version 9.3 program (SAS Institute, USA). Water stability of dry matter content in the experimental diets was tested by ANOVA with repeated measurement design (Cody and Smith 1991). Percentage data was arcsine-transformed prior to statistical analysis.
Results
Water stability of the nutrient content in experimental diets
Significant (P < 0.0001) changes of dry matter content in the experimental diets over all periods of time (Fig. 1) and their significant (P < 0.0001) interaction (experimental diets × time) were observed. The retained dry matter content in all formulated diets substituting U. pinnatifida with the combined U. australis and S. horneri was significantly (P < 0.05) higher than that of the U. pinnatifida at all periods of observation. The retention of dry matter content was the highest in the CUS20 diet and the lowest for the CUS40 diet among all formulated diets at both 12 and 24 h after seawater immersion. However, the CUS60 diet achieved the highest retention of dry matter content and lowest for the CUS0 diet at 48 h after seawater immersion.
Changes in dry matter content (%) in the experimental diets with time after seawater immersion (means of triplicate ± SE). (ANOVA with repeated design: times (P < 0.0001) and their interaction (the experimental diets × time) (P < 0.0001)). Different letters in each time point indicate significant differences (P < 0.05) between the experimental diets within each time point
Growth performance of abalone
Survival of abalone fed all formulated diets ranging from 92.2 to 95.0% was significantly (P < 0.05) higher than that of abalone fed the U. pinnatifida (86.1%) (Table 2). Weight gain and SGR of abalone fed all formulated diets were significantly (P < 0.05) greater than those of abalone fed U. pinnatifida. Abalone fed the CUS100 diet achieved the greatest weight gain and SGR, followed by the CUS80, CUS60, CUS40, CUS0, and CUS20 diets in order.
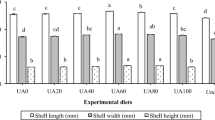
Shell length, width, and height of abalone fed all formulated diets were significantly (P < 0.05) longer, wider, and higher than those of abalone fed the U. pinnatifida (Table 3). Shell length of abalone fed the CUS100 diet was significantly (P < 0.05) longer than that of abalone fed the CUS0 diet, but not significantly (P > 0.05) different from that of abalone fed the CUS20, CUS40, CUS60, and CUS80 diets. Shell length of abalone fed the CUS100 diet was significantly (P < 0.05) wider than that of abalone fed the CUS0, CUS40, and CUS60 diets, but not significantly (P > 0.05) different from that of abalone fed the CUS20 and CUS80 diets. Shell height of abalone fed the CUS100 diet was significantly (P < 0.05) higher than that of abalone fed all other diets. The greatest soft-body weight was observed in abalone fed the CUS100 diet, followed by the CUS80, CUS60, CUS0, CUS40, and CUS20 diets in order. The ratio of the soft-body weight to total weight of abalone fed the CUS100 diet was significantly (P < 0.05) higher than that of abalone fed the CUS0, CUS20, and CUS40 diets, but not significantly (P > 0.05) different from that of abalone fed the CUS60 and CUS80 diets.
Proximate composition of the soft body of abalone
Moisture content of the soft body of abalone fed the CUS0 diet was significantly (P < 0.05) lower than that of abalone fed all other diets (Table 4). However, crude protein content ranging from 19.2 to 19.5%, crude lipid content ranging from 1.2 to 1.4%, and ash content ranging from 2.1 to 2.2% of the soft body of abalone were not significantly (P > 0.05) affected by the experimental diets.
Cumulative mortality of abalone subjected to air stressor
During the 24-h air exposure, the cumulative mortality ranging from 5% in abalone fed the CUS0 diet to 11.7% in abalone fed the CUS80 diet was unaffected by the experimental diets (Fig. 2). There was no significant difference in the cumulative mortality of abalone until 78 h during the 4-day post observation after 24-h air exposure. However, the cumulative mortality (71.7 ± 1.67%) of abalone fed the U. pinnatifida was significantly (P < 0.05) higher than that of abalone fed all other formulated diets at 84 h until the end of the 4-day post observation. Significantly (P < 0.05) lower cumulative mortality was observed in abalone fed the CUS80 diet (51.7 ± 4.41%) compared with that of abalone fed the CUS0 diet (61.7 ± 1.67%), but not significantly (P > 0.05) different from that of abalone fed the CUS20 (53.3 ± 4.41%), CUS40 (53.3 ± 1.67%), CUS60 (53.3 ± 1.67%), and CUS100 (55.0 ± 0.00%) diets at 84 h until the end of the 4-day post observation.
Cumulative mortality (%) of juvenile abalone fed the experimental diets substituting Undaria pinnatifida with the combined Ulva australis and Sargassum horneri for 16 weeks and then subjected to air exposure stressor (means of triplicate ± SE). Different letters in the same elapsed time indicated significant differences (P < 0.05) by Duncan’s multiple range test
Discussion
The repetitive blooms of U. australis (Kim et al. 2017) and S. horneri (Hwang et al. 2016) in the coastline of Jeju Island and Jeolla Province in Korea have created severe ecological and environmental losses. However, the fresh Ulva and Sargassum can be partially utilized as a natural diet for wild abalone (Qi et al. 2010; Robertson-Andersson et al. 2011; Viera et al. 2011). In addition, the fouling macroalgae U. australis (Ansary et al. 2019a) and S. horneri (Ansary et al. 2019b) were successfully used as a replacer for macroalgae (U. pinnatifida) in juvenile abalone (H. discus) feed in the 16-week feeding trial.
SGR values ranging from 0.63 to 0.72% for the initial weight of 6.0 g abalone in this study were superior to those ranging from 0.45 to 0.46%, from 0.19 to 0.34%, from 0.40 to 0.59%, from 0.40 to 0.60%, from 0.22 to 0.63%, and from 0.59 to 0.68% for the same species of abalone with smaller initial weight of 0.43, 1.29, 3.3, 3.3, 3.6, and 5.4 g, respectively (Jung et al. 2016; Kim et al. 2016; Lee et al. 2017, 2018; Choi et al. 2018; Jang et al. 2018), probably indicating that the growth of abalone was relatively well achieved in this study. Weight gain and SGR of abalone were gradually improved with an increased amount of substitution of U. pinnatifida by the combined macroalgae (U. australis and S. horneri). The greatest weight gain and SGR were obtained in abalone fed the CUS100 diet substituting 100% U. pinnatifida with the combined macroalgae when 20% U. pinnatifida was included. This specified that the complete (100%) replacement of U. pinnatifida with the combined macroalgae in the formulated diet was the most suitable for improving growth performance of abalone. This could be explained by the fact that the mixed algal diet produced improved growth performance of abalone over the single algal diet, which may have limited nutrient content and essential amino acids (Shpigel et al. 1999; Viera et al. 2011; Bansemer et al. 2016). O’Sullivan et al. (2010) reported that marine macroalgae are rich in bioactive compounds, which have a potential prebiotic effect. The combined macroalgae (U. australis and S. horneri) are thus able to provide the biologically active compounds not available in the formulated feed to meet a complete range of nutrients and energy requirements of abalone (Kemp et al. 2015) and contribute to the improved growth performance of abalone in this study. Dang et al. (2011) demonstrated that abalone (H. laevigata) may have a better chance to grow properly in a disease-free environment due to the presence of bioactive compounds in macroalgae, which boost up their growth and immunity. Therefore, the suitable combination of different macroalgae in formulated diet is one of the best options rather than to feed a single macroalgae. The combined substitution effect of macroalgae (U. australis and S. horneri) for U. pinnatifida in abalone (H. discus) feed on growth performance was reported for the first time.
However, Ansary et al. (2019b) reported that abalone (H. discus) fed a diet substituting 60% U. pinnatifida with S. horneri achieved the greatest weight gain and SGR, but the diet with 100% substitution of U. pinnatifida with S. horneri produced inferior weight gain and SGR to the 20, 40, 60, and 80% substitution of U. pinnatifida with S. horneri in the diets when 20% U. pinnatifida was included in the experimental diets. Ansary et al. (2019a) also showed that abalone (H. discus) fed the diet substituting 60% U. pinnatifida with U. australis attained the greatest weight gain and SGR, but the diet substituting 100% U. pinnatifida with U. australis produced lower weight gain and SGR compared with those of abalone fed the diets substituting 40 and 80% U. pinnatifida with U. australis when 20% U. pinnatifida was included. In considering these results, the combined macroalgae (U. australis and S. horneri) are more beneficial than either single macroalga (U. australis or S. horneri) alone for the producers to improve growth at the condition of complete (100%) substitution of U. pinnatifia with these fouling macroalgae in abalone (H. discus) feed.
Similarly, Robertson-Andersson et al. (2011) revealed that abalone (H. midae) fed a mixed diet of fresh kelp (Ecklonia maxima) and farmed U. lactuca achieved the greatest weight gain and SGR compared with those abalone fed any of the single macroalgae when abalone were fed with one of the four experimental diets (the combined kelp and U. lactuca, single kelp, single farmed, or wild U. lactuca) for 8 months. Naidoo et al. (2006) also reported that the growth rate of abalone (H. midae) fed the mixture of fresh macroalgae (U. lactuca, Gracilaria gracilis and kelp (E. maxima)) was superior to that of abalone fed either fresh kelp blades, kelp + epiphytes (Carpoblepharis flaccida), commercial abalone feed (Abfeed®), kelp + commercial feed, or three forms of dried kelp (blades/pellets/stipes) for 9 months. Unlike this study, however, Qi et al. (2010) reported that the highest weight gain was obtained in 2-year-old abalone (H. discus hannai) fed a single macroalga (S. japonica), followed by Gracilaria lemaneiformis, the combined S. japonica and G. lemaneiformis, single macroalgae of S. pallidum, and combined S. japonica and S. pallidum, when three different single or two combinations of macroalgae were fed to abalone for 4 months.
Since leaching rate of nutrients during the prolonged stay of feed in water is one of the most important criteria to develop artificial diet for slow-eater abalone (Sales and Janssens 2004), it is critical to correlate water stability of diets with growth performance of abalone and deterioration of water quality in farm. Higher retained dry matter content in all formulated diets compared with the U. pinnatifida at 12, 24, and 48 h after the seawater immersion indicated that the water stability of all formulated diets was superior to the U. pinnatifida, accounting for the superior growth performances of abalone fed the formulated diets to the single macroalga (U. pinnatifida) in this study. Similarly, Ansary et al. (2019b) reported that the water stability of dry matter along with all other nutrient (crude protein, crude lipid, and ash) content in all formulated diets substituting U. pinnatifida with S. horneri was superior to the single macroalga (U. pinnatifida) over all periods of observation for 48 h. Diet stability is generally affected by the different chemicals (Chao et al. 2010; Stone et al. 2013), the size of feed particle (Sales and Britz 2002), and the ratio of agar and gelatin (Knauer et al. 1993).
Higher survival, weight gain, and SGR in abalone fed all formulated diets substituting U. pinnatifida with the combined macroalgae than those of abalone fed single U. pinnatifida in this study agreed with other studies demonstrating the improved growth performance of abalone fed the formulated diets over single macroalgae (Jung et al. 2016; Lee et al. 2016, 2017, 2018; Jang et al. 2018; Ansary et al. 2019a, b). Abalone fed all formulated diets produced higher shell growth (shell length, height, and width) and ratio of the soft body to total body weight than those of abalone fed the U. pinnatifida, being consistent with other studies showing that the growth rate closely affected the biological parameters of abalone (Cho 2010; Myung et al. 2016). Similarly, relatively greater shell growth and heavier soft-body weight of abalone fed the formulated diet over a single macroalga (U. pinnatifida) have also been reported (Jung et al. 2016; Lee et al. 2016, 2017; Jang et al. 2018; Ansary et al. 2019a, b). Viera et al. (2011) also reported that shell growth rate of abalone (H. tuberculata coccinea) was well reflected from growth rate.
There was no difference in proximate composition of the soft body of abalone, except for the moisture content among the experimental diets in this study, being coincident with other studies showing that the chemical composition of the soft body of abalone was not affected by the experimental diets substituting U. pinnatifida with U. australis (Ansary et al. 2019a) and S. horneri (Ansary et al. 2019b). Unlike this study, however, proximate composition of the soft body of abalone was affected by the nutrient content in the experimental diets (Uki et al. 1986; Mai et al. 1995a, b; Cho et al. 2008; Kim et al. 2016; Lee et al. 2016, 2017).
Higher cumulative mortality of abalone fed U. pinnatifida compared with that of abalone fed all other formulated diets at 84 h until the end of 4-day post observation after 24-h air exposure in this study indicated that the administration of the nutrition-balanced diet could improve resistance of abalone subjected to air exposure stressor over a single macroalga (U. pinnatifida). In addition, significantly and slightly lower cumulative mortality of abalone fed the CUS80 diet, and the CUS20, CUS40, CUS60, and CUS100 diets, respectively, compared with the CUS0 diet also indicated that substitution of U. pinnatifida with the combined macroalgae (U. australis and S. horneri) could effectively lower mortality of abalone subjected to air exposure stressor. This probably resulted from the fact that the bioactive compounds in macroalgae (U. australis and S. horneri) and proper nutrient in formulated diets may act as an immune modulator, responsible for the improved physiological health and tolerant capacity against air exposure stressor. Several studies have been conducted to mitigate the effect of air exposure during live transport of abalone (Wells and Baldwin 1995; Sales and Britz 2001); however, post-air exposure and the effects of various re-immersion conditions on the survival and taste properties of abalone remain mysterious (Morash and Alter 2016). Therefore, this research would help to modulate the dietary substances to reduce mortalities during and after live shipment and transport of abalone. Similarly, Ansary et al. (2019a) reported a higher cumulative mortality of abalone fed U. pinnatifida compared with abalone fed the formulated diets substituting U. australis for U. pinnatifida. Lee et al. (2016) also showed lower cumulative mortality of abalone fed the formulated diet substituting 50% fish meal and 100% macroalgae with an equal amount of fermented soybean meal and rice bran, respectively, over a single macroalgae (U. pinnatifida and S. japonica) at 100 h after the 30-h air exposure stressor.
The massive algal blooms of U. australis and S. horneri are now a major global concern; however, this research will be beneficial both economically and environmentally for the abalone growers and to manage hazardous algal pollution in an appropriate way. Considering the output of this study, it can be concluded that U. pinnatifida can be completely (100%) substituted with the combined fouling macroalgae (U. australis and S. horneri) in formulated diet, improving the growth performance of juvenile abalone as well as lowering the cumulative mortality subjected to the air exposure stressor.
References
Anderson DM, Cembella AD, Hallegraeff GM (2012) Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Annu Rev Mar Sci 4:143–176
Ansary MWR, Jeong HS, Lee KW, Kim PY, Kim J, Yun A, Cho SH (2019a) Dietary substitution effect of Ulva australis for Undaria pinnatifida on growth, body composition and air exposure of juvenile abalone, Haliotis discus (Reeve 1846). J Appl Phycol 31:1467–1474
Ansary MWR, Jeong HS, Lee KW, Kim HS, Kim J, Yun A, Cho SH, Kim PY, Kim T (2019b) The effect of substituting Undaria pinnatifida in formulated feeds with Sargassum horneri on growth and body composition of juvenile abalone (Haliotis discus, Reeve 1846). J Appl Phycol. https://doi.org/10.1007/s10811-018-1672-2
AOAC (1990) Official methods of analysis (15th edn). Association of Official Analytical Chemists, Arlington, VA, USA
Baldwin J, Wells RMG, Low M, Ryder JM (1992) Tauropine and D-lactate as metabolic stress indicators during transport and storage of live Paua (New Zealand abalone) (Haliotis iris). J Food Sci 57:280–282
Bansemer MS, Qin J, Harris JO, Howarth GS, Stone DAJ (2016) Nutritional requirements and use of macroalgae as ingredients in abalone feed. Rev Aquac 8:121–135
Britz PJ (1996) Effect of dietary protein level on growth performance of South African abalone, Haliotis midae, fed fishmeal-based semi-purified diets. Aquaculture 140:55–61
Chao WR, Huang CY, Sheen SS (2010) Development of formulated diet for post-larval abalone, Haliotis diversicolor supertexta. Aquaculture 307:89–94
Cho SH (2010) Effect of fishmeal substitution with various animal and/or plant protein sources in the diet of the abalone Haliotis discus hannai Ino. Aquac Res 41:e587–e593
Cho SH, Kim C, Cho YJ, Lee B, Park J, Yoo J, Lee S (2008) Effects of the various dietary additives on growth and tolerance of abalone Haliotis discus hannai against stress. J Aquac 21:309–316
Choi DG, Kim J, Yun A, Cho SH, Jeong HS, Lee KW, Kim HS, Kim PY, Ha MS (2018) Dietary substitution effect of fish meal with tunic meal of sea squirt, Halocynthia roretzi, Drasche on growth and soft body composition of juvenile abalone, Haliotis discus, Reeve 1846. J World Aquacult Soc 49:1095–1104
Cody RP, Smith JK (1991) Applied statistics and the SAS programming language, 3rd edn. Prentice-Hall, Inc, Englewood Cliffs, pp 163–206
Dang VT, Li Y, Speck P, Benkendorff K (2011) Effects of micro and macroalgal diet supplementations on growth and immunity of greenlip abalone, Haliotis laevigata. Aquaculture 320:91–98
Daume S, Davidson M, Ryan S, Parker F (2007) Comparisons of rearing systems based on algae or formulated feed for juvenile greenlip abalone (Haliotis laevigata). J Shellfish Res 26:729–735
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Fleming AE (1995) Digestive efficiency of the Australian abalone Haliotis rubra in relation to growth and feed preference. Aquaculture 134:279–293
Fleming AE, Barneveld RJ, Hone PW (1996) The development of artificial diet for abalone: a review and future directions. Aquaculture 140:5–53
Hwang EK, Lee SJ, Ha DS, Park CS (2016) Sargassum golden tides in the Shinnan-gun and Jeju Island, Korea. Kor J Fish Aquat Sci 49:689–693
Hernández J, Uriarte I, Viana MT, Westermeier R, Farías A (2009) Growth performance of weaning red abalone (Haliotis rufescens) fed with Macrocystis pyrifera plantlets and Porphyra columbina compared with a formulated diet. Aquac Res 40:1–9
Jang B, Kim PY, Kim HS, Lee KW, Kim HJ, Choi DG, Cho SH, Min B, Kim K, Han H (2018) Substitution effect of sea tangle (ST) (Laminaria japonica) with tunic of sea squirt (SS) (Halocynthia roretzi) in diet on growth and carcass composition of juvenile abalone (Haliotis discus, Reeve 1846). Aquac Nutr 24:586–593
Jiang X, Tang Y, Lonsdale DJ, Gobler CJ (2009) Deleterious consequences of a red tide dinoflagellate Cochlodinium polykrikoides for the calanoid copepod Acartia tonsa. Mar Ecol Prog Ser 390:105–116
Jung W, Kim HS, Lee KW, Kim YE, Choi DK, Jang B, Cho SH, Choi CY, Kim B, Joo Y (2016) Growth and body composition effects of tuna byproduct meal substituted for fish meal in the diet of juvenile abalone, Haliotis discus. J World Aquacult Soc 47:74–81
Kemp JOG, Britz PJ, Toledo Agüero PH (2015) The effect of macroalgal, formulated and combination diets on growth, survival and feed utilization in the red abalone Haliotis rufescens. Aquaculture 448:306–314
Kim HG (2006) Mitigation and controls of HABs. In: Graneli E, Turner J (eds) Ecology of harmful algae. Springer, Berlin, pp 327–338
Kim Y, Myung SH, Kim HS, Jung W, Cho SH, Jwa MS, Kim PY, Park M, Kim B (2016) Effect of dietary substitution of sea tangle (ST), Laminaria japonica with rice bran (RB) on growth and body composition of juvenile abalone (Haliotis discus). Aquac Res 47:1202–1208
Kim J, Kwak HS, Kim BG (2017) Effects of various physical and chemical factors on the death of trouble seaweed Ulva australis. Weed Turf Sci 6:222–234
Knauer J, Britz PJ, Hecht T (1993) The effect of seven binding agents on 24 hour water stability of an artificial weaning diet for the South African abalone, Haliotis midae (Haliotidae, Gastropoda). Aquaculture 115:327–334
KOSIS (2018) Korean statistical information service. Daejeon
Lee J, Kim B (2013) Feeding stimulants and feeding preference of Haliotis discus Reeve (Jeju island) to marine algae. Korean J Environ Biol 31:458–470
Lee KW, Kim HS, Yun A, Choi DG, Jang BI, Kim HJ, Cho SH, Joo Y, Kim B, Min B (2016) Effect of the formulated diets on performance and resistance of juvenile abalone [Haliotis discus, (Reeve 1846)] subjected to various stress conditions. J Shellfish Res 35:1–11
Lee KW, Kim HS, Choi DG, Jang BI, Yun A, Cho SH, Min B, Kim K, Han H (2017) Effects of substitution of fish meal (FM) and macroalgae (MA) with soybean meal and rice bran in a commercial juvenile abalone (Haliotis duscus hannai) diet on growth performance. Turk J Fish Aquat Sci 17:519–526
Lee KW, Kim HS, Kim PY, Jeong HS, Kim J, Yun A, Cho SH (2018) Substitution effect of white radish (Raphanus sativus L.)’ by-product and tunic of sea squirt (Halocynthia rorentzi, von Drasche) for Undaria pinnatifida in feed of abalone (Haliotis discus, Reeve 1846). Fish Aquat Sci 21:1–8
Mai K, Mercer JP, Donlon J (1995a) Comparative studies on the nutrition of species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. III. Responses of abalone to various levels of dietary lipid. Aquaculture 134:65–80
Mai K, Mercer JP, Donlon J (1995b) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. IV. Optimum dietary protein level for growth. Aquaculture 136:165–180
Morash AJ, Alter K (2016) Effects of environmental and farm stress on abalone physiology: perspectives for abalone aquaculture in the face of global climate change. Rev Aquac 8:342–368
Myung SH, Jung W, Kim HS, Kim YE, Cho SH, Jwa MS, Kim PY, Kim MK, Park M, Kim B (2016) Effects of dietary substitution of fishmeal with the combined dry microalgae, Nannochloropsis oceanica (NO) biomass residue and casein on growth and body composition of juvenile abalone (Haliotis discus). Aquac Res 47:341–348
Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A comparison of various seaweed-based diets and formulated feed on growth rate of abalone in a landbased aquaculture system. J Appl Phycol 18:437–443
O’Mahoney M, Rice O, Mouzakitis G, Burnell G (2014) Towards sustainable feeds for abalone culture: evaluating the use of mixed species seaweed meal in formulated feeds for the Japanese abalone, Haliotis discus hannai. Aquaculture 430:9–16
O’Sullivan L, Murphy B, McLoughlin P, Duggan P, Lawlor PG, Hughes H, Gardiner GE (2010) Prebiotics from marine macroalgae for human and animal health applications. Mar Drugs 8:2038–2064
Park C, Kim SY (2013) Abalone aquaculture in Korea. J Shellfish Res 32:17–19
Qi Z, Liu H, Li B, Mao Y, Jiang Z, Zhang J, Fang J (2010) Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus hannai Ino. Aquaculture 300:189–193
Robertson-Andersson DV, Maneveldt GW, Naidoo K (2011) Effects of wild and farm-grown macroalgae on the growth of juvenile South African abalone Haliotis midae Linnaeus. Afr J Aquat Sci 36:331–337
Sales J, Britz PJ (2001) Research on abalone (Haliotis midae L.) cultivation in South Africa. Aquac Res 32:863–874
Sales J, Britz PJ (2002) Influence of ingredients particle size and inclusion level of pre-gelatinised maize starch on apparent digestibility coefficients of diets in South African abalone (Haliotis midae L.). Aquaculture 212:299–309
Sales J, Janssens GP (2004) Use of feed ingredients in artificial diets for abalone: a brief update. Nutr Abstr Rev B:13N–21N
Shpigel M, Ragg NL, Lupatsch I, Neori A (1999) Protein content determines the nutritional value of the seaweed Ulva lactuca L. for the abalone Haliotis tuberculata L. and H. discus hannai Ino. J Shellfish Res 18:227–233
Stone DAJ, Harris JO, Wang H, Mercer GJ, Schaefer EN, Bansemer MS (2013) Dietary protein level and water temperature interactions for greenlip abalone Haliotis laevigata. J Shellfish Res 32:119–130
Su L, Shan T, Pang S, Li J (2018) Analyses of the genetic structure of Sargassum horneri in the Yellow Sea: implications of the temporal and spatial relations among floating and benthic populations. J Appl Phycol 30:1417–1424
Uki N, Kemuyama A, Watanabe T (1986) Optimum protein level in diets for abalone. Bull Jpn Soc Sci Fish 52:1005–1012
Viera MP, Courtois de Vicose G, Gomez-Pinchetti JL, Bilbao A, Fernandez-Palacios H, Izquierdo MS (2011) Comparative performance of juvenile abalone (Haliotis tuberculata coccinea Reeve) fed enriched vs non-enriched macroalgae: effect on growth and body composition. Aquaculture 319:423–429
Wells RMG, Baldwin J (1995) A comparison of metabolic stress during air exposure in two species of New Zealand abalone, Haliotis iris and Haliotis australis: implications for the handling and shipping of live animals. Aquaculture 134:361–370
Yaich H, Garna H, Besbes S, Paquot M, Blecker C, Attia H (2011) Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem 128:895–901
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017R1A2B4009773). This work was also supported by a grant from the National Institute of Fisheries Science, Republic of Korea (R2019010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansary, M.W.R., Baek, S.I., Jeong, H.S. et al. Substitution effect of the combined fouling macroalgae Ulva australis and Sargassum horneri for Undaria pinnatifida in formulated diets on growth and body composition of juvenile abalone (Haliotis discus, Reeve 1846) subjected to air exposure stressor. J Appl Phycol 31, 3245–3254 (2019). https://doi.org/10.1007/s10811-019-01812-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01812-x