Abstract
Hypnea musciformis is an edible tropical red seaweed species and still considered as an underexploited seaweed species in Indonesia. In this study, H. musciformis was hydrolyzed using the subcritical water extraction (SCWE) system. Different solid to liquid ratios (S/L ratios 1:150, 1:100, and 1:50) and six different temperature conditions (120–270 °C with 30 °C increments) were applied to obtain the best functional materials. Hypnea musciformis hydrolysate (HMH) was further analyzed for total sugar, protein, phenol, and flavonoid content. The antioxidant capacity and functional material contents varied significantly based on extraction conditions, with HMH hydrolyzed at S/L ratio 1:50 and temperature 210 °C showing the highest antioxidant activity with values of 0.97 ± 0.02 Trolox equivalent (TE) mg g−1, 2.23 ± 0.05 ascorbic acid (AA) equivalent mg g−1, and 0.92 ± 0.00 TE mg g−1for DPPH, total antioxidant, and ABTS, respectively. Pearson analyses showed that the phenolic contents were closely associated with DPPH scavenging activities with a value of 0.983. High-performance liquid chromatography analysis revealed that the highest phenolic acids in H. musciformis were chlorogenic acid (27.98 ± 0.57 mg g−1) and gallic acid (1.22 ± 0.20 mg g−1), with the highest content obtained by SCWE (S/L ratio 1:50) at a temperature of 210 °C. Further, the emulsifying properties of HMH in corn oil and sunflower oil were analyzed. HMH demonstrated thermostable emulsifying properties. Moreover, H. musciformis could be developed as a potential antioxidant additive and also as an emulsifying agent in the food, pharmacy, cosmetics, and other industries. Collectively, this study shows the potential of underexploited red seaweed resources in Indonesia using SCWE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indonesia is an archipelagic country, with more than 70% of the territory consisting of a coastal and marine environment. Geological complexity and strategic location at the center of the triangle of marine diversity has made Indonesia a mega-biodiverse nation with high numbers of marine organisms. The earliest study of Indonesian seaweeds dates back to 1899–1900 (the Siboga expedition), which recorded more than 700 seaweed species. More recently, around 1000 seaweed species have been reported (Hutomo and Moosa 2005). Seaweeds play an important socioeconomic role in Indonesian coastal communities and drive economic growth. Presently, Indonesia is one of the top five seaweed producers in the world. However, considering the high seaweed diversity in Indonesia, only a few species have been commercialized. The most-traded seaweed species in Indonesia are Kappaphycus alvarezii (previously known as Eucheuma cottonii), Eucheuma spinosum, and Gracilaria sp. These seaweeds are commonly used in the hydrocolloid industry to produce agar and carrageenan (Mulyati and Geldermann 2017).
Functional materials derived from seaweeds are also a driving factor for their increased demand in food, pharmaceuticals, cosmetics, and household products (Pangestuti et al. 2018). Seaweeds contain diverse bioactive materials such as phenols, pigments, polysaccharides, proteins, and bioactive peptides (Pangestuti and Kim 2011). These materials are well recognized for their health benefit, emulsifying, stabilizing, and other properties. Hypnea musciformis is an edible red seaweed species and still considered as an underexploited species in Indonesia. The composition of H. musciformis recovered by conventional extraction techniques using different extraction solvents has been previously reported (Rafiquzzaman et al. 2016). However, the use of organic solvents is hazardous and costly. With the increasing demand for seaweeds in several sectors, the recovery of functional materials from seaweeds, including H. musciformis, requires development of a safe and green technology.
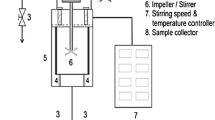
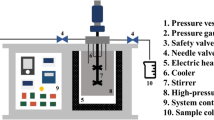
Subcritical water extraction (SCWE) is a green technology that is used to extract functional materials from terrestrial and marine resources. These methods are gaining more recognition as alternative extraction technologies to conventional extraction methods because of the reduced extraction time, efficient and lower extraction cost, and, most importantly, environmental-friendly nature. In these studies, we used SCWE to hydrolyze H. musciformis collected from Tual, Maluku, Indonesia. Three different solid to liquid ratios (S/L ratios 1:50, 1:100, and 1:150) and six different temperature conditions (120–270 °C with 30 °C increments) were applied to obtain H. musciformis hydrolysate (HMH). Antioxidant activities of HMH were evaluated using DPPH, ABTS, and total antioxidant assays. Emulsification properties of HMH were also undertaken to gain insight into the potential of H. musciformis to be applied as an emulsifier. In the present study, we reported the earliest investigation on the characterization of functional materials from underexploited red seaweed resources from Indonesia using SCWE.
Materials and methods
Materials
Hypnea musciformis was collected from Tual, Maluku, Indonesia, in May 2015 (dry season).
Voucher specimen was deposited in the Research Center for Oceanography LIPI with the accession number RSW-HM-1505. All the chemicals used in this study were from Sigma (USA) and Junsei Chemical Co., Ltd. (Japan) and were of analytical grade.
Sample preparation
Seaweeds were washed with tap water. Any epiphytic algae, invertebrates, sand, or debris were gently removed. Samples were dried in an oven at 45 °C for 120 h. The dried seaweeds were ground into a very fine powder (passing through a 710-μm sieve) using a blender (Hanil HMF 3080 stainless steel grinder) and kept at − 20 °C for further analysis.
Seaweed hydrolysis using the SCWE system
A fine powder of H. musciformis was placed in the SCWE reactor and mixed with distilled water (pH 7.2) at 1:50, 1:100, and 1:150 S/L ratios. The SCWE reactor was closed and heated up to six different temperatures (120, 150, 180, 210, 240, and 270 °C). Each hydrolysis process was run for 10 min. After hydrolysis, the hydrolysates were collected, vacuum filtered, and stored at − 20 °C for further analysis.
Determination of hydrolysis efficiency
The hydrolysis efficiency of SCWE was determined, as described previously (Gereniu et al. 2017). The initial weight of H. musciformis (Wi) was obtained from the sample weight before loading into the reactor. The final weight of H. musciformis (Wf) was obtained from the final residue of H. musciformis after incubation for 48 h at 55 °C. The following formula was used for the calculation of hydrolysis efficiency:
Physical properties of HMH
Color of seaweed hydrolysates
The color characteristics of HMH were determined using a reflectance tintometer (Lovibond RT Series, UK). The color values of HMH were expressed as lightness (L* value); green (−) to red (+) (a* value); and blue (−) to yellow (+) (b* value).
pH of seaweed hydrolysates
Following hydrolysis, HMH was cooled and the pH was determined by a pH.
Maillard reaction
The Maillard reaction products (MRPs) were determined by the UV absorbance of samples, as described by Ajandouz et al. (2001). After the hydrolysis processes, filtered HMH were diluted 20 times with distilled water and loaded into 96-well plates. The absorbance was measured at wavelengths of 294 and 420 nm. The absorbance ratios between 294 and 420 nm were calculated to determine the transformation of the UV-absorbing materials into brown polymers. The value of the diluted samples was converted to that of the original reaction solution.
Total protein
The protein concentrations were determined according to Lowry’s method. HMH (200 μL) was mixed with 2 mL alkaline copper sulfate reagent and vortexed for 8 s. After incubation for 10 min at room temperature, 200 μL of 0.2 N Folin–Ciocalteu reagent (FCR) was added to the mixture and incubated for another 30 min. Following these incubation periods, the mixtures were loaded into 96-well plates, and the absorbance value was measured at 660 nm. Bovine serum albumin (BSA) was used as the reference standard. The final results were expressed as gram BSA equivalent per 100 gram dry weight (g (100 g)−1 DW).
Total sugar
The total sugar levels of HMH were measured by the phenol sulfuric acid method of Dubois et al. (1956) with slight modifications (smaller reaction volume). HMH (200 μL) was mixed with 5% phenol (200 μL) and 1 mL of sulfuric acid (H2SO4) and vortexed for 10 s. After incubation for 30 min at 100 °C, the mixture was cooled and reached 25 °C. Following the incubation periods, the mixtures were loaded into 96-well plates and the absorbance was measured at 490 nm. Glucose was used as the reference standard. The final results were expressed as gram glucose equivalent per 100 g dry weight (g (100 g)−1 DW).
Reducing sugar
Reducing sugar analysis was determined using the 3,5-dinitrosalicylic (DNS) acid method, as described previously. Briefly, 1.25 g of sodium hydroxide (NaOH) was mixed with distilled water (125 mL). Once the mixture completely dissolved, 1.25 g of DNS acid, 0.25 g phenols, and 0.0625 g sodium sulfite were added to the reagent solutions. HMH (500 μL) was mixed with 500 μL of the reagent solution. The mixture was incubated at 95 °C for 15 min. After incubation, 500 μL of potassium sodium tartrate tetrahydrate (KNaC4H4O6·4H2O; 40%) was added. The mixture was cooled and loaded into 96-well plates, and the absorbance was measured at 575 nm. Glucose was used as the reference standard.
Total phenolic content
Phenolic content of HMH was measured by Folin–Ciocalteau reagents (FCR) methods, as described previously (Matanjun et al. 2008). HMH (500 μL) was mixed with 0.2 N FCR solution (500 μL) and incubated in the dark for 5 min. After incubation, 500 μL of 7.5% Na2CO3 was added and the mixture was kept in the dark for 2 h. The solution was centrifuged at 2500 rpm for 10 min. The supernatant was then collected and loaded into 96-well plates, and the absorbance was measured at 765 nm using multimode microplate readers. Phloroglucinol was used as the reference standard. The final results were expressed as mg phloroglucinol equivalent per g dry weight (mg PGE g−1 DW).
Total flavonoid content
Flavonoid content of HMH was measured according to methods as described previously (Ozsoy et al. 2008). HMH (200 μL) was mixed with 400 μL of distilled water and 200 μL of 5% NaNO2 and incubated for 10 min. After incubation, 30 μL of 10% AlCl3 and 400 μL of 1 M NaOH were added. The mixture was loaded into 96-well plates, and the absorbance was measured at 510 nm using multimode microplate readers. Quercetin was used as the reference standard. The final results were expressed as mg quercetin equivalent per g dry weight (mg Q g−1 DW).
Antioxidant assay
DPPH radical scavenging assay
HMH (50 μL) was mixed with 950 μL of 0.l mM DPPH. The mixture was incubated in the dark for 30 min. After incubation, the mixture was loaded into 96-well plates and the absorbance was measured at 517 nm using microplate readers. All the measurements were made in triplicate, and methanol was used as the negative control. Trolox was used as the reference standard, and a standard curve was constructed. The final results were expressed as mg Trolox equivalent per g dry weight (mg TE g−1 DW).
ABTS radical scavenging assay
Briefly, ABTS•+ (7 mmol L−1) and potassium persulfate (2.45 mmol L−1) were prepared separately and stored in the dark for at least 16 h and mixed. The ABTS radical solution was diluted with methanol to reach an absorbance value of 0.72 ± 0.02 at 734 nm. Diluted HMH (125 μL) was mixed with 625 μL of ABTS•+ radical solution and stored in the dark for 5 min. After incubation, the mixture was loaded into 96-well plates and the absorbance was measured at 734 nm using microplate readers. All the measurements were made in triplicate, and methanol was used as the negative control. Trolox was used as the reference standard, and a standard curve was constructed. The final results were expressed as mg Trolox equivalent per g dry weight (mg TE g−1 DW).
Total antioxidant capacity
HMH (100 μL) was mixed with 3 mL of radical solution (0.6 M H2SO4, 28 mM Na3PO4, and 4 mM (NH4)6Mo7O24). The HMH and radical solution mixture was incubated at 95 °C for 1.5 h. After incubation, the mixture was loaded into 96-well plates and the absorbance was measured at 695 nm using multimode microplate readers. All the measurements were made in triplicate, and methanol was used as the negative control. Ascorbic acid was used as the reference standard, and a standard curve was constructed. The results were expressed as mg ascorbic acid equivalent per g dry weight (mg AAE g−1 DW).
Emulsifying properties
The ability of HMH to stabilize emulsions was determined as described previously (Fawzy et al. 2017). HMH was mixed with hydrophobic solutions in a ratio of 2:3 (HMH to hydrophobic solutions, v/v) and homogenized with a vortex mixer for 10 min. After the mixture was homogenized, it was incubated for a period of 24 h. The heights of the emulsion were checked, and the emulsification index after 24 h (E24) was determined using the following equation:
The tested hydrophobic compounds used in this study were sunflower oil and corn oil. The ability of HMH to stabilize emulsions after being heated was also determined. Briefly, HMH was preheated at 60 and 100 °C for 2 h and cooled. Then, preheated HMH was mixed with corn oil or sunflower oil and left undisturbed for 24 h to determine the emulsification index.
High-performance liquid chromatography
HMH was further analyzed for phenolic content using an HPLC system (Hitachi America Ltd., USA). The analysis method was programmed based on the method of Vo Dinh et al. (2018). The HMH was analyzed using a Nucleosil C8 column (250 mm × 4.6 mm, 5 μm, Macherey-Nagel, Germany) with linear gradients of water with 0.1% glacial acetic acid (solvent A) and acetonitrile with 0.1% glacial acetic acid (solvent B) at a flow rate of 0.8 mL min−1 (Vo Dinh et al. 2018). The peaks were detected at 280 nm. Each peak was detected and confirmed with the reference phenolic acids. The phenolic standard was used for the quantification by HPLC, standard curve was constructed, and phenolic content of HMH was expressed as mg g−1 DW.
Statistical analysis
All data were presented as the means of three individual experiments, unless otherwise stated. Statistical comparisons between different treatments were carried out by one-way ANOVA with Duncan’s multiple range tests (p < 0.05). Pearson correlation analysis was also conducted to evaluate the correlation between the treatments. SPSS software for Windows ver. 16 (SPSS Inc.) was used for the statistical analysis.
Results and discussion
Yield and characteristics of HMH
In this study H. musciformis was hydrolyzed in the SCWE system at 120–270 °C for 10 min. The hydrolysis efficiency ranged from 61.37 to 81.23% (Fig. 1a). The highest hydrolysis efficiency was obtained from HMH that hydrolyzed at 210 °C. In general, an increase in the hydrolysis efficiency was observed as the temperature increased. Several studies have reported that temperature is one of the crucial factors that affect the efficiency and selectivity in SCWE. A high temperature facilitates deeper penetration of solvent because it disrupts matrix interactions between analytes and samples caused by van der Waals forces, hydrogen bonding, and dipole attraction (Mustafa and Turner 2011). In addition, compared with low temperatures, high temperatures decrease the viscosity of a solvent. This further enhances penetration of the solvent inside the matrix particle, which then improves the diffusion rate of analyte to solvent and finally improves the extraction process. Compared with temperature, S/L ratios have less significant effects, which indicate that the S/L ratios used in this study were adequate to hydrolyze H. musciformis.
The changes in the pH of HMH are shown in Fig. 1b. The pH of solvents before hydrolysis was 7.2, and the pH gradually decreased to 2.84–3.14 at 210 °C and to 3.22–3.44 at 240 °C with extraction. This result might be correlated with the breakdown of sugar into organic acids, which therefore enhanced the acidity of HMH. It has been reported that the fragmentation of carbohydrates in SCWE systems occurs at temperatures above 180 °C (Alvarez et al. 2014). The results in our study are consistent with those of Gao et al. (2014) who reported decreasing pH values of sucrose in SCWE due to the decomposition of fructose and glucose products into acidic materials. At longer residence times, changes in the pH were reported to be larger with a higher conversion of sucrose (Gao et al. 2014). In addition, high temperatures improve the extractability of the solvent, which increases the yield of functional materials in HMH. These functional materials produced at higher temperature increase the dark color of the extract (Fig. 2).
Color is a vital factor in food appearance and acceptance because it affects consumers’ perceptions about flavor and quality of food. The L*, a*, and b* values represent one of the most popular instrumental methods for color measurement based on the primary parameters of lightness (L*), redness (a*), and yellowness (b*). The color analysis of HMH using the L*, a*, and b* values ranged from 18.95 to 50.82 (Fig. 2). The HMH hydrolyzed at 120–150 °C showed low L* values; these might be correlated with the high sugar content obtained at these low temperature. Higher L* values obtained at temperature above 150 °C imply a hydrolysis of polysaccharides generated by high temperature under SCWE conditions. The color changes from yellow to brownish red (Fig. 2d) following SCWE process starting from temperature 180 °C and above, which relates well with a moderate increase of a* value. Interestingly, compared with other conditions, the L values of HMH at 210 °C showed the highest values and then decrease when HMH hydrolyzed at higher temperature. It has been reported elsewhere that, above 200 °C, SCWE produced more caramelized sugars and other functional materials from amino acids and reducing sugar by the Maillard reaction. Therefore, the darker color of HMH processed at higher temperature might be related to the formation of MRPs (Table 1). The absorbance of HMH at 290 and 420 nm was used as an indicator for the intermediate and final stages of MRPs, respectively (Zhou et al. 2016). In addition, MRPs have been known for their antioxidant properties and their ability to retard lipid oxidation.
Total protein, sugar, and reducing sugar properties
We found a slight increase in the protein content of HMH hydrolyzed at 120–150 °C (Fig. 3). However, when H. musciformis was hydrolyzed at higher temperatures (180–210 °C), a significant increase in protein content was observed. The highest protein yield (15.75% ± 0.10) was obtained from the H. musciformis hydrolyzed at 210 °C and at an S/L ratio of 1:50 (Fig. 3). The amount of protein recovered by SCWE can be compared to the amount of protein in H. musciformis (18%) (Siddique et al. 2013). This demonstrated that more than 80% protein in H. musciformis could be recovered by SCWE at 210 °C. These results could be related to the increased solubility of protein. The solubility of protein can be influenced by intrinsic and extrinsic factors. Kramer et al. (2012) reported that pH, ionic strength, solvent type, and hydrolysis temperature are extrinsic factors that affect protein solubility. In general, at lower temperature, protein has lower solubility due to robust aggregation through hydrophobic interactions (Watchararuji et al. 2008; Kramer et al. 2012). An increase in temperature causes an increase in water ionization constant, which increases the protein yield.
The proportions of total sugars ranged from 5.57 to 68.25% (Fig. 4). HMH with the highest sugar content was obtained at lower temperature (120–150 °C). The amount of total sugar found in this study was comparable with total sugar of H. musciformis (19.19 to 67.6%) reported previously (Arman and Qader 2012). The amount of total sugar in HMH decreased with increasing extraction temperature and reached a minimum at 270 °C to an amount of 5.57 ± 0.45 g (100 g)−1 (Fig. 4a). In accordance with the findings in this study, a gradual decrease in the total sugar content has been reported in red seaweeds hydrolyzed by SCWE at higher temperature (Gereniu et al. 2017). The decrease in the total sugar content at temperatures above 150 °C was considered to be due to the hydrolysis of poly- or oligosaccharides and the degradation of monosaccharides generated by the high ionic product of water at high temperature under SCWE conditions (Narita and Inouye 2012). Interestingly, H. musciformis produces a small amount of reduced sugars when hydrolyzed at lower temperature. The reducing sugar content of HMH obtained by SCWE increased with increasing temperature up to 180 °C (52.67 ± 1.14 g (100 g)−1) and drastically decreased at an extraction temperature above 180 °C (Fig. 4b). The reducing sugar in HMH found in this study was higher compared to the reducing sugar of H. musciformis originated from Gujarat, India (3.6 ± 0.3 mg g−1) (Chakraborty and Bhattacharya 2012). Meillisa et al. (2015) reported that the reducing sugar content from the marine polysaccharides recovered by treatment with SCWE increased as the hydrolysis conditions increased up to 180 °C; however, a decreased sugar content was observed at temperatures above 180 °C (Meillisa et al. 2015). The lower amount of reduced sugar observed may be due to the degradation of sugar into other products, including aldehydes and ketones, from which organic acids could be produced.
Total phenolic and flavonoid contents of HMH
Figure 5 shows the total phenolic content (TPC) and total flavonoid content (TFC) of HMH obtained by treatments with SCWE under various temperatures and S/L ratios. The values of TPC and TFC were converted to the gallic acid equivalent (GAE) and quercetin equivalent (QE), respectively. The TPC content in HMH obtained by SCWE increased with increasing S/L ratios and hydrolysis temperature. The TPC and TFC of HMH with S/L ratio 1:50 and hydrolyzed at 210 °C exhibited the highest TPC and TFC contents, 39.57 ± 0.20 mg GAE g−1 and 12.10 ± 0.34 mg QE g−1, respectively. The TPC and TFC of HMH at 210 °C were 8 and 12 times higher than the TPC and TFC of HMH extracted with SCWE at 120 °C. The high TPC of HMH hydrolyzed at 210 °C might correlate with the highest protein content found at the same hydrolysis temperature, suggesting that, at 210 °C, SCWE might precipitate most of the proteins and some of the reversibly bonded phenolic compounds into the solution. In addition, Narita and Inouye (2012) suggested that the high TPC content in SCWE systems might also correlate with the degradation of polyphenolic compounds into smaller and soluble ones. Temperature is one of the most important parameters that affect TPC; at a certain temperature, phenolic compounds are degraded or undergo undesirable reactions due to high hydrolysis temperatures and prolonged times (Khoddami et al. 2013). This might explain the slight decrease of TPC in HMH hydrolyzed at temperatures above 210 °C observed in this study.
The seaweed and solvent quantities are also important factors affecting the yield of functional materials in seaweed. Therefore, in this study, influence of S/L ratios was also studied. The TPC and TFC of HMH increased with increasing S/L ratios from 1:150 to 1:50. Previous studies showed that very low and high S/L ratios are not recommended for extraction of functional materials from their sample matrix. Low S/L ratio is causing excessive swelling of the materials which further creates larger concentration gradient between the solvent and sample matrix. These will subsequently increase the recovery of functional materials up to certain S/L ratio and then decline due to the limit of solvation effects. As an example, Xu et al. (2015) reported that TPC and TFC from marigold by SCWE reached the highest level with the S/L ratio of 1:50 and then decreased slightly as they increased the liquid concentration to the S/L ratio of 1:60 (Xu et al. 2015). In addition, high S/L ratio will also affect SCWE process, as there will be less collision and longer diffusion time for the solvent into the sample matrix. Therefore, it is important to optimize and maintain the suitable S/L ratios during SCWE process.
Rafiquzzaman et al. (2016) extracted H. musciformis using various solvents and reported that the TPC and TFC of H. musciformis was around 20.19–49.77 mg GAE g−1 and 20.65–70.67 mg QE g−1, respectively. The TFC and TPC of H. musciformis found in this study were comparable to earlier reports, suggesting that SCWE is an effective method to obtain phenolic content from H. musciformis. In addition, temperature and S/L ratios used in this study were adequate and suitable to obtain TPC and TFC from H. musciformis.
Antioxidant activity and correlation with HMH functional materials
Seaweed has been reported to contain a wide range of functional materials, some of which have been shown to have antioxidant activity. In this study, the antioxidant activity of the HMH obtained by SCWE was evaluated with DPPH, ABTS, and total antioxidant assays. Antioxidant activity was represented by Trolox equivalents (TE) and ascorbic acid. The results shown in Table 2 indicate that the antioxidant activity of HMH decreases when the hydrolysis temperature is lower, reaches a maximum value at a certain temperature (210 °C), and then decreases again. Under certain temperature conditions, water could extract more antioxidative materials that could not be extracted at lower temperature. A temperature of 210 °C and a 1:50 S/L ratio were found to be the most suitable conditions to hydrolyze H. musciformis using SCWE.
To verify the correlation between HMH functional materials and antioxidant activity, Pearson correlation analysis between total sugar, protein, TFC, TPC, and different antioxidant assays was applied and is presented in Table 3. The Pearson correlation analysis demonstrated that there was a negative correlation between total antioxidant activity and total sugar, with Pearson correlation coefficients of − 0.11, − 0.466, and − 0.528 for total antioxidant, DPPH, and ABTS scavenging activity, respectively. In general, a positive correlation between total protein, TFC, TPC, and antioxidant activity was observed. Notably, we found a very good correlation between the DPPH scavenging assay and TPC with a Pearson correlation coefficient of 0.983 (p < 0.01). In line with the findings in this study, a strong correlation of seaweed TPC and antioxidant activity has been reported in previous studies (Martins et al. 2013; Chakraborty et al. 2015; Rafiquzzaman et al. 2016). These results may indicate that phenolic compounds are a major contributor to the biological activity of HMH. Antioxidant activity of phenolic compounds depends on their structural features. The hydroxyl (–OH) group bound to the aromatic ring of phenolics compounds acts as an electron donor and reacts to a free radical or other reactive species which breaks the cycle of generation of new radicals (Pereira et al. 2009; Pangestuti et al. 2018). This underlies the antioxidant activity of phenolic compounds. In addition, the attachment of –OH groups at the meta position of benzoic acid increases the H-donating radical scavenging activity of phenolic compounds. However, carboxyl group has a negative effect on proton donation due to the electron-withdrawing properties (Fernando et al. 2016). Several techniques such as water extraction, solid–liquid extraction, enzyme-assisted extraction, microwave-assisted extraction, ultrasound-assisted extraction, and SCWE have been carried out in an attempt to extract phenolic compounds from marine algae. Among other techniques, SCWE has currently received more attention. SCWE is achieved at temperatures between 100 and 374 °C under high pressure to keep the water in a liquid state. Vo Dinh et al. (2018) reported that SCWE give higher extracting capability of phenolic compounds than conventional water extraction. It was demonstrated that seaweed hydrolysis by SCWE at temperature above 150 °C increases the contents of both soluble phenolics (i.e., gallic acid) and insoluble phenolics (i.e., ferulic and p-coumaric acids).
In addition to phenolic contents, protein also positively correlates with antioxidant activity of HMH. SCWE can be used as an alternative to enzymatic digestion to facilitate the extraction of protein and peptides. Under appropriate treatment conditions (e.g., temperature, pressure, time, S/L ratios), SCWE could be used as good methods to modify seaweed protein or peptide for specific purposes. Antioxidant activity of seaweed-derived proteins and peptides is mainly due to the complex interactions between their ability to inactivate reactive oxygen species (ROS), scavenge free radicals, chelate prooxidative transition metals, reduce hydroperoxides, enzymatically eliminate specific oxidants, and alter the physical properties of food systems in a way that separates ROS (Elias et al. 2008). In addition, antioxidant activity of protein and peptide is closely related to their structure, with lower molecular weight and high degree of hydrophobicity showing more potent antioxidant activity. Hydrophobicity may contribute to peroxidation inhibition by increasing the solubility of protein and peptide in lipid and thereby facilitating better interaction with radical species.
HPLC analysis of HMH
On the basis of Pearson’s analysis, the antioxidant activity in HMH seemed to be correlated with the amount of phenolic compounds. This strong correlation implies that phenolic compounds are one of the key components in the antioxidant activity of H. musciformis. Therefore, phenolic acid constituents from HMH were quantified by HPLC using phenolic acid standards. The content of phenolic compounds in HMH was estimated based on phenolic acid standard calibration curves. The main constituents of the phenolic acids in H. musciformis are summarized in Fig. 6 and Table 4. The highest phenolic acid in H. musciformis was chlorogenic acid, and the contents obtained by SCWE (S/L 1:50) at temperatures of 180, 210, 240, and 270 °C were 17.91, 27.98, 19.87, and 6.53 mg g−1 DW, respectively. The amount of phenolic acids in HMH generally increased following an increase in temperature up to 180 °C, and a moderate decrease was observed at hydrolysis temperatures above 210 °C suggesting the degradation of phenolic acids at temperature above 180 °C. In accordance with the findings of this study, Fabian et al. (2010) observed a major weight loss of phenolic acids from defatted rice bran, which hydrolyzed using SCWE at temperatures above 207 °C. A decrease in phenolic acid in the SCWE hydrolysates at higher temperature may be related to the conversion of phenolic acid into decarboxylation products and other gaseous products (Fabian et al. 2010). Collectively, SCWE enabled one to recover high amounts of phenolic acids from seaweeds, demonstrating the potential of this technique.
Emulsifying properties
Stability is the most important requirement of functional materials when applied as emulsifiers. Functional materials should be stable under extreme conditions, such as low pH and high temperature, for application as emulsifying agents. In food processing, heating is an important step and temperatures ranging from 60 to 100 °C are commonly applied. Therefore, in the present study, the HMH emulsifying properties were studied using corn oil and sunflower oil at different temperatures (room temperature, 60, and 100 °C). Figure 7 illustrates the emulsifying properties (E24) of HMH. E24 is an emulsifying index that is obtained by determining the height of the sample emulsion after incubation for 24 h.
In the present study, we found that HMH was able to stabilize emulsions formed by sunflower oil and corn oil under different temperature conditions. Notably, the highest and most stable emulsifying activity was obtained from HMH that hydrolyzed at a temperature of 180 °C, with E24 ranging between 46.26 and 53.33%. In addition, HMH was stable against thermal treatments at 60 and 100 °C, suggesting that HMH was thermostable for the preparation of sunflower and corn emulsions. Collectively, these results suggest that HMH possesses the potential to be used as an active emulsifying agent in the food, pharmacy, cosmetics, and other industries.
Conclusions
The results of the present study demonstrated that SCWE enabled the recovery of functional materials from H. musciformis with hydrolysis temperature at 210 °C and S/L ratios 1/50 were found to be the most optimum conditions to obtain functional materials with good antioxidant activity. Meanwhile, HMH hydrolyzed at temperature below 180 °C were found to be the most optimum conditions to obtain sugar and showed stable emulsion-forming properties at different temperatures, making it a potential candidate for emulsifying; however, further analysis on the functional materials (i.e., carbohydrates and protein) is required. The obtained results confirm the importance of these underexploited seaweed resources in Indonesia as a valuable source of functional materials for further use in different application sectors and SCWE as an efficient and environmental-friendly technique for its extraction.
References
Ajandouz E, Tchiakpe L, Ore FD, Benajiba A, Puigserver A (2001) Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci 66:926–931
Alvarez VH, Cahyadi J, Xu D, Saldaña MD (2014) Optimization of phytochemicals production from potato peel using subcritical water: experimental and dynamic modeling. J Supercrit Fluids 90:8–17
Arman M, Qader SAU (2012) Structural analysis of kappa-carrageenan isolated from Hypnea musciformis (red algae) and evaluation as an elicitor of plant defense mechanism. Carbohydr Polym 88:1264–1271
Chakraborty S, Bhattacharya T (2012) Nutrient composition of marine benthic algae found in the Gulf of Kutch coastline, Gujarat, India. J Algal Biomass Util 3:32–38
Chakraborty K, Joseph D, Praveen NK (2015) Antioxidant activities and phenolic contents of three red seaweeds (division: Rhodophyta) harvested from the Gulf of Mannar of peninsular India. J Food Sci Technol 52:1924–1935
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Elias RJ, Kellerby SS, Decker EA (2008) Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr 48:430–441
Fabian C, Tran-Thi NY, Kasim NS, Ju YH (2010) Release of phenolic acids from defatted rice bran by subcritical water treatment. J Sci Food Agric 90:2576–2581
Fawzy MA, Gomaa M, Hifney AF, Abdel-Gawad KM (2017) Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr Polym 157:1903–1912
Fernando IS, Kim M, Son K-T, Jeong Y, Jeon Y-J (2016) Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. J Med Food 19:615–628
Gao D, Kobayashi T, Adachi S (2014) Kinetics of sucrose hydrolysis in a subcritical water-ethanol mixture. J Appl Glycosci 61:9–13
Gereniu CRN, Saravana PS, Getachew AT, Chun B-S (2017) Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J Appl Phycol 29:1609–1621
Hutomo M, Moosa MK (2005) Indonesian marine and coastal biodiversity: present status. Indian J Mar Sci 34:88–97
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375
Kramer RM, Shende Varad R, Motl N, Pace CN, Scholtz JM (2012) Toward a molecular understanding of protein solubility: increased negative surface charge correlates with increased solubility. Biophys J 102:1907–1915
Martins CDL, Ramlov F, Carneiro NPN, Gestinari LM, dos Santos BF, Bento LM, Lhullier C, Gouvea L, Bastos E, Horta PA (2013) Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J Appl Phycol 25:1179–1187
Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming CH (2008) Antioxidant activities and phenolics content of eight species of seaweeds from North Borneo. J Appl Phycol 20:367–373
Meillisa A, Woo H-C, Chun B-S (2015) Production of monosaccharides and bio-active compounds derived from marine polysaccharides using subcritical water hydrolysis. Food Chem 171:70–77
Mulyati H, Geldermann J (2017) Managing risks in the Indonesian seaweed supply chain. Clean Techn Environ Policy 19:175–189
Mustafa A, Turner C (2011) Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta 703:8–18
Narita Y, Inouye K (2012) High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem 135:943–949
Ozsoy N, Can A, Yanardag R, Akev N (2008) Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem 110:571–583
Pangestuti R, Kim S-K (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3:255–266
Pangestuti R, Siahaan E, Kim S-K (2018) Photoprotective substances derived from marine algae. Mar Drugs 16:399
Pereira DM, Valentão P, Pereira JA, Andrade PB (2009) Phenolics: from chemistry to biology. Molecules 14:2201–2211
Rafiquzzaman S, Ahmad MU, Lee JM, Kim EY, Kim YO, Kim DG, Kong IS (2016) Phytochemical composition and antioxidant activity of edible red alga Hypnea musciformis from Bangladesh. J Food Process Preserv 40:1074–1083
Siddique MAM, Aktar M, bin Mohd Khatib MA (2013) Proximate chemical composition and amino acid profile of two red seaweeds (Hypnea pannosa and Hypnea musciformis) collected from St. Martin’s island, Bangadesh. Journal of Fisheries Sciences 7:178–186
Vo Dinh T, Saravana PS, Woo HC, Chun BS (2018) Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep Purif Technol 196:287–299
Watchararuji K, Goto M, Sasaki M, Shotipruk A (2008) Value-added subcritical water hydrolysate from rice bran and soybean meal. Bioresour Technol 99:6207–6213
Xu H, Wang W, Jiang J, Yuan F, Gao Y (2015) Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS·+ assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J Food Sci Technol 52:3803–3811
Zhou Y-Y, Li Y, Yu A-N (2016) The effects of reactants ratios, reaction temperatures and times on Maillard reaction products of the L-ascorbic acid/L-glutamic acid system. Food Sci Technol (Campinas) 36:268–274
Acknowledgements
Pangestuti was supported by the National Research Foundation (Rep. of Korea) through the Postdoctoral Fellowship Program for Foreign Researchers 2017–2018. The authors acknowledge Dept. of Food Science and Technology, Pukyong National University, and Indonesian Institute of Science (LIPI) for all the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pangestuti, R., Getachew, A.T., Siahaan, E.A. et al. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J Appl Phycol 31, 2517–2528 (2019). https://doi.org/10.1007/s10811-019-1754-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-1754-9