Abstract
In this study, two strains of Kappaphycus alvarezii were treated with pressurized hot water extraction (PHWE) at conditions ranging from 150 °C/1 MPa to 300 °C/10 MPa using three different solvents including water, 1% formic acid, and 1% sodium hydroxide. Hydrolysates obtained from the extraction were investigated for their hydrolysis efficiency, pH, radical scavenging activity (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) and 2,2-diphenyl-1-picrylhydrazyl (DPPH)), total phenolic content (TPC), total flavonoid content (TFC), total sugar, reducing sugar, monosaccharide composition, Maillard reaction products (MRPs), total protein, emulsifying properties, and foaming properties. The highest hydrolysis efficiency ranged from 81.47 to 97.18% showing a consistent increase with increasing temperature and pressure conditions. Major monosaccharide compounds recovered from the hydrolysates were glucose and galactose with concentrations of 156.21 and 155.49 mg L−1, respectively. Radical scavenging activity, polyphenol content, and proteins increased from 150 °C/1 MPa to 270 °C/8 MPa but decreased at 300 °C/10 MPa due to decomposition and protein denaturation. Foaming and emulsifying properties were influenced by variation of protein with relation to temperature and protein–polysaccharide electrostatic interactions. Thus, this study showed that the best condition for all sugars and foaming properties is 150 °C/1 MPa, while antioxidants, TPC, TFC, total protein, and emulsifying properties are best recovered at 270 °C/8 MPa for both strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The marine alga Kappaphycus alvarezii, commercially known as Eucheuma cottonii, is a macroalgal species of the class Rhodophyceae (red algae) which has fleshy thalli and plastic-like morphology that normally appears green, yellowish brown, or reddish in color. The variation in color is due to concentration of the pigment phycoerythrin that determines the three different strains (green, brown, and red). Kappaphycus alvarezii can grow up to 2 m, and it is a widely cultured species due to the low cost of farming materials and fast growth, known to double its biomass in 15 days. Over the last decades, K. alvarezii has become one of the most economically important edible seaweeds because of its increasing demand as the major source of κ-carrageenan (Munoz et al. 2004). κ-Carrageenan is a disaccharide commonly used in the food and cosmetic industries for its gelling, stabilizing, thickening, and emulsifying properties. Recently, the Food and Agriculture Organization (FAO) (2014) reported a significant increase in the global production of this species from 1990 to 2012. For centuries, this edible red macroalga has become a vital part of the diet in the countries of Southeast Asia including China, Japan, and Korea essentially for its dietary fiber and antioxidant properties. Antioxidants are important nutraceuticals that have various functions, including defense against oxidative damage and participation in the major signaling pathways of cells (Kumar et al. 2008). The major function of antioxidants is mainly to prevent damage of cells by the action of reactive oxygen species (ROS). Hence, the demand for K. alvarezii in most parts of the world is not only for food production but also for its health benefits including antioxidant properties.
According to Tan et al. (2013), Hurtado et al. (2014), and Msuya et al. (2014), K. alvarezii is widely cultivated throughout the paleotropical regions in the world with Indonesia and Philippines currently being the overall leading producers of κ-carrageenan. In 1980, the farming of this highly commercialized macroalgae was introduced to the Solomon Islands. From reports by FAO (2014), production in Solomon Islands had increased quite steadily from 3260 t in 2005 to 13,000 t in 2012, with an anticipated further increase in the later years. The main producers in Solomon Islands are seaweed farmers from Waghena in Choiseul province and Manaoba in Malaita province. The seaweed samples used in this study were from the seaweed farms in Waghena located (latitude 7° 26′ 00″ S, longitude 157° 46′ 00″ E) in the Katupika region of Choiseul province.
With the increasing significance of K. alvarezii in different sectors such as food, cosmetic, and pharmaceutical industries, extraction of functional materials from the algal biomass requires an efficient and eco-friendly technology. The pressurized hot water extraction (PHWE) technique was selected because of the significant reduction of the quantity of solvents used and faster time of extraction compared to other solvent extraction techniques. According to Michalak and Chojnacka (2014), PHWE uses only deionized water which is environmentally friendly and involves elevated temperature that enables high analytic solubility by increasing both solubility and mass transfer rate. The increase surface contact and deeper penetration of solvent into the sample matrix enhance a high extraction yield.
In theory, the phase in PHWE that involves water pressurized at a temperature and pressure under critical level conditions is normally described as the subcritical region (Thiruvenkadam et al. 2015). In the subcritical region, seaweed–water matrix is treated to a pressurized temperature between 100 and 374 °C with a pressure of up to 22 MPa, and the water is maintained in the liquid state by operating with a constant pressure above the vapor pressure. Addition to that, the subcritical condition also facilitates an increase in dielectric constant and decrease in density which cause hydrocarbons to become more water soluble allowing complex reactions like decomposition and depolymerization to occur (Jazrawi et al. 2013). Technically, PHWE is a highly efficient, inexpensive, and environmentally friendly technique that provides a better alternative to the traditional methods. Thus, using this green technology, the aim of this study was to extract monosaccharides and bioactive compounds from two seaweed strains of the species K. alvarezii and to identify the best conditions using various solvents that produced the highest yields of important functional materials that are safe for human consumption.
Materials and methods
Kappaphycus alvarezii was harvested and sun dried at the seaweed farms in Waghena, Solomon Islands. The dried seaweed samples (~3000 g dry weight) were shipped to our laboratory, and high purity nitrogen gas (99.99%) was supplied by KOSEM (Republic of Korea). All reagents were of analytical high-performance liquid chromatography (HPLC) grade, and standards such as catechin, L-gulose, L-mannose, D-(+)-glucose, D-(+)-galactose, L-(+)-arabinose, and D-(+)-xylose were purchased from Sigma-Aldrich Chemical Co. (USA).
Sample preparation
The sun-dried seaweed samples were thoroughly washed under tap water to rehydrate and eliminate excessive salts and epiphytes. They were then cut into small pieces, packed in aluminum boxes, and later freeze dried for 48 h at −80 °C using Eyela FDU-2100 (Tokyo Rikakikai Co., Ltd., Japan) fitted with a square drying chamber (Eyela DRC-1000, Tokyo Rikakikai Co., Ltd., Japan). The dried samples were then ground using a mechanical blender (PN SMKA-4000 mixer, PN Co., Korea) and sieved to an evenly fine size by a stainless steel sieving mesh of size 710 μm.
Pressurized hot water extraction
PHWE was performed in a 200-cm3 batch reactor made of Hastelloy C276 (continuous-type supercritical water system, Phosentech, South Korea) with a temperature control (Saravana et al. 2016). Four gram of finely ground sample material was loaded into the reactor with 160 mL of distilled water. Formic acid (1%) and sodium hydroxide (1%) catalyst were prepared separately in distilled water. The reactor was tightly sealed with a cap and heated using an electric heater to the desired temperature (150 to 300 °C). The required pressures were determined based on saturated vapor to be between 1 and 10 MPa for the temperature range studied. The temperature and pressure in the reactor were controlled using a temperature controller and pressure gauge, respectively (Meillisa et al. 2015). The hydrolyzed sample was agitated using a four-blade impeller at 150 rpm. The reaction time was kept for 5 min after reaching its desired temperature. Hydrolysate samples from the reactor were collected immediately using a refrigerated circulated bath connected to the batch. It was then filtered using 0.45-μm Whatman nylon membrane filter paper and stored at 4 °C for further analysis. The sample residue from the hydrolysates was dried and weighed to determine the percentage (%) efficiency (yield) of each hydrolysate.
Proximate analysis
Carbohydrate content was determined by calculating the percentage difference from all other constituents (Abd-Rahim et al. 2014). An indirect protein determination method for measuring ammonium nitrogen in Kjeldahl digester was used for measuring total nitrogen and protein content calculated using a nitrogen conversion factor of 6.25. Lipid content was determined by the Soxhlet system (AOAC 1997), while total ash was measured by calcinations in a muffle furnace at 550 °C until constant weight was obtained (AOAC 1997). Similarly, moisture content was determined by incubation at 105 °C after a constant dry weight was obtained.
Hydrolysis efficiency
Determination of the hydrolysate extraction efficiency was calculated according to Dong et al. (2011) with some modifications. The following formula was used for the calculation:
The initial weight of K. alvarezii hydrolysate was obtained before loading sample into the reactor. The hydrolysate filtrate was kept in an oven at 60 °C for 24 h to eliminate moisture, and the weight of dry extract was attained by subtracting the weight of dried filtrate after incubation from the total initial weight.
pH measurement
The pH measurements of K. alvarezii hydrolysates were obtained using a pH meter (Mettler Toledo Five Easy Plus, Switzerland) at room temperature. The equipment was calibrated using technical buffer solutions pH 4.01, 7.00, and 9.1 prior to the measurements.
Radical scavenging activity
DPPH
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assays were conducted using a modified method as described by Khalaf et al. (2008). Briefly, 6 mM DPPH assay was prepared by dissolving 0.0237 g in 100 mL of concentrated methanol. The mixture was well vortexed and wrapped in aluminum foil to prevent increasing absorbance. For analysis of hydrolysate samples, 100 μL of crude extract was mixed with 3.9 mL of methanol DPPH and vortexed for 1 min. A negative control was included by replacing the sample with methanol (100 μL). The mixtures were closed with aluminum foil caps and incubated in dark at room temperature for 30 min. Eighty percent methanol was used as control, and the absorbance of the samples and negative control was measured at 517 nm (Shimadzu 1240 UV-Vis spectrophotometer). The DPPH radical scavenging activity was quantified in percentage (%) using the following formula:
where A c is the absorbance of the negative control at 517 nm and A s is the absorbance of the sample or crude extract at 517 nm. All sample analysis was conducted in triplicates, and scavenging activity was expressed as percentage (%) radical scavenging activity.
ABTS
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was conducted using the method described by Sachindra et al. (2010) with minor modifications. Briefly, the assay was prepared by mixing 7 mM ABTS solution and 2.45 mM of potassium persulfate together and kept in dark for 10–15 h. Analysis of hydrolysate samples was conducted by mixing 100 μL of diluted crude extract with 3.9 mL of the ABTS assay and vortexed for 1 min. The mixtures were closed with aluminum caps and kept in the dark for 6 min, and the absorbance was measured at 734 nm. Eighty percent methanol was used as control.
where A c is the absorbance of the control at 734 nm, while A s is absorbance of the sample (the extracts) at 734 nm.
Total phenolic content
The total phenolic content (TPC) was determined by the Folin–Ciocalteu reagent (FCR) method as described by Wang et al. (2009) with minor modifications. Basically, 1 mL diluted sample was mixed with 1 mL FCR (1:10 v/v) and incubated at room temperature for 4 min. Of 7.5% sodium carbonate (Na2CO3) anhydrous solution, 0.8 mL was added, and the mixture was incubated in the dark for 2 h. The absorbance was measured at 765 nm, and gallic acid dissolved in ethanol was used as standard.
Total flavonoid content
The total flavonoid content (TFC) was determined according to a modification of the method described by Ozsoy et al. (2008). Briefly, 0.5 mL of diluted sample was added to 2.5 mL of deionized water. An aliquot of 0.15 mL of sodium nitrite (NaNo2) was added to the mixture and allowed to stand in ambient temperature for 6 min. Again, an aliquot of 0.5 mL aluminum chloride (AlCl3) was added to the mixture and allowed to stand in ambient temperature for 5 min. Finally, 1 mL of sodium hydroxide (NaOH) was added to the mixture, and the absorbance was measured at 510 nm. Catechin was used as standard.
Total sugars
Measurement of total sugars was conducted using the phenol sulfuric acid method described by Dubois et al. (1956) with slight modifications. The mixture was prepared by adding 0.5 mL of hydrolysate sample to 2.5 mL of concentrated sulfuric acid and to this mixture; 0.5 mL of (5%) phenol was added, and it was incubated in a 100 °C water bath for 30 min. The mixture was allowed to cool to room temperature, and the absorbance was measured at 490 nm. D-Glucose was used as a standard to produce a standard calibration curve, and each sample analysis was done in triplicates. All values in the results were expressed in milligram per 100 g (mg (100 g)−1).
Reducing sugar
Analysis for reducing sugar was performed using the 3,5-dinitrosalicyclic (DNS) acid method (Miller 1959) with minor variations. The DNS reagent solution was prepared by dissolving 1 g of sodium hydroxide (NaOH) in 100 mL distilled water. After the mixture was completely dissolved, 1 g of DNS was added followed by 200 mg of phenol and 50 mg of sodium sulfite (Na2SO3). Analysis for reducing sugar was conducted by adding 3 mL of DNS reagent to 3 mL of the sample. The mixture was then heated in a water bath at 90 °C for 10 min until it developed a red brown color. Finally, 1 mL of 40% potassium sodium tartrate (Rochelle salt) solution was added to stabilize the color and allowed to cool at room temperature. The absorbance was recorded at 575 nm, and each sample analysis was done in triplicates. D-Glucose was used as a standard to produce a standard curve, and all values in the results were expressed in milligram per 100 g (mg (100 g)−1).
Monosaccharide composition
The presence of glucose and galactose in the hydrolysate of two seaweed strains was quantified using HPLC fitted with a light scattering detector. The analysis process was conducted with a Jasco HPLC (Easton, USA) model 400 by means of a ChromNav analysis software, and high-purity nitrogen (99.9%) from KOSEM Co. was used as carrier gas (Saravana et al. 2016). For analysis of monosaccharides, a sugar column (SP0810) Shodex (Japan) 8.0 mm ID × 300 mL, thermocalibrated to 80 °C, was used. HPLC grade water for elution was filtered with cellulose acetate filter (0.22 μm), and a flow rate of 1.0 mL min−1 was maintained. The hydrolysates were diluted using HPLC grade water and filtered through 0.2-μm nylon filter before analysis by HPLC.
Maillard reaction product determination
The melanoids (Maillard reaction product (MRP)) were determined in terms of browning intensity of the sample extracts. Wavelengths of 360 and 420 nm were used to measure the browning intensity of the extracted samples immediately after filtration. The values were expressed as arbitrary absorbance units (A.U.).
Total protein
Analysis of total protein content was conducted using a slightly modified Lowry’s method described by McDermid and Stuercke (2003). Briefly, two reagents were prepared: Reagent 1 consists of 48 mL of 2% sodium carbonate in 0.1 N sodium hydroxide mixed with 1 mL of 1% sodium potassium tartrate in water and 1 mL 0.5% copper(II) sulfate pentahydrate [CuSO4·5H2O] in water), while reagent 2 consists of one part Folin phenol [2 N] and one part water. An aliquot of 0.2 mL sample containing protein was collected in a test tube and filled to 1 mL with deionized water. A total of 4.5 mL of reagent 1 was added to the mixture and incubated for 10 min. After incubation, 0.5 mL of reagent 2 was added to the mixture and incubated again for 30 min. The absorbance was measured at 660 nm, and bovine serum albumin (BSA) was used as standard to plot a standard curve.
Emulsification properties
Emulsification properties of K. alvarezii hydrolysates were determined according to a modified method of Cooper and Goldenberg (1987). Soybean oil was added to seaweed hydrolysate (1 g mL−1), ratio 3:2 (hydrocarbon/protein concentrate). The mixture was stirred vigorously for 2 min using a vortex at room temperature and left undisturbed for 5 to 20 min. Oil emulsion and aqueous layers were measured at different time intervals, and emulsifying index (EI) was calculated as follows:
Emulsifying index was noted with respect to time (5, 10, 15, 20 min) and represented accordingly.
Foaming properties
Foaming capacity and stability of K. alvarezii hydrolysates were conducted according to a modified method of Nath and Rao (1981). To determine the foaming capacity (FC), 20 mL of seaweed hydrolysate (protein concentrate) (1 g mL−1) was vortexed for 2–5 min to incorporate air at room temperature and transferred immediately to a 100-mL measuring cylinder. The volume increase was recorded within 30 s and expressed as percentage foam capacity.
where V 1 is the volume of protein solution before whipping and V 2 is the volume of protein solution after whipping.
Foaming stability (FS) was determined by measuring the decrease in volume of foam as a function of time from 10 to 30 min.
Statistical analysis
All statistical data for mean values were analyzed by one-way ANOVA. Values are expressed as mean ± standard deviation (SD; n = 3 replicates) (SPSS software; version 20 for Windows, IBM, USA). Statistical analysis was performed using the Tukey test with a significance level of P = 0.05.
Results and discussion
This study investigated the monosaccharide and antioxidant yields from two seaweed strains of the species K. alvarezii using different temperature and pressure conditions and three different solvents with the primary objective to identify the conditions and solvent that give the best yields.
Proximate composition
The proximate compositions of each strain of K. alvarezii are shown in Table 1. The brown strain was found to contain 67.08 ± 0.11% carbohydrate, 15.48 ± 0.23% ash, 11.33 ± 0.31% moisture, 5.64 ± 0.38% protein, and 0.45 ± 0.21% lipid, while the red strain contained 67.74 ± 0.55% carbohydrate, 14.51 ± 0.52% ash, 12.61 ± 0.46% moisture, 4.24 ± 0.27% protein, and 0.50 ± 0.25% lipid. These results were slightly different from a previous study by Abd-Rahim et al. (2014) who conducted proximate analysis on K. alvarezii species from the Sabahan coast in Malaysia. The previous study found a total of 59.58 ± 0.88% carbohydrate, 19.70 ± 0.09% ash, 14.23 ± 0.32% moisture, 5.74 ± 0.89% protein, and 0.75 ± 0.22% lipid. Actually, the components of seaweed can vary according to season, age of population, species, geographic, location, and temperature. The present study showed that carbohydrate which consisted mainly of polysaccharides was the highest composition in both seaweed strains.
Hydrolysis efficiency
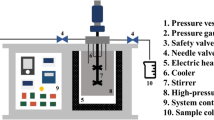
The conditions used in PHWE ranged from 150 °C/1 MPa to 300 °C/10 MPa between 30 and 85 min. Temperature and pressure conditions were monitored as shown in the schematic diagram (Fig. 1). The product obtained after reaching room temperature was normally a mixture of water and solids sorted in a matrix consisting of two layers due to precipitation of particles after extraction. The upper layer comprised of a less viscous aqueous solution with very low turbidity, while the lower layer was predominantly wet seaweed residue. The seaweed hydrolysate obtained was brownish in color when extracted with water and formic acid but appeared as dark brownish with sodium hydroxide. The aroma on the other hand was somehow toasty for lower temperature conditions with water and formic acid but turned pungent as thermal conditions increased further. This change in aroma with increasing temperature was also reported by Saravana et al. (2016) for hydrolysates of Saccharina japonica using PHWE. However, sodium hydroxide generally has a pungent aroma that grew stronger with increasing temperature and pressure conditions.
The highest hydrolysis efficiency for both seaweed strains ranged from 81.47 to 97.18% with formic acid, while the lowest ranged from 77.72 to 93.69% with sodium hydroxide (Fig. 2a, b). The capability of carboxylic acids such as formic acid to degrade the substrate and increase subcritical hydrolysis efficiency was reported by Lee and Jeffries (2011). In the present study, it is shown that hydrolysis efficiency for both seaweed strains increased consistently with increasing temperature. This was characterized by enhanced mass transfer rate, increase in solubility of the analytes, and decrease in solvent viscosity and surface tension that occur due to increasing temperature and pressure under subcritical conditions (Herrero et al. 2015). Previous work by Asaduzzaman and Chun (2014) with Scomber japonicus using the same PHWE apparatus reported similar results for hydrolysis efficiency. Thus, increasing temperature and pressure conditions facilitate deeper penetration of solvent into the sample matrix which enhanced greater surface contact and improved mass transfer to the solvent.
Hydrolysis efficiency and pH of each seaweed strain with three different solvents at temperature and pressure conditions ranging from 150 °C/10 bar to 300 °C/100 bar. a Hydrolysis efficiency of the brown strain. b Hydrolysis efficiency for the red strain. c pH of water, 1% HCOOH, and 1% NaOH for the brown strain. d pH of water, 1% HCOOH, and 1% NaOH for the red strain; n = 3, error bars are standard deviation
pH analysis
Measurements obtained for pH of K. alvarezii hydrolysates are shown in Fig. 2c, d. pH increased with increasing temperature with water and formic acid but decreased with sodium hydroxide. The low pH at lower temperatures was due to breakdown of sugars to organic acids followed by chain reactions of these acids creating the acidity that enhanced the rate of subsequent reactions as an autocatalyst process (Sasaki et al. 1998). However, the constant increase in pH with increasing temperature could be due to decomposition of acidic compounds to other substances. Moreover, it may also be attributed to the formation of salts and degradation of organic matter. Sodium hydroxide, on the other hand, showed a decrease in pH as temperature increased most probably due to the presence of acidic materials such as phenolic compounds (Pourali et al. 2010). In fact, pH influenced the decomposition rate of the sample; therefore, it is an important parameter for extraction of functional materials from their natural sources.
Antioxidant activity
Due to variation of oxidation processes, the use of one antioxidant method to evaluate antioxidant activity cannot clearly demonstrate the real antioxidant properties (Hifney et al. 2016); therefore, ABTS and DPPH were prepared to estimate antioxidant activities in K. alvarezii hydrolysates. According to Chew et al. (2011), ABTS+ and DPPH radical scavenging capacity was used to measure the antioxidant capacity, while TPC and TFC were used for the determination of phenolic and flavonoid compounds in the crude extracts, respectively. Phenolic acids and flavonoids are subgroups of polyphenols which are important antioxidants because their redox potentials can allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, and metal chelators (Tsao and Deng 2004). For both seaweed strains (Fig. 3a–d), antioxidant activity of K. alvarezii with ABTS+ and DPPH generally increased from 150 °C/1 MPa to around 240 °C/3 MPa but decreased slightly as temperature increased further. The moderate decrease after 240 °C/3 MPa was most probably due to decomposition of antioxidant compounds by elevated temperatures. Similar results were reported by Asaduzzaman and Chun (2014) for antioxidant analysis of freeze-dried and SC-CO2 residues of Scomber japonicus skin using the same assays. TPC and TFC (Fig. 4a–d) also increased from 150 °C/1 MPa to 240 °C/3 MPa and decreased as temperature increased further. However, the yields produced by formic acid were relatively higher than the other solvents maybe because most polyphenols are stable in acid. The increase in TPC and TFC yield with increasing temperature was reported previously by Wang et al. (2010) for extraction of antioxidant ingredients from the red alga Palmaria palmata using enzymatic extraction. In theory, most antioxidants in seaweed are polar compounds that dissolve easily in water following the principle for solvent extraction which states that solvents only dissolve substances with similar polarities (Meillisa et al. 2015). Hence, the increasing thermal conditions in the subcritical region enabled deeper penetration of water into the sample matrix which improved mass transfer and enhanced radical scavenging capacity of the hydrolysate.
Effect of increasing temperature and pressure conditions on radical scavenging capacity of the hydrolysates using ABTS+ and DPPH assays. The three solvents were used to investigate the impact of pH on antioxidant activity of K. alvarezii hydrolysates. a ABTS+ for the brown strain. b ABTS+ for the red strain. c DPPH for the brown strain. d DPPH for the red strain; n = 3, error bars are standard deviation
Effect of increasing temperature and pressure conditions on total phenolic and total flavonoid contents of K. alvarezii hydrolysates. The sample was extracted with different solvents to investigate the stability of phenolic compounds in acid and basic medium. a Total phenolic content for the brown strain. b Total phenolic content for the red strain. c Total flavonoid content for the brown strain. d Total flavonoid content for the red strain; n = 3, error bars are standard deviation
Comparing the two radical scavenging assays, ABTS+ showed higher antioxidant activity for the two seaweed strains than DPPH. Similar results were reported by Floegel et al. (2011) for comparability of antioxidant capacity measurements obtained by ABTS+ and DPPH assays from 50 most popular antioxidant-rich foods in the US diet. In the present study, ABTS+ showed slightly higher antioxidant capacity of 80.50 to 86.98% with the red strain than the brown strain which ranged from 77.53 to 86.68%. DPPH on the other hand also showed higher antioxidant capacity with the red strain ranging from 7.69 to 82.85% compared to the brown strain which ranged from 23.10 to 78.25% antioxidant activity. Regarding the polyphenolic content in the seaweed, both strains showed more than 30% TPC and TFC recovered with water and formic acid, but it was present in very low amounts with sodium hydroxide. Thus, this confirms that most polyphenols are more stable in acid than the basic medium.
Total sugar and reducing sugar
The highest yields for both total sugar and reducing sugar were recovered with water at condition 150 °C/1 MPa for the brown strain (Fig. 5a–d). All sugars were higher at milder conditions but decreased gradually as temperature and pressure increased. Generally, water produced higher yields than the other solvents except reducing sugar from the red strain. The latter was relatively higher with formic acid than with water, while sodium hydroxide showed quite lower yields for both strains most probably due to variation in depolymerization. Similar results were reported by Meillisa et al. (2015), for Saccharina japonica with 1% formic acid using subcritical water hydrolysis. However, the major setback of using acid catalyst in hydrolysis is the fact that acid solvents produce by-product compounds that inhibit cell growth and ethanol production from the hydrolysates (Meinita et al. 2012). Therefore, PHWE treatment without acid or base catalyst is a promising step towards bioethanol production.
Reducing sugar and total sugar of K. alvarezii hydrolysates extracted under different conditions. The trend showed the effect of increasing temperature on the content of sugar in the seaweed hydrolysates. a Reducing sugar for the brown strain. b Reducing sugar for the red strain. c Total sugar for the brown strain. d Total sugar for the red strain; n = 3, error bars are standard deviation
Monosaccharide composition
The monosaccharide yield from K. alvarezii hydrolysates generally showed a decrease as temperature and pressure conditions increased (Fig. 6a–d). The highest yields obtained were 156.21 and 155.49 mg L−1 from the red strain at condition 150 °C/1 MPa with 30-min extraction time for glucose and galactose, respectively. The concentration of glucose in water was higher than the other two solvents in all conditions. Galactose was present in both strains only at conditions 150 °C/1 MPa to 240 °C/3 MPa in water and formic acid, but it was not found in sodium hydroxide. Glucose on the other hand was present in all conditions with all three solvents, but the concentration was low in sodium hydroxide. Lechat et al. (1997) determined the monosaccharide composition in the cell wall of K. alvarezii, and galactose and glucose were identified as the major monosaccharides. The other monosaccharides identified were mannose, xylose, and arabinose, but they were present in very minimal amounts. Similarly, in the current study, these minor monosaccharides were identified only as trace amounts when analyzed on HPLC.
Effects of increasing temperature and pressure conditions on monosaccharide composition of K. alvarezii hydrolysates. a Composition of galactose in the brown strain. b Composition of galactose in the red strain. c Composition of glucose in the brown strain. d Composition of glucose in the red strain; n = 3, error bars are standard deviation
Maillard reaction product determination
MRP is basically the development of the brown color in different intensities that can be used to observe non-enzymatic reactions, including the Maillard reaction. The Maillard reaction involves reactions of amines, amino acids, peptides, and proteins with reducing sugars and vitamin C, known as caramelization (Bastos et al. 2012). The possible end products of this reaction are melanoidins which are long polymeric compounds that act as brown pigments giving cooked food its brown color. The enhanced intensity of the brown color is a direct result of advanced phases of the reaction. In fact, MRPs are very important in food processing since they greatly influence essential food quality attributes such as flavor, aroma, color, and texture. MRPs are usually observed at absorbance 360 and 420 nm to determine the development of the browning reaction in the products. Hence, according to the measurements at 360 and 420 nm (Tables 2 and 3), the red macroalgae K. alvarezii hydrolysates possess reasonable amounts of MRPs. Generally, the highest MRP content was at 300 °C/10 MPa (4.00 A.U. at 360 nm, 3.612 A.U. at 420) with formic acid for the red strain, while the lowest was at 150 °C/1 MPa (3.64 A.U. at 360 nm, 1.51 A.U. at 420 nm) with water also for the red strain. This indicated that the red strain produced a broader range of MRP compared to the brown strain. The results highlighted above revealed evidence of caramelization in the K. alvarezii hydrolysates with absorbance values that increased consistently with increasing temperature and pressure conditions. Similar results of increasing absorbance with increasing temperature were reported by Plaza et al. (2010) and Saravana et al. (2016). MRPs occur in a wide range of compounds including some which have health benefits and others which are toxic and harmful to the human body.
Total protein
Total protein for both strains increased from 150 °C/1 MPa to 270 °C/8 MPa but decreased slightly as temperature increased further (Fig. 7a, b). This was in agreement with Watchararuji et al. (2008) who reported that protein increased with increasing temperature up to 220 °C for rice bran by subcritical water hydrolysis. Generally, the increase in protein with increasing temperature in PHWE is due to a change in polarity of water in the subcritical region. According to Thiruvenkadam et al. (2015), the increase in dielectric constant and the decrease in density (1 g cm−3 at 25 °C to 0.75 g cm−3 at 300 °C) compared to ambient conditions consequently enable hydrocarbons to become more water soluble. This is characterized by breakdown of hydrogen bonding in the water molecules which changes the polarity of water in the subcritical region from complete polarity to moderately non-polar. This condition tends to enhance the attraction of water towards non-polar hydrocarbons thus increases miscibility and enhances hydrocarbon solubility in water. However, the decrease of proteins after 270 °C/8 MPa was most probably due to denaturing as a result of exceedingly high temperatures. Actually, proteins can be denatured by heat when their resistance to thermal denaturation capacity is exceeded (Haque et al. 2016). Thus, this study showed that the best condition for utilizing proteins from K. alvarezii by PHWE is around 270 °C/8 MPa.
Relationship of total protein content with emulsifying properties of K. alvarezii hydrolysates. a Total protein in the brown strain. b Total protein in the red strain. c Emulsifying properties for the brown strain. d Emulsifying properties for the red strain; n = 3, error bars are standard deviation
Emulsifying properties
Emulsifying properties are important and useful in the food industry for production and storage. Based on the graphs in (Fig. 7c, d), conditions 300 °C/10 MPa with water and 270 °C/8 MPa with formic acid showed high emulsifying properties for the brown and red strains, respectively. Emulsifying index (EI) was measured with relation to time from 5 to 20 min in soybean oil, and the trend showed a moderate increase with increasing temperature and pressure conditions for both strains. The increase of emulsifying index is most probably due to the increase in protein with elevated temperatures. This modest increase was described by Lam and Nickerson (2013) as the result of hydrolysis that increases protein solubility by revealing hidden hydrophobic groups which increase the surface hydrophobicity and reduce its molecular weight allowing for better adherence to the oil–water interface. Technically, emulsifying index is the parameter used to characterize the biosurfactant in emulsification which is normally the hydrophobic phase in hydrophilic phase. According to Nishinari et al. (2014), emulsifiers must have both hydrophobic and hydrophilic groups to interact with oil and water; hence, the higher emulsion index indicates a higher stability of the emulsion.
Foaming properties
Foam production involves the generation of a protein film surrounding a gas bubble and the packing of gas bubbles into an overall structure (Foegeding et al. 2006). The FC and FS of K. alvarezii with water, formic acid, and sodium hydroxide are shown in Fig. 8a–d. Generally, both FC and FS decreased with increasing temperatures when using water and formic acid but showed very less to no activity with sodium hydroxide. The steady decrease in foaming properties maybe possible due to protein denaturation at the air/water interface as reported by Mutilangi et al. (1996); however, since there was no indication of protein denaturation until condition 270 °C/8 MPa, the decrease is most probably due to dwindling sugars with increasing temperature as indicated in all the graphs of Figs. 5 and 6. Actually, foam formation does not only depend on aerated protein/water interactions, but it is also initiated from electrostatic combinations of protein and polysaccharides at favorable conditions. Chiu et al. (2009) in a work on comparing emulsifying properties of Maillard reactions by green and red seaweeds reported that protein–polysaccharide complexes caused positive effects on forming property. Wang et al. (2015) also indicated that the interactions between protein and polysaccharide are mostly instigated from electrostatic interactions between the oppositely charged regions of the biopolymers and positively charged proteins which can strongly bind to anionic polysaccharides resulting in the formation of both soluble and insoluble complexes under pH lower than the isoelectric point (pI) of the protein. However, increased foaming properties formed by protein and polysaccharide-soluble complexes at pH higher than pI was also reported. As a matter of fact, foam formation is an important quality attribute that can provide a range of unique textures with many foods like cake, bread, ice cream, and confectionary products (Campbell and Mougeot 1999); thus, the results in the present study showed that 150 °C/1 MPa is the best condition for foam formation.
Effects of increasing temperature and pressure conditions on foaming properties of K. alvarezii hydrolysates. a Foaming capacity for the brown strain. b Foaming capacity for the red strain. c Foaming stability for the brown strain. d Foaming stability for the red strain; n = 3, error bars are standard deviation
Conclusion
In this study, PHWE of K. alvarezii influenced the hydrolysis efficiency, pH, radical scavenging activity, TPC, TFC, total sugar, reducing sugar, monosaccharide composition, MRP, total protein, emulsifying properties, and foaming properties. Pressurized hot water at elevated temperatures increased hydrolysis efficiency with all three solvents for both strains. The best condition for antioxidant, TPC, TFC, and total protein content was 270 °C/8 MPa. These were reduced at 300 °C/10 MPa due to decomposition and protein denaturation. Furthermore, the maximum yields for total sugar, reducing sugar, and monosaccharides were recovered at 150 °C/1 MPa for both seaweed strains, and all sugars were decomposed by increasing temperature. Foaming and emulsifying properties on the other hand were determined by variation of protein with relation to polysaccharide content, pH, and temperature. The highest foaming properties were recovered at 150 °C/1 MPa, while the best conditions for emulsifying activities were at 270 °C/8 MPa with water and 300 °C/10 MPa with formic acid for the brown and red strain, respectively. pH of hydrolysates increases with increasing temperature for water and 1% formic acid but decreases with 1% sodium hydroxide. Generally, the present study showed that bioactive compounds in the hydrolysates increased with increasing temperature and the red strain produced the highest yields of protein and sugars in water and formic acid solvents. Thus, with the high yields of monosaccharides, MRP, foaming properties, and emulsifying properties, the K. alvarezii species from Solomon Islands has great potential to be utilized as a stabilizer, thickener, and emulsifier for production of high-quality edible products in the food industry.
References
Abd-Rahim F, Wasoh H, Zakaria MR, Ariff A, Kapri R, Ramli N, Siew-Ling L (2014) Production of high yield sugars from Kappaphycus alvarezii using combined methods of chemical and enzymatic hydrolysis. Food Hydrocolloid 42:309–315
AOAC (1997) Association of official analytical chemists. Official methods of the analysis of the association of official analytical chemists, 16th edn. AOAC, Washington
Asaduzzaman A, Chun B-S (2014) Hydrolyzates produced from mackerel Scomber japonicus skin by the pressurized hydrothermal process contain amino acids with antioxidant activities and functionalities. Fisheries Sci 80:369–380
Bastos DM, Monaro É, Siguemoto É, Séfora M (2012) Maillard reaction products in processed food: Pros and cons. In: Valdez B (ed) Food industrial processes – methods and equipment. InTech doi:10.5772/31925
Campbell GM, Mougeot E (1999) Creation and characterisation of aerated food products. Trends Food Sci Tech 10:283–296
Chew K, Khoo M, Ng S, Thoo Y, Wan Aida W, Ho C (2011) Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Food Res Int 18:1427–1435
Chiu T-H, Chen M-L, Chang H-C (2009) Comparisons of emulsifying properties of Maillard reaction products conjugated by green, red seaweeds and various commercial proteins. Food Hydrocoll 23:2270–2277
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microb 53:224–229
Dong X, Zhao M, Shi J, Yang B, Li J, Luo D, Jiang G, Jiang Y (2011) Effects of combined high-pressure homogenization and enzymatic treatment on extraction yield, hydrolysis and function properties of peanut proteins. Appl Environ Microb 12:478–483
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
FAO (2014) World review of fisheries and aquaculture. State of World Fisheries and Aquaculture: opportunities and challenges. Rome, Food and Agriculture of the United Nations Part 1:3–93
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24:1043–1048
Foegeding EA, Luck P, Davis J (2006) Factors determining the physical properties of protein foams. Food Hydrocoll 20:284–292
Haque MA, Timilsena BYP, Adhikari B (2016) Food proteins, structure, and function. In: Reference Module in Food Science. Elsevier. http://dx.doi.org/10.1016/B978-0-08-100596-5.03057-2.
Herrero M, del Pilar S-CA, Cifuentes A, Ibáñez E (2015) Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trend Anal Chem 71:26–38
Hifney AF, Fawzy MA, Abdel-Gawad KM, Gomaa M (2016) Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll 54:77–88
Hurtado AQ, Gerung GS, Yasir S, Critchley AT (2014) Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol 26:707–718
Jazrawi C, Biller P, Ross AB, Montoya A, Maschmeyer T, Haynes BS (2013) Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res 2:268–277
Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H (2008) Antioxidant activity of some common plants. Turk J Biol 32:51–55
Kostas ET, White DA, Du C, Cook DJ (2016) Selection of yeast strains for bioethanol production from UK seaweeds. J Appl Phycol 28:1427–1441
Kumar KS, Ganesan K, Rao PS (2008) Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty—an edible seaweed. Food Chem 107:289–295
Lam RS, Nickerson MT (2013) Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem 141:975–984
Lechat H, Amat M, Mazoyer J, Gallant DJ, Buléon A, Lahaye M (1997) Cell wall composition of the carrageenophyte Kappaphycus alvarezii (Gigartinales, Rhodophyta) partitioned by wet sieving. J Appl Phycol 9:565–572
Lee J-W, Jeffries TW (2011) Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresource Technol 102:5884–5890
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
Meillisa A, Woo H-C, Chun B-S (2015) Production of monosaccharides and bio-active compounds derived from marine polysaccharides using subcritical water hydrolysis. Food Chem 171:70–77
Meinita MDN, Hong Y-K, Jeong G-T (2012) Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioproc Biosyst Eng 35:93–98
Michalak I, Chojnacka K (2014) Algal extracts: technology and advances. Eng Life Sci 14:581–591
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Msuya FE, Buriyo A, Omar I, Pascal B, Narrain K, Ravina JJM, Mrabu E, Wakibia JG (2014) Cultivation and utilisation of red seaweeds in the Western Indian Ocean (WIO) region. J Appl Phycol 26:699–705
Munoz J, Freile-Pelegrín Y, Robledo D (2004) Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán, México. Aquaculture 239:161–177
Mutilangi W, Panyam D, Kilara A (1996) Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci 61:270–275
Nath J, Rao MN (1981) Functional properties of guar proteins. Journal Food Sci 46:1255–1259
Nishinari K, Fang Y, Guo S, Phillips G (2014) Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocoll 39:301–318
Ozsoy N, Can A, Yanardag R, Akev N (2008) Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem 110:571–583
Plaza M, Amigo-Benavent M, Del Castillo MD, Ibáñez E, Herrero M (2010) Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int 43:2341–2348
Pourali O, Asghari FS, Yoshida H (2010) Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem Eng J 160:259–266
Sachindra N, Airanthi M, Hosokawa M, Miyashita K (2010) Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Tech 47:94–99
Saravana PS, Choi JH, Park YB, Woo HC, Chun BS (2016) Evaluation of the chemical composition of brown seaweed (Saccharina japonica) hydrolysate by pressurized hot water extraction. Algal Res 13:246–254
Sasaki M, Kabyemela B, Malaluan R, Hirose S, Takeda N, Adschiri T, Arai K (1998) Cellulose hydrolysis in subcritical and supercritical water. J Supercrit Fluid 13:261–268
Tan J, Lim P-E, Phang S-M (2013) Phylogenetic relationship of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Malaysia. J Appl Phycol 25:13–29
Thiruvenkadam S, Izhar S, Yoshida H, Danquah MK, Harun R (2015) Process application of subcritical water extraction (SWE) for algal bio-products and biofuels production. Appl Energ 154:815–828
Tsao R, Deng Z (2004) Separation procedures for naturally occurring antioxidant phytochemicals. J Chromatogr B 812:85–99
Wang T, Jónsdóttir R, Kristinsson HG, Hreggvidsson GO, Jónsson JÓ, Thorkelsson G, Ólafsdóttir G (2010) Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. Lwt-Food Sci Technol 43:1387–1393
Wang T, Jonsdottir R, Ólafsdóttir G (2009) Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–248
Wang Z, Zhang S, Vardhanabhuti B (2015) Foaming properties of whey protein isolate and λ-carrageenan mixed systems. J Food Sci 80:N1893–N1902
Watchararuji K, Goto M, Sasaki M, Shotipruk A (2008) Value-added subcritical water hydrolysate from rice bran and soybean meal. Bioresource Technol 99:6207–6213
Acknowledgements
This work was financially supported by Korea International Cooperation Agency through a project implemented by Overseas Fisheries Cooperation Center in collaboration with Pukyong National University to support the Department of Fisheries in Solomon Islands National University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gereniu, C.R., Saravana, P., Getachew, A. et al. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J Appl Phycol 29, 1609–1621 (2017). https://doi.org/10.1007/s10811-017-1052-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1052-3