Abstract
Polycyclic aromatic hydrocarbons (PAHs) have high risks for human and living organisms due to their mutagenic and carcinogenic properties. Here, the effect of different concentrations of fluorene as a persistent toxic PAH on growth parameters and antioxidant systems in the green microalga Chlorella vulgaris was investigated. Intriguingly, cell number as well as dry and fresh weight of the alga were raised at 2 ppm fluorene compared to the control sample. However, with the increasing levels of fluorene from 10 to 50 mg L−1, the growth parameters gradually decreased. Accordingly, cells of C. vulgaris were found to enhance the activity of ROS scavenging enzymes after 7 days of exposure to fluorene in a concentration-dependent manner. Exposure to 25 and 50 mg L−1 fluorene was led to a significant decrease at chlorophyll content, whereas the concentration of carotenoids was not changed. Total phenol and flavonoid contents were markedly raised in 50 mg L−1 of fluorene compared to the control. Although flow cytometry assessment showed no substantial reduction in the viability at 50 mg L−1 fluorene-treated samples for 24 h, chlorophyll fluorescence was noticeably reduced. The results of SEM analysis revealed that the 50 mg L−1 fluorene treatment clearly damaged the algal cells after 24 h. The ability of the alga for biodegradation of fluorene was assessed by GC-MS. Consequently, a number of produced intermediate compounds were identified. These findings displayed that C. vulgaris had not only notable resistance against fluorene but also noteworthy potential for its degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) represent a group of aromatic compounds consisting of two or more fused benzene rings (Kirso and Irha 1998). The distribution of PAHs as a class of ubiquitous, toxic, and persistent environmental pollutants in atmosphere, freshwater, and marine sediments is rapidly increasing as a result of wide-ranging industrial and anthropogenic activities (Kirso and Irha 1998; Hong et al. 2008; Cheema et al. 2010). PAHs in contaminated water ecosystems have serious effects on the growth and development of aquatic organisms and also threat human health indirectly via food consumption (Kim et al. 2013; Wei et al. 2014). Toxicity of PAHs is highly variable depending on the type, concentration, exposure time, environmental conditions, and sensitivity of species (Semple et al. 1999; Lei et al. 2006, 2007; Patel et al. 2016; Subashchandrabose et al. 2017).
Fluorene is the representative of 3-ring and low molecular weight PAHs, showing high concentration in riverine and freshwater (Casellas et al. 1997; Salehi-Lisar and Deljoo 2015). Thus, fluorene have received increasing consideration for the environmental recovery in the contaminated aquatic ecosystems. There are a number of treatment approaches including physical, chemical, and biological techniques for removal of PAH contaminants from the environment. Among various procedures, biological methods are considered as eco-friendly, low-cost, and effective ways for the cleanup of pollutants (Rawat et al. 2011; Vafaei et al. 2013).
In the last decades the application of micro- or macroalgae for removal of pollutants from environment (phycoremediation) has attracted growing attention (Rawat et al. 2011; Kalhor et al. 2016). Microalgae, as the major primary producers in aquatic ecosystems have a vital role in removing toxic metals and organic pollutants (Wang et al. 2008; Qian et al. 2011; Rai et al. 2013; Suman et al. 2015). Furthermore, microalgae are widely used as indicators for toxicity assays in contaminated sites because of their sensitivity to a broad range of pollutants (Çelekli et al. 2016; Shen et al. 2016). So far, some capable microalgal species have been identified for uptake and degradation of PAHs as hazardous organic pollutants (Kirso and Irha 1998; Hong et al. 2008; Shen et al. 2016; Asghari et al. 2018). Chlorella vulgaris is a single-celled green freshwater microalga with notable ability for adaptation to various environmental conditions (Safi et al. 2014; Cheng et al. 2016). The current study aimed to investigate the potential of C. vulgaris for remediation of fluorene under experimental condition. The changes in some physiological parameters of the microalga such as photosynthetic pigment content as well as enzymatic and non-enzymatic antioxidant functions along with total phenol and flavonoid contents were evaluated. Finally, some intermediate compounds resulting from the biodegradation of fluorene by the microalga were identified using GC-MS. The obtained results of the present work shed more light on the cleanup process of PAHs in wastewater by algal species.
Materials and methods
Preparing microalga cultures
The green microalga Chlorella vulgaris was obtained from the Culture Collection of Algae of Bushehr Shrimp Research Institute, Iran. The algal cells were cultured under sterile conditions in 1-L Erlenmeyer flasks under illumination of daylight fluorescent lamps at a photon flux density of 80 μmol photons m−2 s−1 with a 12:12-h (light/dark) photoperiod at a temperature of 25 ± 2 °C. The stock of algal cultures was aerated continuously by means of a mechanical pump. BG11 medium was prepared according to Stanier et al. (1971). Algal samples at the logarithmic phase were harvested and utilized for the experiments.
Experimental setup
To perform experiments, fifteen 250-mL Erlenmeyer flasks, each containing 100 mL BG11 medium, were sterilized. Before the algal inoculation, the proper amounts of fluorene (using a 1000 mg L−1 stock solution in acetone) were spiked into Erlenmeyer flasks to obtain the desired concentrations of fluorene (2, 10, 25, and 50 mg L−1) in the culture media. A BG11 control medium was also made without fluorene. The media were inoculated with exponentially growing algae after complete evaporation of acetone on a rotary evaporator at 120 rpm for 48 h at 25 °C. Then, the algal cells were exposed to different assessment test suspensions in exponential growth phase. After 7 days of culture, the algal cells were centrifuged at 2500×g for 10 min at 4 °C, washed in sterile deionized water, and used for different assays under the same laboratory conditions.
Determination of growth parameters
To measure growth parameters of C. vulgaris, fresh weight, dry weight, optical density, and cellular density were examined. Algal growth was assessed by measuring the optical density at 600 nm every 24 h. Cell counting was performed using a hemocytometer during the logarithmic growth phase, and a clear linear correlation was presumed between cellular density and optical density (OD600). The regression equation between OD600 (x) and cellular density (y × 106 mL−1) was calculated on the basis of standard curve as y = 42.025x − 1.6709 (R2 = 0.997). Accordingly, the cell number of algae was recorded in the culture media after 0, 24, 48, 72, 96, 120, 144, and 168 h of exposure to fluorene.
Algal cells were harvested after 7 days of cultivation by centrifugation at 2500×g for 10 min at 4 °C and then were washed with sterile distilled water. Subsequently, fresh weight of the samples was measured. Afterward, dry weight of the samples was determined after drying the samples in the oven at 37 °C for 24 h.
Enzyme analysis
The harvested cells were frozen in liquid nitrogen and were homogenized in a prechilled mortar and pestle with 50 mM potassium phosphate buffer (pH 7.0). The homogenate was centrifuged at 10,000×g for 10 min at 4 °C. The resulting supernatant was immediately used for measurement of the protein content (Bradford 1976) as well as determination of the antioxidant enzyme activities including superoxide dismutase (Winterbourn et al. 1976), peroxidase (APX) (Nakano and Asada 1981), and catalase (CAT) (Maehly and Chance 1955).
Analysis of photosynthetic pigments
Assessing the photosynthetic pigments including chlorophyll a, b and total carotenoids was done based on the method described by Lichtenthaler (1987). The algal cells were homogenized in 100% methanol at 4 °C for 24 h in the dark. The homogenate was centrifuged for 10 min at 10,000×g. Subsequently, quantitative determination of the pigments was by the means of a UV/V spectrophotometer at 470, 665, and 653 nm.
Analysis of total phenol and flavonoid contents
Total phenol and flavonoid contents of the algal cells were extracted with 100% methanol. The total phenol content of the algal extracts was quantified using Folin-Ciocalteu procedure according to the method described by Meda et al. (2005). Accordingly, 100 μL of algal extract was mixed with 2.8 mL of deionized water, 100 μL of Folin-Ciocalteu reagent and 2 mL of 2% sodium carbonate aqueous solution. The samples were incubated for 30 min at room temperature in the dark. Finally, the absorbance was determined at 720 nm. Gallic acid was used for the preparation of calibration curve and total phenol content of the extracts was expressed as mg g−1 FW.
For assessing total flavonoid content, a reaction mixture was prepared using 500 μL of the algal extract, 1.5 mL of 100% methanol, 100 μL of 10% aluminum chloride solution, 100 μL of 1 M potassium acetate, and 2.8 mL of distilled water. The mixture was incubated at 25 °C for 40 min, and then, the absorbance of the solutions was measured at 415 nm. The total flavonoid content of the extracts was reported as mg g−1 FW with reference to quercetin equivalent (Chang et al. 2002).
Microscopic observations
The morphological features of algal cells were characterized by a scanning electron microscope (SEM, MIRA3 FEG-SEM). After 24 h of exposure to 50 mg L−1 fluorene, treated and untreated cells were harvested by centrifugation at 2500×g for 10 min at 4 °C. The cell pellets were washed three times with BG11 and then were freeze-dried for 4 h. After subjecting to gold sputtering, the morphology of the cells was analyzed.
Flow cytometric analysis
Flow cytometry (fluorescence-activated cell sorting (FACS)) assay was done to evaluate the effect of fluorene on cell viability of C. vulgaris treated with 50 mg L−1 fluorene for 24 h. Approximately 1.0 × 106 cells were centrifuged at 2500×g for 10 min at 4 °C. The supernatant was discarded, and the cell pellet was washed three times with phosphate buffer solution (PBS, pH 7.0). Afterwards, the samples were exposed to 5 μL propidium iodide (PI) for 30 min in the dark. Finally, the samples were transferred to FACS tubes and immediately analyzed. The fluorescent emission of PI was collected from ~ 10,000 events per cell sample in FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, USA) FL2 channel and chlorophyll fluorescence was obtained in FL3 channel.
GC-MS analysis
GC-MS analysis was performed to identify the possible intermediate compounds resulting from the biodegradation of fluorene by C. vulgaris. About 3.0 × 107 cells were exposed to 100 mL BG11 medium containing 10 mg L−1 of fluorene. BG11 media with and without fluorene were used as control samples for GC-MS analysis. After 7 days, microalgae were separated from the culture medium by centrifugation at 2500×g for 10 min at 4 °C. The organic compounds were extracted from the medium using 25 mL of diethyl ether for three times. The separated organic phase was evaporated and the remaining material was dissolved in 100 μL of absolute methanol and was then analyzed by GC–MS. The analysis of samples was carried out by means of an Agilent 6890 GC system and Gas Chromatograph interfaced to a Mass Spectrometer (GC-MS) equipped with a Shimadzu GC-MS-QP 5050A gas chromatograph fitted with a DB-1 (polydimethylsiloxane, 60 m × 0.25 mm i.d.) capillary column. Carrier gas was helium with a column flow rate of 0.9 mL min−1. The oven temperature was programmed from 50 °C (hold time 5 min) to 290 °C with an increase of 7 °C min−1 and held at 290 °C for 10 min. The injector and detector temperatures were set at 260 °C and 290 °C, respectively. A volume of 1 μL was injected onto the capillary column in the split mode. Mass spectra were acquired at an electron energy of 70 eV, a rate of 1.6 scans s−1 and a mass/charge range of 30–650 m/z.
Statistical analysis
Data with three replications were analyzed by one-way analysis of variance (ANOVA) with Duncan multiple comparison tests using SPSS 21 software. Values were considered statistically significant at P < 0.05.
Results and discussion
Effect of fluorene on growth of C. vulgaris
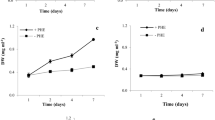
The influences of different concentrations of fluorene on cell density, fresh weight, and dry weight of C. vulgaris is shown in Figs. 1 and 2. Interestingly, the number of viable cells was increased after treatment with 2 mg L−1 fluorene in comparison to control medium from the second day of growth. Based on the Duncan’s post hoc analysis, the increasing cell number was significant from the fourth day of growth (Fig. 1). Furthermore, an increase of 7 and 13% was noticed in the dry weight and fresh weight of the algae, respectively, after 7 days of exposure to 2 mg L−1 of fluorene (P < 0.05) (Fig. 2). The growth of microalgae was significantly inhibited in the treated samples with 25 and 50 mg L−1 concentrations of fluorene compared to the control sample (Figs. 1 and 2). The cells did not divide in 72 h in the media containing 25 and 50 mg L−1 of fluorene, but an increase in the number of viable cells was significant from the fourth day (Fig. 1). In addition, dry weight and fresh weight of microalgae were noticeably diminished in both 25 and 50 mg L−1 concentrations.
It could be concluded that the optimal growth of C. vulgaris was due to the ability of the cells for consumption of fluorene as a carbon source at low concentrations (El-Sheekh et al. 2013; Kalhor et al. 2017). An increase has been formerly reported in the cellular density and dry mass of C. vulgaris exposed to 10 and 20 g L−1 concentrations of crude oil (Kalhor et al. 2016). The inhibitory effects of the higher concentrations of PAHs on the growth of Chlorella and some other algal species could be associated with the cell toxicity (Soto et al. 1975; Karydis 1981; Munoz et al. 2003; Subashchandrabose et al. 2017).
Enzymatic antioxidant system response to fluorene
The activities of APX, CAT, and SOD were assessed in C. vulgaris after 7 days of treatment with different concentrations of fluorene (0, 2, 10, 25, and 50 mg L−1). APX is an essential enzyme of the antioxidant defensive system which scavenges the toxic levels of H2O2 in photosynthetic organisms (Jaleel et al. 2009). Exposure of the algal cells to fluorene caused a significant increase in the activity of APX just in high concentrations (25 and 50 mg L−1) (Fig. 3a) (P < 0.05). This response of APX is similar to the report of Rai et al. (2013) showing increasing activity of APX in C. vulgaris with the elevating concentration of a heavy metal (chromium).
CAT is another important enzyme for the detoxification of reactive oxygen species (ROS) that quenches hydrogen peroxide to water and oxygen (Cheng et al. 2016). The activity of CAT was peaked in the media containing 25 and 50 mg L−1 fluorene after 7 days of exposure (Fig. 3b). Increment in the activity of CAT in C. vulgaris and some other microalgae has been reported under environmental stressful conditions (Lei et al. 2006; Qian et al. 2012; Sáenz et al. 2012). However, APX and CAT activities were not significantly changed at low concentrations of fluorene.
SOD has been proposed to modulate the amount of superoxide radicals by their converting to oxygen and H2O2 (Jaleel et al. 2009). SOD activity was considerably increased with the rising concentrations of fluorene from 10 to 50 mg L−1, while notable difference was not observed in the samples treated with 2 mg L−1 of fluorene (P < 0.05) (Fig. 3c). Environmental pollutants such as heavy metals and organic compounds may lead to the formation of ROS in microalgae which can result in oxidative damage in cell structures. Under such stress condition, microalgae would require efficient defense mechanisms to overcome oxidative stress and ROS detoxification (Cheng et al. 2016; Nazari et al. 2018; Fazelian et al. 2019). According to our findings, the assayed antioxidant enzymes were in maximum level of activity in presence of 25 and 50 mg L−1 of fluorene, while the low concentrations of fluorene showed no significant effect on their activity. Actually, the stimulation of APX activity along with CAT and SOD can be regarded as a protective response against oxidative damage of fluorene in C. vulgaris.
The content of chlorophylls and carotenoids
After 7 days of exposure of C. vulgaris to various concentrations of fluorene, the content of the photosynthetic pigments was determined. The amounts of chlorophyll a, b and total chlorophyll declined in the cells treated with 25 and 50 mg L−1 of fluorene compared to the control sample (P < 0.05). A decrease in the content of chlorophyll a was measured up to 48 and 67% at 25 and 50 mg L−1 concentrations treatments, respectively. Additionally, a decrease was recorded for the treatments with 25 and 50 mg L−1 by 45 and 64% in the content of chlorophyll b, respectively (Fig. 4). However, different concentrations of fluorene showed no significant effect on the total carotenoids quantity (Fig. 4). Reduction in the quantity of chlorophyll a, b and total chlorophyll has been reported after exposure of C. vulgaris to different concentrations of some heavy metals (chromium and cadmium) (Rai et al. 2013; Cheng et al. 2016). The amount of the photosynthetic pigments in plants is considered as one of the connected factors to oxidative stress (Vafaei et al. 2013). The reduction in chlorophyll content in high concentrations of fluorene was possibly the consequence of a decrease in microalgal biomass and/or high ROS levels which in turn reduced carbon fixation. This phenomenon could be a protective response to prevent the accumulation of ROS by-product in chloroplasts (Liu et al. 2009; Tarrahi et al. 2017). Additionally, the decrease in the amounts of chlorophyll observed at the high concentrations of fluorene was probably due to the decreasing number of algal cells with respect to the control sample.
Total phenol and flavonoid contents
The amount of phenols and flavonoids was not significantly affected at 2–25 mg L−1 of fluorene after 7 days of exposure. They increased in the cells treated with 50 mg L−1 of fluorene and reached to their highest quantities (P < 0.05) (Fig. 5).
Plants have a non-enzymatic defense system, besides the antioxidant enzymes, against environmental stresses, which mainly consists of phenols (Bautista et al. 2016; Mahjouri et al. 2018b). Phenolic compounds as a large group of secondary metabolites play a key role in scavenging free radicals. It has been proposed that peroxidases could detoxify H2O2 in the presence of phyto-phenolics, which lead to the formation of phenoxyl radicals. These radicals can be reduced by ascorbate (Mallick 2004; Mahjouri et al. 2018a). Significant enhancement in total phenol and flavonoid amounts in C. vulgaris after exposure to 50 mg L−1 fluorene could be a protection response against the harmful effects of the free radicals. These results are in agreement with the reports showing accumulation of phenols and flavonoids in response to oxidative stress in different algal species (Comotto et al. 2014; Çelekli et al. 2016; Fazelian et al. 2019).
Flow cytometric analysis
One of the main approaches in toxicity analyses is the estimation of cell viability indicating cellular reaction to a toxicant (Mahjouri et al. 2018b). Therefore, the viability of the algal cells was analyzed by flow cytometry after 24-h exposure to 50 mg L−1 fluorene using red fluorescent nucleic acid dye PI. Undamaged membranes of living cells are impermeable to PI, while PI can enter into the cells by the death of cells and as the consequence of a loss in the cell membrane integrity. PI intercalates with double-stranded DNA molecules and emits red fluorescence (Suman et al. 2015). In flow cytometry graphs, upper left and right quadrants demonstrate the percentage of dead cells and lower left and right quadrants represent the percentage of live cells and lower right quadrant indicates chlorophyll fluorescence (Fig. 6). The viability of the control sample was almost 100% and all of the cells displayed chlorophyll fluorescence (Fig. 6a). Approximately 5.5% decline in cell viability was observed when cells were exposed to 50 mg L−1 of fluorene and 36.91% of the cells did not show chlorophyll fluorescence (Fig. 6b). Therefore, it can be concluded that 50 mg L−1 of fluorene had a low effect on cell viability while the amount of chlorophyll fluorescence was significantly influenced. Hitherto, significant reduction in the cell viability of C. vulgaris treated with toxic nanoparticles was identified by flow cytometry (Suman et al. 2015; Nazari et al. 2018).
Microscopic analysis
The cells of C. vulgaris were examined under SEM for analyzing their surface morphology. The SEM observations displayed that 24-h exposure to 50 mg L−1 of fluorene caused noticeable changes in the cell structure, whereas cell surfaces remained intact in the control sample (Fig. 7). In agreement with our results, the atypical morphological symptoms were revealed in various microalgae treated with different hazardous nanoparticles and PAHs (Suman et al. 2015; Asghari et al. 2018; Nazari et al. 2018; Fazelian et al. 2019). These findings confirmed that fluorene, as a PAH, possess a significant influence on the cell structure.
Analysis of biodegradation activity of the algae
GC-MS analysis showed some intermediate products during biodegradation of fluorene by C. vulgaris after 7 days. The constituents were identified by matching their spectra with those recorded in the Mass library (WILEY229 and NIST21&107). Based on the GC–MS analysis, four compounds were detected (Table 1). Accordingly, a possible degradation pathway of fluorene was proposed (Fig. 8). The degradation of PAHs in algal cells is mostly carried out by dioxygenase enzyme system (Warshawsky et al. 1995; Haritash and Kaushik 2009; Patel et al. 2016). As shown in Fig. 8, one of the primary steps in the fluorene biodegradation assumed to be the formation of N-C band in the middle ring which yielded the N-hydroxymethylcarbazol compound. Possibly, primary oxidation of polycyclic compounds by oxidizing enzymes led to opening the aromatic rings. The next step was probably the addition of nitrogen atom in reduced form into the aromatic structure under influence of culture medium constitutes. Afterward, dibutyl phthalate and 1,2-benzenedicarboxylic acid, dioctyl ester were created via oxidation as two other by-products probably by ring-cleaving dioxygenase system. It has been reported that these compounds act in the degradation pathways of benzopyrene by Arthrobacter oxydans (B4) (Takáčová et al. 2014) and pyrene by Mycobacterium sp. KR2 (Haritash and Kaushik 2009). Finally, further oxidation could result in opening benzene ring and the formation of hexadecanoic acid, ethyl ester. In accordance with our data, Patel et al. (2016) reported that cyanobacterium Anabaena fertilissima produced tetradecanoic acid and benzene ring-containing compounds after exposure to 5.0 and 10.0 mg L−1 anthracene for 16 days. In addition, erucic acid was recorded in the treatments exposed to 1.5, 3, and 6 mg L−1 of pyrene after 16 days. Our results have demonstrated that C. vulgaris was capable of uptaking and degrading fluorene presumably with a similar mechanism.
Conclusion
Our results conclusively showed that C. vulgaris possesses high resistance against fluorene as a persistent environmental pollutant. The study of morphological features of the microalga confirmed some changes at the cell surface after treatment with fluorene. However, stimulation of antioxidant enzymes activity including SOD, CAT, and APX along with the increasing amounts of phenols and flavonoids confirmed the protective role of antioxidant systems in response to oxidative stress emerged by fluorene in the cells of C. vulgaris. On the other hand, fluorene significantly dropped the content of the chlorophylls in a dose-dependent manner which seemed to be also a defense mechanism. Likewise, the results of flow cytometric analysis revealed that applied concentrations of fluorene had low impacts on viability of the cells, while they significantly diminished the chlorophyll fluorescence. The potential of the alga in bioremoval of fluorene was confirmed by identifying some biodegradation byproducts. Taken all together, the noticeable ability of C. vulgaris for growth in the fluorene-contaminated environments makes the algal species attractive for further bioremediation studies.
References
Asghari S, Movafeghi A, Lisar SYS, Barar J, Omidi Y (2018) Effects of phenanthrene on growth parameters and antioxidant systems in the green microalga Chlorella vulgaris. Biointerface Res Appl Chem 8:3405–3411
Bautista I, Boscaiu M, Lidón A, Llinares JV, Lull C, Donat MP, Mayoral O, Vicente O (2016) Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol Plant 38:9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Casellas M, Grifoll M, Bayona JM, Solanas AM (1997) New metabolites in the degradation of fluorene by Arthrobacter sp. strain F101. Appl Environ Microbiol 63:819–826
Çelekli A, Gültekin E, Bozkurt H (2016) Morphological and biochemical responses of Spirogyra setiformis exposed to cadmium. Clean-Soil Air Water 44:256–262
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Cheema SA, Khan MI, Shen C, Tang X, Farooq M, Chen L, Zhang C, Chen Y (2010) Degradation of phenanthrene and pyrene in spiked soils by single and combined plants cultivation. J Hazard Mater 177:384–389
Cheng J, Qiu H, Chang Z, Jiang Z, Yin W (2016) The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. Springerplus 5:1290
Comotto M, Casazza AA, Aliakbarian B, Caratto V, Ferretti M, Perego P (2014) Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Sci World J 2014:1–9
El-Sheekh MM, Hamouda RA, Nizam AA (2013) Biodegradation of crude oil by Scenedesmus obliquus and Chlorella vulgaris growing under heterotrophic conditions. Int Biodeterior Biodegradation 82:67–72
Fazelian N, Movafeghi A, Yousefzadi M, Rahimzadeh M (2019) Cytotoxic impacts of CuO nanoparticles on the marine microalga Nannochloropsis oculata. Environ Sci Pollut Res 26:17499–17511
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Hong Y-W, Yuan D-X, Lin Q-M, Yang T-L (2008) Accumulation and biodegradation of phenanthrene and fluoranthene by the algae enriched from a mangrove aquatic ecosystem. Mar Pollut Bull 56:1400–1405
Jaleel CA, Riadh K, Gopi R, Manivannan P, Ines J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Kalhor AX, Nassab ADM, Abedi E, Bahrami A, Movafeghi A (2016) Biodiesel production in crude oil contaminated environment using Chlorella vulgaris. Bioresour Technol 222:190–194
Kalhor AX, Movafeghi A, Mohammadi-Nassab AD, Abedi E, Bahrami A (2017) Potential of the green alga Chlorella vulgaris for biodegradation of crude oil hydrocarbons. Mar Pollut Bull 123:286–290
Karydis M (1981) The toxicity of crude oil for the marine alga Skeletonema costatum (Greville) Cleve in relation to nutrient limitation. Hydrobiologia 85:137–143
Kim K-H, Jahan SA, Kabir E, Brown RJ (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Kirso U, Irha N (1998) Role of algae in fate of carcinogenic polycyclic aromatic hydrocarbons in the aquatic environment. Ecotoxicol Environ Saf 41:83–89
Lei A, Hu Z, Wong Y, Tam NF (2006) Antioxidant responses of microalgal species to pyrene. J Appl Phycol 18:67–78
Lei A-P, Hu Z-L, Wong Y-S, Tam NF-Y (2007) Removal of fluoranthene and pyrene by different microalgal species. Bioresour Technol 98:273–280
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu H, Weisman D, Ye Y-B, Cui B, Huang Y-H, Colón-Carmona A, Wang Z-H (2009) An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci 176:375–382
Maehly A, Chance B (1955) Assay of catalases and peroxidases, in methods in enzymology. Methods Enzymol 2:764–775
Mahjouri S, Movafeghi A, Divband B, Kosari-Nasab M (2018a) Toxicity impacts of chemically and biologically synthesized CuO nanoparticles on cell suspension cultures of Nicotiana tabacum. Plant Cell Tissue Organ Cult 135:223–234
Mahjouri S, Movafeghi A, Divband B, Kosari-Nasab M, Kazemi EM (2018b) Assessing the toxicity of silver nanoparticles in cell suspension culture of Nicotiana tabacum. Biointerface Res Appl Chem 8:3252–3258
Mallick N (2004) Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: response of the antioxidant system. J Plant Physiol 161:591–597
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Munoz R, Guieysse B, Mattiasson B (2003) Phenanthrene biodegradation by an algal-bacterial consortium in two-phase partitioning bioreactors. Appl Microbiol Biotechnol 61:261–267
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nazari F, Movafeghi A, Jafarirad S, Kosari-Nasab M, Divband B (2018) Synthesis of reduced graphene oxide-silver nanocomposites and assessing their toxicity on the green microalga Chlorella vulgaris. Bionanoscience 8:997–1007
Patel JG, Kumar JN, Kumar RN, Khan SR (2016) Biodegradation capability and enzymatic variation of potentially hazardous polycyclic aromatic hydrocarbons—anthracene and pyrene by Anabaena fertilissima. Polycycl Aromat Comp 36:72–87
Qian H, Li J, Pan X, Sun L, Lu T, Ran H, Fu Z (2011) Combined effect of copper and cadmium on heavy metal ion bioaccumulation and antioxidant enzymes induction in Chlorella vulgaris. Bull Environ Contam Toxicol 87:512–516
Qian H, Li J, Pan X, Sun Z, Ye C, Jin G, Fu Z (2012) Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ Toxicol 27:229–237
Rai U, Singh N, Upadhyay A, Verma S (2013) Chromate tolerance and accumulation in Chlorella vulgaris L.: role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour Technol 136:604–609
Rawat I, Kumar RR, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88:3411–3424
Sáenz ME, Di Marzio WD, Alberdi JL (2012) Assessment of Cyfluthrin commercial formulation on growth, photosynthesis and catalase activity of green algae. Pestic Biochem Physiol 104:50–57
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278
Salehi-Lisar SY, Deljoo S (2015) The physiological effect of fluorene on Triticum aestivum, Medicago sativa, and Helianthus annus. Cogent Food Agric 1:1020189
Semple KT, Cain RB, Schmidt S (1999) Biodegradation of aromatic compounds by microalgae. FEMS Microbiol Lett 170:291–300
Shen C, Miao J, Li Y, Pan L (2016) Effect of benzo [a] pyrene on detoxification and the activity of antioxidant enzymes of marine microalgae. J Ocean U China 15:303–310
Soto C, Hellebust JA, Hutchinson TC (1975) Effect of naphthalene and aqueous crude oil extracts on the green flagellate Chlamydomonas angulosa. II. Photosynthesis and the uptake and release of naphthalene. Can J Bot 53:118–126
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171
Subashchandrabose SR, Logeshwaran P, Venkateswarlu K, Naidu R, Megharaj M (2017) Pyrene degradation by Chlorella sp. MM3 in liquid medium and soil slurry: possible role of dihydrolipoamide acetyltransferase in pyrene biodegradation. Algal Res 23:223–232
Suman T, Rajasree SR, Kirubagaran R (2015) Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol Environ Saf 113:23–30
Takáčová A, Smolinská M, Ryba J, Mackuľak T, Jokrllová J, Hronec P, Čík G (2014) Biodegradation of benzo[a]pyrene through the use of algae. Cent Eur J Chem 12:1133–1143
Tarrahi R, Khataee A, Movafeghi A, Rezanejad F, Gohari G (2017) Toxicological implications of selenium nanoparticles with different coatings along with Se4+ on Lemna minor. Chemosphere 181:655–665
Vafaei F, Movafeghi A, Khataee A, Zarei M, Lisar SS (2013) Potential of Hydrocotyle vulgaris for phytoremediation of a textile dye: inducing antioxidant response in roots and leaves. Ecotoxicol Environ Saf 93:128–134
Wang HY, Zeng XB, Guo SY, Li ZT (2008) Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics 29:39–46
Warshawsky D, Cody T, Radike M, Reilman R, Schumann B, LaDow K, Schneider J (1995) Biotransformation of benzo [a] pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem Biol Interact 97:131–148
Wei H, Song S, Tian H, Liu T (2014) Effects of phenanthrene on seed germination and some physiological activities of wheat seedling. C R Biol 337:95–100
Winterbourn CC, McGrath BM, Carrell RW (1976) Reactions involving superoxide and normal and unstable haemoglobins. Biochem J 155:493–502
Acknowledgments
We thank the University of Tabriz and Research Center for Pharmaceutical Nanotechnology at the Tabriz University of Medical Sciences for all supports provided.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asghari, S., Rajabi, F., Tarrahi, R. et al. Potential of the green microalga Chlorella vulgaris to fight against fluorene contamination: evaluation of antioxidant systems and identification of intermediate biodegradation compounds. J Appl Phycol 32, 411–419 (2020). https://doi.org/10.1007/s10811-019-01921-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01921-7