Abstract
Nanotechnology has quite a lot of applications in various fields of industrial sectors like food and agriculture. Although nanotechnology can improve the quality of life, its possible associated risks should be assessed. Here copper oxide nanoparticles (CuO NPs) were synthesized by chemical (polymer pyrolysis) and biological (green) methods with an average size of 30 and 44 nm, respectively. Afterwards, a cell biology approach was applied to evaluate the toxic effects of chemically and biologically synthesized CuO nanoparticles on tobacco cell suspension cultures. Both types of CuO nanoparticles significantly dropped the viability of the cells in a dose and time dependent manner. Accordingly, tobacco cells were found to increase the activity of antioxidant enzymes after 48 h of exposure to nanoparticles. The production of reactive oxygen species (ROS) and malondialdehyde (MDA) in a dose dependent manner was also observed. Assessment of the toxicity of CuO NPs revealed that chemically synthesized NPs were more toxic than biologically synthesized ones. It can be concluded that the organic components of the plant extract as capping agents that remain on the surface of green synthesized CuO NPs may reduce their toxicity effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The onset of nanotechnology has provided enormous opportunities for the development of new products with a wide range of application areas of economic and scientific importance. Nanoparticles (NPs), with a diameter scales between 1 and 100 nm, are being used in several fields including drug and gene delivery, bioimaging, catalysis and environmental decontamination (Pissuwan et al. 2011; Wang et al. 2013). The exceptional properties of these materials, for example, large specific surface area and greater reactivity, have raised questions concerning potential adverse effects on living organisms and environment (Borm et al. 2006). In order to elucidate these anxieties and to ensure the development of safe and publicly accepted nano-based products, specific aspects and possible risks of engineered NPs must be carefully examined. Since plants are essential components of all ecosystems, understanding the interactions between plants and engineered nanoparticles are required for monitoring the environmental consequences of nanotechnology.
Copper oxide (CuO) NPs are one of the major and frequently used engineered metal oxide NPs with significant commercial applications including gas sensors, solar cells, heat transfer nanofluids, catalysts, semiconductors (Tran and Nguyen 2014), and also are a promising antimicrobial agent (Azam et al. 2012). Different chemical methods have been described for the synthesis of CuO NPs for a variety of applications (Zhang et al. 2014). However, in the chemical synthesis procedures, the applications of hazardous and expensive chemicals as well as having high energy consumption have a negative outcome on the environment. As an alternative technique, the biological (green) synthesis of NPs using plant extracts has received a great deal of attention because of its numerous advantages such as being cost effective, environmental-friendly and suitable for large-scale production (Iravani 2011). Accordingly, synthesis of CuO NPs using extracts of various plants has been described (Acharyulu et al. 2014; Nasrollahzadeh et al. 2015). We herein report the green synthesis of CuO NPs using aqueous leaf extract of Berberis thunbergii (Berberidaceae) which is a garden plant and tolerant to adverse environmental factors such as drought, and urban pollution. This plant species is known as medicinal plant and has a number of biological effects including anti-inflammatory and antimicrobial activities (Li et al. 2007; Villinski et al. 2003). Its leaves contain a large amount of flavonoid pigments (Pietrowska-Borek et al. 2010) as well as alkaloids (Khamidov et al. 1997), which may act as reducing and capping agents for synthesis of CuO NPs.
The expanding production and widespread applications of CuO NPs lead to environmental exposure and therefore raise concerns about their adverse effects on living organisms. Several studies have been demonstrated their toxicity on different organisms (Hou et al. 2017). CuO NPs were detected in the xylem sap of Zea mays plants by combining transmission electron microscopy, selected-area electron diffraction, and energy dispersive spectroscopy, confirmed that these NPs can penetrate the root system, reach the xylem, and be translocated to the aerial parts (Wang et al. 2012). It has also been demonstrated that CuO NPs can be taken up, transported, and biotransformed by rice plants under hydroponic conditions (Peng et al. 2015). The results of another study suggested that CuO NPs can be absorbed by the roots and translocated to the shoots in Cu-tolerant plant Elsholtzia splendens (Shi et al. 2014). The growth inhibition of soybean and chickpea seedlings (Adhikari et al. 2012), as well as reduction in the root length of wheat (Dimkpa et al. 2013), have been reported after treatment with CuO NPs. Copper-based NPs not only reduced the size of lettuce and alfalfa plants but also altered nutrient content and enzyme activity in both plant species (Hong et al. 2015). Evaluating the toxicity of CuO NPs on rice seedlings (Shaw and Hossain 2013) and aquatic plant Spirodela polyrrhiza (Khataee et al. 2017) confirmed the induction of oxidative damages.
Hitherto, most of the toxicity studies have been focused on chemically synthesized nanoparticles, which were mainly produced by toxic compounds. Recently, the toxicity of some green synthesized nanoparticles has also been reported. The toxic impacts of the biologically synthesized NPs were reported to be not as much of the chemically synthesized NPs (Krishnaraj et al. 2012; Saif et al. 2016). However, there is an insufficient amount of data available to compare the toxic effects of chemically and biologically synthesized NPs. Thus, detailed descriptions and comprehensive studies about the toxicity of NPs with different synthesis procedures are required. In this study, green synthesis of CuO NPs using aqueous leaf extract of B. thunbergii, as well as chemical synthesis using polymer pyrolysis method was conducted. Further, a cell biology approach has been used to evaluate the impacts of synthesized CuO NPs on tobacco cell suspension cultures, considering their effect on cell viability and oxidative stress. Accordingly, the activity of antioxidant enzymes such as catalase (CAT, EC 1.11.1.6), superoxide dismutase (SOD, EC 1.15.1.1) and peroxidase (POD, EC 1.11.1.7) as well as total phenol and flavonoid were assessed. The different effects of chemically and biologically synthesized CuO NPs were compared.
Materials and methods
Synthesis and characterization of CuO nanoparticles
Chemically synthesized CuO nanoparticles (CS-CuO NPs) were prepared by polymer pyrolysis technique. The aqueous solution of CuSO4⋅5H2O (0.2 M) was mixed with 1 g of PEG (4000) (polyethylene glycol) and heated to 80 °C with constant stirring. The pH of mixture was adjusted to 8 by ammonia and the obtained gel was dried at 90 °C for 24 h and calcinated at 500 °C for 4 h.
The leaf extract of B. thunbergii was used for production of green synthesized-CuO nanoparticles (GS-NPs). A quantity of 10 g of the leaves was shaken with 100 ml of deionized water for 2 h and sonicated for 20 min at 35 °C. The sample was shaken overnight, and then sonication and shaking processes were repeated. The retained mixture was filtered and centrifuged. For synthesis of GS-CuO NPs, 1 g of CuSO4⋅5H2O, 10 ml of leaf extract and 25 ml of distilled water were mixed for 5–6 min. After pH adjustment to 6–7 using 1 M NaOH, the reaction mixture was subjected to microwave heating (360 W for 3 min). The precipitate was centrifuged and dried at 70 °C for 4 h and was heated at 400 °C for 4 h. The prepared nanoparticles were used as GS-CuO NPs.
Characteristics of the synthesized NPs were determined using different methods including scanning electron microscopy (SEM), energy-dispersive X-ray (EDX) spectroscopy, X-ray powder diffraction (XRD), Fourier transform infra-red (FT-IR) spectroscopy and dynamic light scattering (DLS).
Cell cultivation, treatments, and microscopic observations
Seeds of Nicotiana tabacum cv. Virginia were obtained from East Azerbaijan Research and Education Center for Agriculture and Natural Resources. The seeds were sterilized using 70% ethanol for 2 min followed by 5% bleach for 15 min and were washed several times with sterile distilled water. Then, they were germinated on Murashige and Skoog (MS) medium, solidified with 0.8% agar. For callus induction, cotyledon explants of 3-week-old seedlings were cut and placed on MS medium supplemented with 2% sucrose and 1 mg L−1 of 2,4-D (2,4-dichlorophenoxyacetic acid) and maintained in dark condition (Khodakovskaya et al. 2012). Tobacco cell suspension cultures were initiated from three-month-old calli in the liquid MS medium (2% sucrose, 1 mg L−1 of 2,4-D) and were cultured under constant shaking (110 rpm) at 25 °C in the dark. Cells were subcultured every 7 days by adding 10 mL of the cell suspension to a 250 mL flask containing 40 mL of fresh MS medium.
Experiments were conducted in the exponential phase of growth at day 4 of culture. Cells were treated with different concentrations of CuO NPs solution (0, 10, 25, 50, 75 and 100 mg L−1) with a pH of 5.8. After 48 h of incubation, cells were harvested for different assays and were frozen at − 80 °C or used directly for the antioxidant enzyme assays.
The morphology of CuO NPs-treated and untreated tobacco cells were investigated using SEM and light microscopy.
Cell viabilities assay
Cell viabilities were estimated using modified triphenyl-tetrazolium chloride (TTC) reduction assay (Sobkowiak and Deckert 2003). A quantity of 1 ml of cell culture was incubated in 1 mL of TTC solution (0.1% TTC in 50 mM potassium phosphate buffer, pH 7.5) for 24 h at 25 °C in dark. Afterwards, TTC solution was aspirated and cells were incubated in 2 mL of 96% ethanol for 15 h at 55 °C. The absorbance of the supernatant was read at 485 nm after 15 min of centrifugation at 10,000 g.
Antioxidant enzyme activity
An amount of 0.2 g of fresh tobacco cells was homogenized in pre-chilled mortar and pestle with 1 mL of 50 mM potassium phosphate buffer (pH 7). The homogenate was centrifuged at 10,000 g at 4 °C for 20 min. The resulting supernatant was used to measure the activities of superoxide dismutase (Winterbourn et al. 1976), Peroxidase (Chance and Maehly 1955) and catalase (Simon et al. 1974) as well as protein content (Bradford 1976).
Hydrogen peroxide content
The concentration of H2O2 was determined using the method of Velikova et al. (2000). An amount of 0.3 g of cell samples was homogenized with a 0.1% trichloroacetic acid (TCA) solution using a pre-chilled mortar and pestle in an ice bath. The homogenate was centrifuged at 12,000 g for 15 min at 4 °C. Subsequently, 0.5 mL of supernatant was added to 0.5 mL of 10 mM potassium phosphate buffer (pH 7) in addition to 1 mL of 1 M potassium iodide reagent and was incubated at 25 °C for 15 min. The absorbance was measured at 390 nm and the concentration of H2O2 was determined using a standard curve.
Malondialdehyde determination (MDA assay)
For the determination of lipid peroxidation, MDA assay was used (Boominathan and Doran 2002). An amount of 0.3 g of tobacco cells was homogenized with 1 mL of 0.1% trichloroacetic acid in pre-chilled mortar and pestle. Centrifugation was done at 12,000 g for 15 min and 0.5 mL of the supernatant was mixed with 1.5 mL of 20% (w/v) trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid. The mixture was heated (95 °C, 30 min) and then was shortly placed in an ice-bath. The cooled mixture was centrifuged (10,000 g, 15 min) and the absorbance of the supernatant was measured at 532 nm. Production of MDA was estimated as a malondialdehyde equivalent using a standard curve.
Total phenolic and flavonoid contents
For evaluation of the total phenolic and flavonoid contents, 0.3 g of tobacco cells was homogenized using 1 ml of methanol. A method of Folin–Ciocalteu was applied to calculate the total phenolic content (Singleton et al. 1999). Accordingly, 2.5 mL of distilled water, 0.1 mL of Folin–Ciocalteu reagent as well as 0.1 mL of methanolic extract of cells were mixed. After 6 min, 0.15 mL of 20% Na2CO3 were added and the mixture was incubated at 25 °C for 30 min. Finally, the absorbance was measured at 760 nm. The phenolic content in extracts was stated with reference to gallic acid equivalent.
In order to determine the flavonoid content, 0.5 mL of the extract and 0.5 mL of 2% AlCl3 solution dissolved in methanol were incubated for 1 h at 25 °C and the absorbance was measured at 415 nm. The flavonoid content in extracts was stated as the quercetin equivalent (Quettier-Deleu et al. 2000).
DPPH assay
DPPH free radical scavenging assay was settled as stated by Choochote et al. (2014). Briefly, 0.5 mL of extract (0.3 g of tobacco cells were extracted using 1 ml of methanol) was added to 0.3 ml of DPPH solution (1 mM in methanol) and the total volume of the reaction mixture was reached to 3 ml with methanol. After 15 min dark incubation, the absorbance of the reaction mixture was measured by a spectrophotometer at 517 nm. The mixture containing 2.7 ml methanol and 0.3 ml of DPPH solution assayed as DPPH blank sample. The inhibition percentage was calculated by the following equation:
Statistical analysis
All results are presented as the mean ± standard error (SE). The One Way ANOVA test of significance was used to compare the different conditions by Duncan’s Test (SPSS software, version 22). The result was considered significant if p ≤ 0.05 when compared to the control.
Results and discussion
Characterization of CuO nanoparticles
The SEM images of CS-CuO and GS-CuO NPs (Fig. 1a, b) clearly display the formation of spherical copper oxide nanoparticles. The results reveal an average size of 30.81 nm for CS-CuO and 44.14 nm for GS-CuO NPs (Fig. 1c, d). EDX spectrum of CuO NPs (given in Fig. 1e, f) approves the 100% formation of CuO nanoparticles and no detectable impurities were observed in the samples.
The XRD patterns of prepared CS-CuO and GS-CuO NPs confirm that the formation of CuO NPs from each method is in monoclinic phase and are comparable with JCPDS No. 45-0937 (Fig. 2). XRD analysis shows intense peaks at 2θ = 32.72°, 35.52°, 38.92°, 49.02°, 53.68°, 58.5°, 61.72°, 66.48°, 68.24°, 72.62° and 75.2° that are assigned to (110), (002), (111), (202), (020), (202), (113), (311), (113), (311) and (222) plane orientation, respectively. The width of XRD diffraction peaks for CS-CuO NPs in comparison with the GS-CuO NPs is wider, illustrating the smaller crystallite size of CS-CuO NPs. This finding is in fair agreement with the SEM and DLS analysis. Hence, the average crystallite size of synthesized CuO-NPs seemed to be in appropriate size range for uptake by tobacco cells.
FTIR spectroscopy was employed to identify the biomolecules of B. thunbergii leaf extract (Fig. 3a). FTIR spectrum indicates the peak shifts after reaction of CuSO4 with leaf extract. In the spectrum (Fig. 3b), peaks of GS-CuO NPs at 535.08, 1111.82, 1384.81, 1604.45 and 3431.44 cm−1 are observed. The existence of a broad peak at 3431.44 cm−1 confirms the presence of OH groups on the surface of CuO NPs. Further, the absorption bands at 1604.45 and 1384.81 cm−1 can be attributed to the C=O stretching and –CH3 group, respectively. A band at 1111.82 cm−1 corresponds to the C–O bond. The existence of band at 535.08 cm−1 is connected to the vibrations of Cu–O, confirming the formation of CuO NPs (Javed et al. 2017; Padil and Černík 2013).
Size distribution of CS-CuO NPs in distilled water ranged from 25 to 100 nm (Fig. 4a) and zeta potential was 7 mV. In the case of GS-CuO NPs, DLS analysis showed distribution size range from 43 to 86 nm and zeta potential measurements showed the positively charged particles (21 mV) in distilled water, which corroborates the stability of the suspension (Fig. 4b).
Cell viability and morphology
In the first step, the toxicity of CuO NPs on tobacco cells was evaluated by TTC staining method. The viability of tobacco cells significantly decreased in dose- as well as time-dependent manners, growing in the presence of various concentrations of CS-CuO NPs (0–100 mg L−1) for 0–48 h (Fig. 5a). The toxicity of CS-CuO NPs began to appear at 25 mg L−1 concentration after exposure for 6 h. The viability was significantly diminished by 59.75% at the lowest CS-CuO NPs concentration (10 mg L−1) and only about 2.39% of tobacco cells were alive after a 48-h treatment at the highest NPs concentration (100 mg L− 1). The toxicity of GS-CuO NPs was also assessed on tobacco cells at 0 − 100 mg L− 1 during the time periods of 0–48 h (Fig. 5b). Similar to CS-CuO NPs, cell viability upon GS-CuO NP exposure was concentration- and time-dependent. Low concentration of GS-CuO NPs (10 mg L− 1) had no effect on viability even after 48 h of treatment. Concentrations of 25 and 50 mg L− 1 of GS-CuO NPs had the lowest effect on cell viability in comparison with CS-CuO NPs. However, high concentrations of GS-CuO and CS-CuO NPs (75 and 100 mg L−1) approximately showed same behavior during the treatment (Fig. 5a, b). The compared results of 48 h cell viability of CS-CuO and GS-CuO NPs was illustrated in Fig. 5c.
Tobacco cell viability measured using TTC assay after exposure to different conditions. Cell viability after exposure to 0–100 mg L−1 of CS-CuO (a) and GS-CuO NPs (b) for 6, 12, 24, and 48 h. Cell viability after 48 h exposure to CS-CuO and GS-CuO NPs at different concentrations (c). Data are showed as mean values ± standard error (n = 3)
The changes in surface morphology of the tobacco cells were studied after treatments with CuO NPs using SEM and light microscopy (Fig. 6). The control tobacco cells showed a well-defined shape with a normal smooth surface (Fig. 6a, b). NPs treatment at 100 mg L−1 concentration for 48 h, led to changes in cell morphology and cell death. Remarkable deformations such as cell shrinkage and cellular membrane collapse were clearly evident (Fig. 6c–f).
Hydrogen peroxide and MDA production
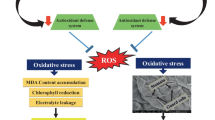
The effects of NPs on the oxidative stress in plants have been examined by measuring either H2O2 or ROS in general. On the other hand, the quantity of lipid peroxidation is commonly used as a symptom of oxidative damage (Rico et al. 2015). In this study, to determine whether CuO NPs can induce the generation of excess H2O2 in treated cells, H2O2 accumulation has been evaluated after 48 h of tobacco cells exposure to CS-CuO and GS-CuO NPs. Exposure to different concentrations of CS-CuO NPs caused an elevation in H2O2 level which was significantly enhanced with the increasing concentration of NPs (Fig. 7a). In comparison to CS-CuO NPs, only higher concentrations of GS-CuO NPs (75 and 100 mg L−1) significantly increased H2O2 levels (Fig. 7b). Recent studies have shown that different NPs could lead to production of ROS in a concentration dependent manner (Prakash and Chung 2016; Zhao et al. 2017). Enhanced production of ROS during diverse stresses can pose a special threat to cell survival through lipid peroxidation, oxidation of proteins, damage to DNA and activation of programmed cell death pathway (Sharma et al. 2012).
Lipid peroxidation is one of the most examined consequences of ROS action on membrane integrity. We analyzed the final product of lipid peroxidation, malondialdehyde (MDA) in CuO-treated tobacco cells (Fig. 7c, d). The MDA content was mounted notably from 25 to 100 mg L−1 for CS-CuO NPs to the highest concentration equal to 4.37 nmol g−1 FW and 4.79 nmol g−1 FW respectively. In contrast, for GS-CuO NPs treatment, MDA production increased just at the highest concentration (100 mg L−1, 4.77 nmol g−1 FW). Induction of lipid peroxidation under CuO NPs stress matches with increased production of H2O2. Increased peroxidation of lipids has been reported in plants growing under NPs stresses (Poborilova et al. 2013). For instance, the content of MDA was significantly elevated after CuO NPs treatment in Lemna minor (Song et al. 2016).
Enzymatic antioxidant defense system response to CuO NPs
Mounting concentrations of CS-CuO NPs generated a significant change in SOD activity (Fig. 8a). SOD activity was increased considerably when tobacco cells were exposed to different concentrations of CS-CuO NPs. In the case of GS-CuO NPs, the SOD activity was not significantly affected at low concentrations, while higher activities were observed at 75 and 100 mg L−1 of NPs in the treated cells compared with the control (Fig. 8b). SOD has been suggested to have a critical role in plant stress tolerance and provides the first line of defense against the ROS-induced damages (Gill and Tuteja 2010). A significant increase in SOD activity in plants in response to some nanoparticles toxicity has been reported (Song et al. 2013). Nano-CuO confronted oxidative damages through enhanced ROS scavenging antioxidant enzymes activity including SOD in rice seedlings (Shaw and Hossain 2013). SOD activity of L. minor was also increased after CuO NPs treatment (Song et al. 2016).
Effect of CS-CuO NPs (a, c, e) and GS-CuO NPs (b, d, f) on the activity of the antioxidant enzyme system (SOD, POD and CAT) of tobacco cell suspension cultures treated for 48 h. Different letters indicate significant differences at p ≤ 0.05 according to Duncan’s Test. The error bars represent standard error of the mean (n = 3)
POD activity showed a significant enhancement in tobacco cells after treatment with different concentrations of CS-CuO NPs (Fig. 8c). At 25 and 100 mg L−1 concentrations, 2.09 and 3.9 times rise in enzyme activity was recorded. However, GS-CuO NPs caused an increase in POD activity just in high concentrations (75 and 100 mg L−1) (Fig. 8d). This response of POD is in agreement with other reports investigated CuO toxicity in plants (Dimkpa et al. 2012; Kim et al. 2012). Actually, some peroxidases play an important role in lignin biosynthesis and might build up a physical barrier against the poisoning of heavy metals (Rai et al. 2004).
Catalases are highly expressed enzymes as an integral part of the antioxidative system in plant cells (Mhamdi et al. 2010). CAT activity was increased when tobacco cells were exposed to CS-CuO NPs as well as GS-CuO NPs (Fig. 8e, f). This significant increase in the CAT activity suggests a continuous decontamination of H2O2 when tobacco cells were exposed to high concentrations of CuO NPs. In accordance with this finding, it was reported that CAT activity in cucumber (Kim et al. 2012), wheat (Dimkpa et al. 2012) and S. polyrrhiza (Khataee et al. 2017) were enhanced by CuO NPs. Similarly, the activities of CAT were enhanced by CeO2 NPs in wheat (Du et al. 2015) and by Se NPs in L. minor (Tarrahi et al. 2017).
ROS generation is one of the important toxicological mechanisms, by which NPs cause oxidative damage and cell death in plants. Thus, measuring the activity of antioxidant enzymes such as SOD, POD or CAT can be considered as a crucial biomarker of nanoparticle toxicity (Rico et al. 2015). According to our results, after treatments with both types of CuO NPs, the activities of all examined antioxidant enzymes were increased by the elevating concentrations of NPs. Indeed, they were operative during 48 h treatment of NPs stress period in scavenging ROS. In contrast to CS-CuO NPs, GS-CuO NPs just in high concentrations could encourage the enhancement of antioxidant enzymes activity in tobacco suspension cell culture. Most probably, natural plant compounds maintained on the surface of the GS-CuO NPs reduce the toxic effects of these materials.
Total phenol and flavonoid content and antioxidant capacity
Phenolics are a diverse group of secondary metabolites including flavonoids, tannins, hydroxy cinnamate esters, and lignin which exhibit antioxidant properties (Grace and Logan 2000). Moreover, it has been proposed that phytophenolics can detoxify H2O2 as electron donors for phenol peroxidases (guaiacol peroxidases), which results in the formation of respective phenoxyl radicals (Michalak 2006). Indeed, they assist as ROS scavengers by neutralizing radicals before damaging cells and thus are important for plant resistance under various stresses. According to our results, the total phenolic content was notably dropped at the presence of CS-CuO NPs above 25 mg L−1 in the tobacco cell cultures. On the other hand, GS-CuO NPs showed less phenolic compounds at 75 and 100 mg L−1 when compared with the control cells (Fig. 9 a, b). Furthermore, the highest and lowest amounts of flavonoid were recorded at 25 and 75 mg L−1 of CS-CuO NPs (13.82 and 12.3 µg g−1 FW) respectively, while the flavonoid content was decreased at 75 and 100 mg L−1 of GS-CuO NPs which was equivalent to 11.79 and 11.39 µg g−1 FW (Fig. 9c, d). Induction in the production of phenolic and flavonoid compounds has been identified in plants as a response to NPs (Poborilova et al. 2013; Zafar et al. 2016). However, reduction in phenolic and flavonoid contents have been previously observed in Calendula officinalis treated with silver NPs (Ghanati and Bakhtiarian 2014) and in Arthrospira platensis after treatment with TiO2 NPs (Comotto et al. 2014).
Content of phenolics, flavonoids and inhibition percent of DPPH in tobacco cells treated by different concentrations of CS-CuO NPs (a, c, e) and GS-CuO NPs (b, d, f) after 48 h of exposure. Different letters indicate significant differences at p ≤ 0.05 according to Duncan’s Test. The error bars represent standard error of the mean (n = 3)
In DPPH assay, a decrease in the inhibitory percent of DPPH was also observed after 48 h for both nanoparticles (Fig. 9e, f). Since phenolics are considered as the main contributors to the total non-enzymatic antioxidant capacity of plant extracts (Heo et al. 2007), most probably reduction in phenolic compounds caused dropping in the radical scavenging capacity. Our results are in fair agreement with another study that showed the reduction in radical scavenging capacity after treatment of C. officinalis plants with different concentrations of toxic silver NPs (Ghanati and Bakhtiarian 2014).
Conclusion
Our results revealed that B. thunbergii extract is capable of producing CuO NPs in a simple, rapid and biocompatible manner. The FTIR results confirmed the formation of CuO NPs with organic compounds holding polar groups on their surface. According to acquired toxicity experiments in this study, CuO NPs significantly dropped the viability of tobacco cells in a dose and time dependent manner. They caused oxidative stress in tobacco cells, which was evident from notably mounted amounts of H2O2 and lipid peroxidation. The findings give the impression that just like other oxidative stresses in plants, the stimulation of SOD activity along with POD and CAT, played a protective role against oxidative damage of CuO NPs in tobacco cells. This phenomenon seems to prevent the accumulation of ROS. Thus, it can be inferred that the activity of these enzymes may serve as useful biomarkers for CuO NPs toxicity in plants. On the other hand, the antioxidant capacity of tobacco cells along with total phenolic and flavonoid contents were significantly decreased after being exposed to CuO NPs. However, it seems that CuO NPs did not activate the production of non-enzymatic antioxidant molecules in tobacco cell suspension cultures. The comparison of toxicity results for CuO NPs, demonstrated that CS-CuO NPs were more toxic than GS-NPs. Indeed, it can be concluded that the active components of the plant extract remain on the surface of synthesized CuO NPs as capping agents and reduce the toxicity of these particles. Therefore, green synthesis of NPs refers to a more environment-friendly method that should be considered in the forthcoming studies.
Abbreviations
- CS-CuO NPs:
-
Chemically synthesized copper oxide nanoparticles
- GS-CuO NPs:
-
Green synthesized copper oxide nanoparticles
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive Oxygen Species
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
References
Acharyulu N, Dubey R, Swaminadham V, Kollu P, Kalyani R, Pammi S (2014) Green synthesis of CuO nanoparticles using Phyllanthus amarus leaf extract and their antibacterial activity against multidrug resistance bacteria. Int J Engin Res Technol 3:639–641
Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Rao AS (2012) Effect of copper oxide nanoparticle on seed germination of selected crops. J Agric Sci Technol 2:815–823
Azam A, Ahmed AS, Oves M, Khan M, Memic A (2012) Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int J Nanomedicine 7:3527–3535. https://doi.org/10.2147/IJN.S29020
Boominathan R, Doran PM (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol 156:205–215. https://doi.org/10.1046/j.1469-8137.2002.00506.x
Borm PJ et al (2006) The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol 3:1743–8977. https://doi.org/10.1186/1743-8977-3-11
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chance B, Maehly A (1955) [136] Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Choochote W, Suklampoo L, Ochaikul D (2014) Evaluation of antioxidant capacities of green microalgae. J Appl Phycol 26:43–48. https://doi.org/10.1007/s10811-013-0084-6
Comotto M, Casazza AA, Aliakbarian B, Caratto V, Ferretti M, Perego P (2014) Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Sci World J 2014:9. https://doi.org/10.1155/2014/961437
Dimkpa CO et al (2012) CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14:1–15. https://doi.org/10.1007/s11051-012-1125-9
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013) Fate of CuO and ZnO nano-and microparticles in the plant environment. Environ Sci Technol 47:4734–4742. https://doi.org/10.1021/es304736y
Du W, Gardea-Torresdey JL, Ji R, Yin Y, Zhu J, Peralta-Videa JR, Guo H (2015) Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: a life cycle field study. Environ Sci Technol 49:11884–11893. https://doi.org/10.1021/acs.est.5b03055
Ghanati F, Bakhtiarian S (2014) Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop J Pharm Res 13:1783–1789. https://doi.org/10.4314/tjpr.v13i11.2
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Grace SC, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos Trans R Soc Lond B 355:1499–1510. https://doi.org/10.1098/rstb.2000.0710
Heo HJ, Kim YJ, Chung D, Kim D-O (2007) Antioxidant capacities of individual and combined phenolics in a model system. Food Chem 104:87–92. https://doi.org/10.1016/j.foodchem.2006.11.002
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ Sci Proc Impacts 17:177–185. https://doi.org/10.1039/C4EM00551A
Hou J, Wang X, Hayat T, Wang X (2017) Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ Pollut 221:209–217. https://doi.org/10.1016/j.envpol.2016.11.066
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/C1GC15386B
Javed R, Mohamed A, Yücesan B, Gürel E, Kausar R, Zia M (2017) CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tiss Organ Cult 131:611–620. https://doi.org/10.1007/s11240-017-1312-6
Khamidov I, Aripova S, Karimov A, Yusupov M (1997) Berberis alkaloids. XL. An investigation of the alkaloids of Berberis thunbergii. Chem Nat Compd 33:599–599. https://doi.org/10.1007/BF02254817
Khataee A, Movafeghi A, Mojaver N, Vafaei F, Tarrahi R, Dadpour MR (2017) Toxicity of copper oxide nanoparticles on Spirodela polyrrhiza: assessing physiological parameters. Res Chem Intermed 43:927–941. https://doi.org/10.1007/s11164-016-2674-9
Khodakovskaya MV, de Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6:2128–2135. https://doi.org/10.1021/nn204643g
Kim S, Lee S, Lee I (2012) Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut 223:2799–2806. https://doi.org/10.1007/s11270-011-1067-3
Krishnaraj C, Jagan E, Ramachandran R, Abirami S, Mohan N, Kalaichelvan P (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658. https://doi.org/10.1016/j.procbio.2012.01.006
Li A-R, Zhu Y, Li X-N, Tian X-J (2007) Antimicrobial activity of four species of Berberidaceae. Fitoterapia 78:379–381. https://doi.org/10.1016/j.fitote.2007.03.001
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220. https://doi.org/10.1093/jxb/erq282
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Nasrollahzadeh M, Maham M, Sajadi SM (2015) Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J Colloid Interface Sci 455:245–253. https://doi.org/10.1016/j.jcis.2015.05.045
Padil VVT, Černík M (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine 8:889–898. https://doi.org/10.2147/IJN.S40599
Peng C et al (2015) Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants. Environ Pollut 197:99–107. https://doi.org/10.1016/j.envpol.2014.12.008
Pietrowska-Borek M, Chadzinikolau T, Kozlowska M (2010) Effect of urban pollution on 4-coumarate: CoA ligase and flavonoid accumulation in Berberis thunbergii. Dendrobiology 64:79–85
Pissuwan D, Niidome T, Cortie MB (2011) The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release 149:65–71. https://doi.org/10.1016/j.jconrel.2009.12.006
Poborilova Z, Opatrilova R, Babula P (2013) Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ Exp Bot 91:1–11. https://doi.org/10.1016/j.envexpbot.2013.03.002
Prakash MG, Chung IM (2016) Determination of zinc oxide nanoparticles toxicity in root growth in wheat (Triticum aestivum L.) seedlings. Acta Biol Hung 67:286–296. https://doi.org/10.1556/018.67.2016.3.6
Quettier-Deleu C et al (2000) Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol 72:35–42. https://doi.org/10.1016/S0378-8741(00)00196-3
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167:1159–1169. https://doi.org/10.1016/j.plantsci.2004.06.016
Rico C, Peralta-Videa J, Gardea-Torresdey J (2015) Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. In: Siddiqui MH, Al-Whaib M (eds) Nanotechnology and plant sciences. Springer, Cham, pp 1–17
Saif S, Tahir A, Asim T, Chen Y (2016) Plant mediated green synthesis of CuO nanoparticles: comparison of toxicity of engineered and plant mediated CuO nanoparticles towards Daphnia magna. Nanomaterials 6:1–15. https://doi.org/10.3390/nano6110205
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93:906–915. https://doi.org/10.1016/j.chemosphere.2013.05.044
Shi J et al (2014) Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicology 8:179–188. https://doi.org/10.3109/17435390.2013.766768
Simon L, Fatrai Z, Jonas D, Matkovics B (1974) Study of peroxide metabolism enzymes during the development of Phaseolus vulgaris. Biochem Physiol Pflanz 166:387–392. https://doi.org/10.1016/S0015-3796(17)30073-2
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Sobkowiak R, Deckert J (2003) Cadmium-induced changes in growth and cell cycle gene expression in suspension-culture cells of soybean. Plant Physiol Biochem 41:767–772. https://doi.org/10.1016/S0981-9428(03)00101-3
Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, Lee EJ (2013) Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol Environ Saf 93:60–67. https://doi.org/10.1016/j.ecoenv.2013.03.033
Song G et al (2016) Effects of CuO nanoparticles on Lemna minor. Bot Stud 57:3. https://doi.org/10.1186/s40529-016-0118-x
Tarrahi R, Khataee A, Movafeghi A, Rezanejad F, Gohari G (2017) Toxicological implications of selenium nanoparticles with different coatings along with Se 4+ on Lemna minor. Chemosphere 181:655–665. https://doi.org/10.1016/j.chemosphere.2017.04.142
Tran TH, Nguyen VT (2014) Copper oxide nanomaterials prepared by solution methods, some properties, and potential applications: a brief review. Int Sch Res Notices. https://doi.org/10.1155/2014/856592
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Villinski J, Dumas E, Chai H-B, Pezzuto J, Angerhofer C, Gafner S (2003) Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm Biol 41:551–557. https://doi.org/10.1080/13880200390500768
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46:4434–4441. https://doi.org/10.1021/es204212z
Wang H et al (2013) Graphene-based materials: fabrication, characterization and application for the decontamination of wastewater and wastegas and hydrogen storage/generation. Adv Colloid Interface Sci 195:19–40. https://doi.org/10.1016/j.cis.2013.03.009
Winterbourn CC, McGrath BM, Carrell RW (1976) Reactions involving superoxide and normal and unstable haemoglobins. Biochem J 155:493–502. https://doi.org/10.1042/bj1550493
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535. https://doi.org/10.3389/fpls.2016.00535
Zhang Q et al (2014) CuO nanostructures: synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog Mater Sci 60:208–337. https://doi.org/10.1016/j.pmatsci.2013.09.003
Zhao J et al (2017) Uptake, distribution and transformation of CuO NPs in a floating plant Eichhornia crassipes and related stomatal responses. Environ Sci Technol 51:7686–7695. https://doi.org/10.1021/acs.est.7b01602
Acknowledgements
The authors thank the University of Tabriz (Iran) and the Hayyan Biotechnology Laboratory for scientific and financial supports.
Author information
Authors and Affiliations
Contributions
AM and SM was responsible for the design and overall investigation. BD helped in synthesis and analysis of nanoparticles. SM and MK-N conducted the cell culture experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. I. Beruto.
Rights and permissions
About this article
Cite this article
Mahjouri, S., Movafeghi, A., Divband, B. et al. Toxicity impacts of chemically and biologically synthesized CuO nanoparticles on cell suspension cultures of Nicotiana tabacum. Plant Cell Tiss Organ Cult 135, 223–234 (2018). https://doi.org/10.1007/s11240-018-1458-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1458-x