Abstract
During seaweed mariculture the stocking density increases with growth season, which would potentially affect the inorganic carbon availability for photosynthesis. In the present study, the red macroalgal species, Pyropia haitanensis, collected from the cultivation site located at Nan’ao Island, Shantou, China (23o20’N, 116o40’E), was cultured under high and low stocking density level (5 and 1 g L−1) and two different carbon concentrations (ca. 390 and 20 ppm CO2 in air), to investigate how the stocking density and carbon supply affect the growth and photosynthesis of this alga. The relative growth rate (RGR), nitrate reductase (NR) activity, and photosynthetic rates of high stocking density-grown P. haitanensis thalli were decreased compared with low stocking density-grown thalli. The lowered carbon supply in culture inhibited the RGR, NR activity, photosynthetic rates, and photosystem II activity of the algae. It was shown that high stocking density exhibited similar negative effect compared with reduction of carbon supply on the photosynthesis of P. haitanensis. Moreover, high stocking density aggravated the effect resulting from decreased carbon supply on photosynthesis. We proposed that it is necessary to strive to maintain the relative low stocking density to obtain a sustained high rate of biomass increase in P. haitanensis mariculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the nature, the biomass of seaweeds is highly related to stocking density (Demetropoulos and Langdon 2004). The growth of seaweeds is inhibited by low irradiance and dissolved growth substances in high-density algal mats; therefore, it will result in a balance between density and biomass (Scrosati 2005). Moreover, previous studies have shown that photosynthetic performance of seaweeds varied greatly among different densities (Binzer and Middelboe 2005; Richards et al. 2011). Increasing density of seaweeds reduced light saturation of maximum photosynthetic rate, light use efficiency, and dark respiration rate but significantly increased light saturation point (Binzer and Sand-Jensen 2002; Copertino et al. 2009; Richards et al. 2011). In some situations, a severe low dissolved inorganic carbon (Ci) concentration also occurs in the condition of high density and slow seawater exchange (Richards et al. 2011; Zou 2014; Jiang et al. 2016a, b). Seaweed cultivation beds are often subject to low carbon, as intense photosynthetic activity depletes dissolved Ci (DIC) in the water. The DIC content of natural seaweed vegetation bed was significantly higher (more than five times) than the content of seaweed farm (Chung et al. 2013). Several studies concerned the impacts of carbon limitation on seaweeds and showed that decreased CO2 in seawater increased pigments content (García-Sánchez et al. 1994; Mercado et al. 1999; Andría et al. 2001; Zou 2014; Jiang et al. 2016a, b), Rubisco content and carbonic anhydrase (CA) activity (García-Sánchez et al. 1994; Andría et al. 2001; Zou et al. 2003), but lowered the growth and photosynthesis (Mercado et al. 1999; Zou 2014; Jiang et al. 2016a, b).
Pyropia haitanensis (Bangiales, Rhodophyta) is the principal species in aquaculture along the coast of South China. Pyropia cultivations mainly adopt the pillar, semi-floating and all floating raft culture. Farming sites are selected in water flow rate is 10–30 cm s−1. At Shen’ao Bay, Nan’ao Island, Shantou, China, the local people choose pillar type to culture P. haitanensis. When the mean seawater temperature is 25–27 °C (usually in September), P. haitanensis conchospores are transplanted to polyethylene ropes, and the quantity of conchospores is ∼1.2 million m−2. The maximum density of young P. haitanensis thalli can reach up to ∼300 cm−1 before the polyethylene ropes are suspended at surface seawater layer. After P. haitanensis thalli grow to 3–6 cm, the maximum growth length can reach up 5 cm day−1. The maximum length of P. haitanensis will reach up to 25 cm, and biomass will up to ∼0.3 kg m−2 after 40 days since conchospores have been fixed. During P. haitanensis mariculture, the thalli probably experience lowered inorganic carbon and irradiance due to high mat density. Our previous study had found that under growth condition of decreased CO2 supply, the photosynthetic pigment contents of P. haitanensis were increased at lowered sunlight, while the growth rate and photosystem activity were decreased at relative high sunlight (Jiang et al. 2016a). Considering that P. haitanensis experience varied stocking density and lowered inorganic carbon supply in the field during mariculture, we cultured P. haitanensis outdoor with two different stocking densities and two different carbon supply levels in the present study. We specially paid attention to understand the effects of decreased carbon and increased stocking density on growth rate and photosynthetic capacity in P. haitanensis, thereby getting some knowledge about the response and acclimation behaviors of the algal growth and photosynthesis to varied mat density and its synergically effect with low carbon level.
Materials and methods
Thalli of Pyropia haitanensis TJ Chang et BF Zheng were collected during December 2014 from a cultivation field at the Shen’ao Bay, Nan’ao Island, Shantou, China (23° 20′ N, 116° 40′ E). The thalli used in the experiment were 5 to 8 cm long. The thalli were gently rinsed to remove sediments and epiphytes. Only unwounded and healthy thalli were selected. Samples were transported to the laboratory in a plastic bucket with some seawater (at ca. 4 °C) and were maintained in plexiglass aquaria with filtered natural seawater (salinity ca. 33 psu) at 20 ± 0.5 °C. The algae received an irradiance of about 100 μmol photons m−2 s−1 (PAR) with a 12 h/12 h of light/dark period. The seawater was continuously aerated and renewed every day. The thalli used for experimental treatments were acclimated for 2 days in the above conditions. The irradiance was quantified by means of a quantum sensor (QSL2100, USA).
Culture treatments
The thalli were cultured at two inorganic carbon (Ci) concentrations and two levels of stocking density. For the treatment of ambient carbon supply (AC), the culture seawater was aerated continuously with ambient air (the CO2 concentration in the air was ca. 390 ppm; the seawater contain ca. 12.8 μM CO2, 2495 μM HCO3 −, 370 μM CO3 2−). For the decreased carbon (DC) treatment, the culture media had low Ci seawater and were aerated continuously with ambient air through 5 M NaOH solution (the CO2 concentration in the air was ca. 20 ppm, Zou et al. 2003; the seawater contain ca. 0.7 μM CO2, 405 μM HCO3 −, 190 μM CO3 2−). The low Ci seawater was prepared from Ci-free seawater as described as below. Ci was removed from the sterilized natural seawater by reducing pH to less than 4.0 with the addition of 0.5 M HCl, and then sparging with high purity N2 gas for at least 5 h. Finally, the pH in the seawater was adjusted to 8.2 with freshly prepared 0.5 M NaOH solution (Zou 2014; Jiang et al. 2016b). The treatment of high density (HD) means that stocking density was 5 g L−1 (FW), and the treatment of low density (LD) means that stocking density was 1 g L−1 (FW). Three replicate cultures were carried out for each growth treatment.
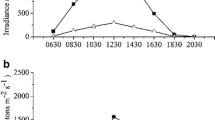
Seaweeds were inoculated in 12 jars (diameter is 21 cm) containing 10 L of filtered seawater cultured under open conditions. The thalli were kept in suspension by continuous sparging and aeration at 0.4 L min−1, which was continuously controlled using the flowmeter (Huanming LZB-3; Yuyao KingTai Instrument Co., Ltd., Yuyao, China). The photon irradiance conditions in the algal mats were measured at 2 h intervals between 06:30 and 20:30 in a typical day during the culture. The values of irradiance were at their maximum about 12:30, and the maximum value of HD and LD was about 699 and 827 μmol photons m−2 s−1, respectively (Fig. 1). For all of the treatments, the culture seawater was supplemented 20 μM H2PO4 − and 100 μM NO3 −. The seawater was replaced every day. The fresh medium had been pre-aerated with the air containing the appropriate [CO2] for at least 5 h. The algal thalli were harvested for use after 18 days of cultures. The daily mean temperature of the seawater was ca. 19 °C during the period of cultivation.
Growth rates
Biomass was measured in each culture to estimate growth. The relative growth rate (RGR), expressed as percentage of increase in fresh weight (FW) biomass per day (% day−1), was estimated assuming exponential growth during the culture period according to the formula: RGR = [(lnW t − lnW 0)/t] × 100, where W 0 represents the initial and W t the final FW of the algae, and t is the time of culture in days. The algal samples were softly blotted the excess water on filter papers before FW weighting.
Biochemical components
To determine pigment contents, about 0.1 g FW per sample were extracted in 100% methanol with mortar and pestle. The crude extracts were extracted at 4 °C in darkness for 24 h. The extract was centrifuged at 5000×g for 10 min, and then the supernatant was used to determine the contents of chlorophyll a (Chl a) and carotenoid (Car) with an ultraviolet spectrophotometer (UV-1800; Shimadzu, Japan). The concentrations of Chl a and Car were calculated spectrophotometrically using the equation by Porra (2005) and Parsons and Strickland (1963). To determine phycoerythrin (PE) and phycocyanin (PC), about 0.2 g FW per sample were extracted in 0.1 M phosphate buffer (pH 6.8) at 4 °C with mortar and pestle. The extracts were then centrifuged at 10,000×g for 20 min, and then the supernatant was used to determine the contents of PE and PC with an ultraviolet spectrophotometer (UV-1800; Shimadzu, Japan). The PE and PC contents were quantified according to Beer and Eshel (1985).
Nitrate reductase activity
Nitrate reductase (NR) activity was assayed using fresh material directly from the cultures according to the in situ procedure of Corzo and Niell (1991). The algal samples were collected 6–8 h into the light period, and the measurement was carried out immediately (Zou 2005). Approximately 0.15 g of tissue was placed in 10 mL of incubation medium. The final composition of the incubation medium was 0.1 M phosphate buffer (pH 7.5), 0.5 mM Na-EDTA, 0.1% 1-propanol, 50 mM NaNO3, and 0.01 mM glucose. N2 flushing for 2 min was performed after introducing the sample. Then, the sample was incubated in the dark at 30 °C for 30 min. Two milliliter of the supernatant was transferred into a colorimetric tube, and 1 mL of sulfonamide reagent (1%) and 1 mL of hydrochloric acid naphthalene ethylenediamine reagent (0.1%) were added. After 15 min, the mixture was used to determine the contents of NO2 −-N using an ultraviolet spectrophotometer at 543 nm wavelength. Each sample had three replicates. NR activity was expressed in micromoles NO2 − per gram per hour.
Chlorophyll fluorescence
Measurements of chlorophyll fluorescence were made using a Pulse-amplitude modulated fluorometer (Junior-PAM, Walz, Germany). The maximal quantum yield of photosystem II (PS II) of P. haitanensis thalli (dark-adapted for 10 min) was determined as F v /F m , where F v indicates variable fluorescence (F v = F m − F o ) (Cosgrove and Borowitzka 2011). A saturating pulse of white light (approx. 3000 μmol photons m−2 s−1) was applied to obtain the maximal fluorescence, F m (the fluorescence yield when all the PS II reaction centers are reduced). The initial fluorescence, F o (fluorescence intensity with all PS II reaction centers open, while the photosynthetic membrane is in the non-energized state), was obtained at a pulsed irradiance of approximately 0.1 μmol photons m−2 s−1. The rapid light curves (RLCs) consisted of the fluorescence response to eight different and increasing actinic irradiance levels as in our previous study. Relative electron transport rate (ETR, μmol electrons m−2 s−1) at a given actinic irradiance was estimated as ETR = Y× PAR × 0.84 × 0.5, where Y is the effective quantum yield, PAR is the actinic irradiance (μmol photons m−2 s−1), 0.84 is the default value for the PSII absorption factor, and 0.5 is the fraction of incident PAR absorbed by chlorophyll associated to PSII. The parameters of the RLCs were calculated following the model equation from Jassby and Platt (1976): rETR = rETRm × tanh (α × I/rETRm), where rETR m is the saturated maximum relative electron transport rate, α is the initial slope of the RLCs, and I is the incident irradiance. Non-photochemical quenching indicating the fluorescence intensity with all PS II reaction centers closed in any light adapted state was determined from the equation from Bilger and Björkman (1990): NPQ = F m /F m ′ − 1, where F m ′ is the maximal yield in a saturation pulse during actinic illumination.
Photosynthetic oxygen evolution
The rate of respiration and net photosynthesis of P. haitanensis thalli were determined with the Clark-type oxygen electrode (YSI Model 5300, USA) at 20 °C. The P. haitanensis thalli were cut into small segments (ca. 0.3 × 0.3 cm), and then these pieces were incubated in 100 μmol photons m−2 s−1 for at least 1 h to minimize the effect of cutting damage. The mediums were the outdoor culture seawater, respectively, and aerated with carbon levels were same as the experiment treatment. The 50 mg algal samples were allowed to equilibrate for about 15 min before the respiration, and net photosynthetic rates were measured. Respiration was recorded when the oxygen uptake stabilized, usually within 5–10 min. Immediately following the respiration measurement, the irradiance-saturated net photosynthetic rates were determined at 500 μmol photons m−2 s−1 (Zou and Gao 2002).
To obtain the photosynthetic rate versus Ci concentration relationship (P-C curve), a sample of about 50 mg FW thalli segments was incubated in the electrode chamber containing 8 mL Ci-free seawater until no further O2 evolved. The Ci-free seawater was prepared prior to the measurements according to our previous studies (Zou 2014). Ci was removed from the sterilized nature seawater by reducing pH to less than pH 4.0 with the addition of 0.5 M HCl and then sparged with high-purity N2 gas for at least 5 h. Finally, TRIS buffer was added to give a final concentration of 25 mM, and the pH in the seawater was adjusted to 8.2 again with freshly prepared 0.5 M NaOH solution. Different aliquots of 50–200 mM NaHCO3 stock solution were then injected into the chamber, to create different Ci concentrations as desired. For the P-C parameters, the Ci-saturated maximum rate of photosynthesis (V max) and the concentration of Ci (K 1/2(Ci)) supporting half of V max were estimated from double reciprocal plots of the rates of O2 evolution and the Ci concentrations.
Statistical analyses
The data were expressed as the means ± standard deviation (S.D., n ≥ 3). Statistical significance of the data was tested with analysis of variance (ANOVA) or t test using SPSS for Window version 19.0. Tests for normality and homogeneity of variance were performed to check assumptions of parametric analysis. The significant level was set at P < 0.05.
Results
Growth rates
The mean relative growth rate (RGR) of Porphyra haitanensis grown at high stocking density reduced by ca. 68% (under ambient carbon supply) and ca. 54% (under decreased carbon supply), respectively, compared with the rate of algae grown at low stocking density. Under the low-density condition, the RGR of P. haitanensis thalli grown at decreased carbon was significantly lowered (reduced by ca. 37%) compared with the thalli grown at ambient carbon. Moreover, when P. haitanensis thalli cultured at high density level, the mean of RGR was similar between the low carbon-grown thalli and ambient carbon-grown thalli (P > 0.05) (Fig. 2).
Biochemical composition
Regardless of the carbon concentrations, the Chl a, Car, PE, and PC contents of P. haitanensis thalli were similar between the thalli grown at low and high densities (P > 0.05). P. haitanensis thalli showed similar (P > 0.05) Car contents when the thalli grown at the two different carbon levels; however, the Chl a, PE, and PC contents of decreased carbon-grown thalli were higher than ambient carbon-grown thalli (P < 0.05), irrespective of the stocking densities (Table 1).
Nitrate reductase activity
The nitrate reductase (NR) activity exhibited a lowered value (P < 0.05) in the thalli cultured at high density than at low density. Additionally, NR activity displayed a lowered value (P < 0.05) in the thalli cultured at decreased carbon than at ambient carbon (Fig. 3).
Chlorophyll fluorescence characteristics
Figure 4 shows that the rapid light curves (RLCs) in P. haitanensis thalli differed clearly with different growth treatments. The maximum quantum yield of photosystem II (F v /F m ) and non-photochemical quenching (NPQ) were similar between thalli grown at low and high stocking densities (P > 0.05). However, the initial slope of the RLC (α) and maximum relative electron transport rate (rETRm) of high stocking density-grown thalli were significantly reduced compared with low density-grown thalli (P < 0.01), when P. haitanensis thalli grown at decreased carbon condition. The F v /F m value of the algae grown at decreased carbon was reduced (P < 0.05) relative to the value of ambient carbon-grown algae; however, the NPQ value of decreased carbon-grown thalli was greater (P < 0.05) than the value of ambient carbon-grown thalli, irrespective of the stocking densities (Table 2).
Relative electron transport rates (rETR) as a function of actinic irradiance (rapid light curves, RLCs) of Pyropia haitanensis grown at ambient carbon supply and decreased carbon supply seawater and at high stocking density (a) and low stocking density (b). Vertical bar represents ±SD of the means (n = 6)
Photosynthetic responses
Table 3 shows the net photosynthetic (P n ) and respiratory (R d ) rates of P. haitanensis thalli grown at different stocking density and carbon supply conditions. Under the two carbon conditions, the P n in the thalli grown at low density was higher (P < 0.05) than the thalli grown at high density; however, the high stocking density reduced (P < 0.05) the R d . The P n of ambient carbon-grown algae was significantly higher (P < 0.05) than the rate of decreased carbon-grown algae; however, the R d was significantly reduced (P < 0.05) in decreased carbon-grown algae compared with ambient carbon-grown algae, irrespective of the stocking densities.
The photosynthetic responses to Ci concentration (P-C curves) are shown in Fig. 5, and the parameters of P-C curves are presented in Table 4. The curves of the algae cultured under different stocking density and carbon supply conditions were highest in ambient carbon with regard to low density. Under the two carbon conditions, the maximum Ci-saturated photosynthetic rate (V max) of algae grown at low stocking density was much higher (P < 0.05) than algal thalli grown at high stocking density. Under the low stocking density condition, the V max of algae grown at ambient carbon was significantly greater (P < 0.01) than the rate of algae grown at decreased carbon supply. The values of the apparent half-saturating Ci concentration (K 1/2 (Ci)) were similar (P > 0.05) among all the stocking density and carbon supply growth conditions.
The P n , R d , and V max of P. haitanensis thalli grown at high stocking density and ambient carbon condition were similar (P > 0.05) relative to the thalli grown at low density and decreased carbon condition. Moreover, the P n of the thalli grown at high stocking density and decreased carbon was the lowest among the four different treatments (Tables 3 and 4).
Discussion
Growth and biochemical components
Population density acts as an ecological factor affecting the algal growth. The present results showed that the relative growth rates (RGR) were reduced in Porphyra haitanensis grown at high stocking density relative to the algae grown at low stocking density. Although the RGR of low density treatments with more than twice higher that in high density treatments, the final densities were significantly different between low density (ca. 1.6 g L−1) and high density (ca. 5.9 g L−1). The previous studies had also reported that density was negatively correlated with growth in a red alga (Kappaphycus striatum; Hurtado et al. 2008) and a green alga (Ulva rigida; Viaroli et al. 1996). High-density populations may form diffusion boundary layers (DBL) that can reduce the NO3 − to algal thalli surfaces (Hurd 2000; Richards et al. 2011). Moreover, the thalli grown at high density displayed lowered photosynthetic rates. Therefore, the DBL and decreased photosynthetic rates were factors to inhibit RGR of P. haitanensis grown at high stocking density.
When the CO2 level for aeration in culture was lowered, the RGR was considerably decreased. In previous studies, the reduction of RGR in low carbon condition had been reported in other macroalgal species (García-Sánchez et al. 1994; Andría et al. 2001; Zou 2014; Jiang et al. 2016a, b). When carbon level was decreased in seawater, the lowered RGR was related to the lack of sufficient carbon for growth under severe Ci limitation. Such a decrease of growth was also associated with low photosynthetic rates of the algae grown at low carbon supply.
The present results showed that the photosynthetic pigment (chlorophyll a, carotenoids, phycoerythrin, and phycocyanin) contents of P. haitanensis were similar between algae grown at high and low stocking densities. Generally, the different grown conditions exist in varied algal mats density, and even these can result in changed physiology of algae. Therefore, the results indicated that the mechanisms for photosynthetic pigment processes of P. haitanensis were not activated under the high stocking density.
The cellular components are affected by the carbon dissolving levels in seawater. The present result showed that the decreased carbon level in seawater increased photosynthetic pigment contents of P. haitanensis thalli. The electron flow between PS II and PS I and carbon fixation pathway is related to the concentration of photosynthetic units and their minimal turnover time (Falkowski and Raven 1997). In this study, the increased photosynthetic pigment contents of the thalli would be beneficial to compensate for a part of the decreased electron transport in the low carbon condition. Thereby, the results provide evidence that P. haitanensis thalli increased the pigment contents in an effort to acclimate to the decreased carbon condition. These results agreed with previous studies in other macroalgal species which showed that the pigment contents were increased by the low carbon growth condition (García-Sánchez et al. 1994; Huertas et al. 2000; Andría et al. 2001; Zou 2014; Jiang et al. 2016a, b).
Nitrate reductase activity
When the stocking density was increased, the activity of nitrate reductase (NR) activity of algae was considerably reduced. It might suggest that high stocking density reduced the capacity of nitrogen metabolism, which would be a reason for explaining lowered RGR of P. haitanensis grown at high density. Light is an important factor involved in NR regulation (Lillo 1994). Therefore, the lowered NR activity might be related to relative low light level in high stocking density algal mats.
The present results showed that the decrease of carbon reduced the activity of NR. Similar result was found in Grateloupia livida (Jiang et al. 2016b). Furthermore, the opposite pattern was obtained regarding the high levels of carbon. For example, the activity of NR was increased in Porphyra leucosticta (Mercado et al. 1999), Hizikia fusiforme (Zou 2005), and Ulva rigida (Gordillo et al. 2001) grown at high levels of CO2 compared with the activity of those algae grown at normal CO2 level. The results implied that the reduction of carbon level (decreased carbon supply and/or increased consumption of Ci in high density) in seawater had an adverse effect on activity of NR of P. haitanensis.
Photosynthetic characteristics
The present results showed that the algae grown at high stocking density reduced the net photosynthetic rates (P n ), maximum relative electron transport rate (rETRm), and respiration rates (R d ). The similar results had also been shown in previous studies, where the maximum rate of photosynthesis and apparent photosynthetic efficiency were decreased by the high density in Undaria pinnatifida, Cystophora scalaris, Xiphophora gladiata (Richards et al. 2011), and turf algae (Copertino et al. 2009). When P. haitanensis thalli were cultured at high stocking density, Ci and irradiance in culture seawater would be decreased. The photosynthetic pigments were similar between the algae grown at high stocking density and low stocking density, implying that the different stocking densities had no effects on the concentration of light-harvesting pigments and the photosystem II reaction center densities. However, our previous study found that the photosynthetic system activity of P. haitanensis grown at low irradiance was increased through promoting the synthesis of photosynthetic pigments (Jiang et al. 2016a). Therefore, we speculated that the delicate irradiance differences (the maximum value was ca. 15%) between the high and low stocking density could not influence the photosynthesis of P. haitanensis. Moreover, the DBL in high density algal mats reduced the flux of O2 and dissolved Ci to and from algal thalli surfaces, and then these resulted in lower photosynthetic performance compared with the individuals (Richards et al. 2011). Therefore, these suggested that probable inorganic carbon shortage and DBL effect in high populations were factors to inhibit photosynthesis of P. haitanensis.
The maximum quantum yield of photosystem II (PS II; F v /F m ) and P n of reduced carbon-grown P. haitanensis thalli were reduced, which might be caused by the inhibition of PS II activity. The result was consistent with the observation of Zou (2014) and Jiang et al. (2016a, b), where the F v /F m and F v ′/F m ′ were decreased under the low carbon condition in macroalgae. In the course of carbon starvation, the endogenous electron acceptors reduce and oxygen becomes the main available electron acceptor. At the same time, oxygen also can act as electron acceptor in photorespiration and the Mehler reaction (Durchan et al. 2001). The activity of PS II and photosynthetic rates will be inhibited by the active oxygen radicals (e.g. superoxide, hydroxyl radicals, and hydrogen peroxide) which produce in the Mehler reaction. When carbon limitation occurs in seawater, the more photosynthetic pigments of algae absorb more light than can be utilized by carbon assimilation. However, the excess energy will be dissipated by the increased non-photochemical quenching (NPQ) in algae.
Under high stocking density and lowered carbon conditions, the maximum Ci-saturated photosynthetic rates (V max) of P. haitanensis thalli were decreased. However, the apparent half-saturating Ci concentrations (K 1/2 (Ci)) of the thalli grown at the four different culture conditions were similar. It was also reported that the photosynthetic rates were reduced at decreased carbon in U. conglobata (Zou 2014), Gracilaria sp. (Mercado et al. 1999), and P. leucosticta (Andría et al. 2001). Our previous studies had demonstrated that photosynthesis of P. haitanensis depended on the external carbonic anhydrase (CA) activity that catalyzed dehydration of extracellularly HCO3 − to CO2 and then that formed CO2 is taken up into the cells. There were two major steps in P. haitanensis thalli involved in the supply of CO2 to Rubisco from the external pool of HCO3 −: first, the external conversion of HCO3 − into CO2 by external CA, and second, the transport of Ci within the cell close to the carboxylating site of Rubisco for final photosynthetic CO2 fixation (Zou and Gao 2002). Moreover, it had reported that internal CA is involved in Ci transport processes inside the algal cell (Sültemeyer 1998; Zou and Gao 2002). Therefore, the lower photosynthesis rates of thalli grown at high stocking density and lowered carbon supply might relate to reduction of potential of Rubisco carboxylating. In the present study, P. haitanensis thalli exhibited similar K 1/2 (Ci) values between the algal thalli cultured at high stocking density and the thalli cultured at lowered carbon. This suggested that the different culture environments had no effects on Ci affinity of the algal thalli and also implied that the capacities of using the external HCO3 − and the transport of Ci toward Rubisco within the cell were likely to remain similar levels under high density and low carbon supply condition. However, the activities of CA (including extracellular and internal activities) and Rubisco carboxylating capacity of the P. haitanensis thalli are yet to be addressed.
In the present study, the P n and V max were similar between the thalli grown at high stocking density with ambient carbon conditions and low density with decreased carbon supply. This result indicated that high stocking density populations exhibited similar negative effect compared with reduction of carbon supply on the photosynthesis of P. haitanensis. When carbon supply was decreased in seawater, the P n of the thalli grown at high density was lowest among the different treatments, implying that lowered carbon and high density existed synergistic inhibition on the photosynthetic rates. In the nature sea area, the biomass and photosynthesis of this important economical alga would be lessened in seawater with decreased availability of Ci, which could occur in cultivation with high stocking density and reduced seawater circulation.
In conclusion, the data obtained from the RGR, photosynthetic rates, and photosystem II activity of P. haitanensis support that the growth and photosynthesis of the algae were depressed with either the high stocking density or decreased carbon conditions. Additionally, the decreased carbon supply and the simultaneous high stocking density aggravated the depression effects of growth and photosynthetic rates in P. haitanensis. Therefore, we propose that more labor would be required to choose relative fast seawater exchange of cultivation site and timely reduce the density during mariculture.
References
Andría JR, Brun FG, Pérez-Lloréns JL, Vergara JJ (2001) Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot Mar 44:361–370
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar Freshw Res 36:785–792
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Binzer T, Middelboe AL (2005) From thallus to communities: scale effects and photosynthetic performance in macroalgae communities. Mar Ecol Prog Ser 287:65–75
Binzer T, Sand-Jensen K (2002) Importance of structure and density of macroalgae communities (Fucus serratus) for photosynthetic production and light utilization. Mar Ecol Prog Ser 235:53–62
Chung IK, Oak JH, Lee JA, Shin JA, Kim GJ, Park KS (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES J Mar Sci 11:1–7
Copertino MS, Cheshire A, Kildea T (2009) Photophysiology of a turf algal community: integrated responses to ambient light and standing biomass. J Phycol 45:324–336
Corzo A, Niell FX (1991) Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J Exp Mar Biol Ecol 146:181–191
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Demetropoulos CL, Langdon CJ (2004) Enhanced production of Pacific dulse (Palmaria mollis) for co-culture with abalone in a land-based system: effects of stocking density, light, salinity, and temperature. Aquaculture 235:471–488
Durchan M, Vacha F, Krieger-Liszkay A (2001) Effects of severe CO2 starvation on the photosynthetic electron transport chain in tobacco plants. Photosynth Res 68:203–213
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell Science, Malden, pp 128–135
García-Sánchez MJ, Fernândez JA, Niell FX (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194:55–61
Gordillo FJL, Niell FX, Figueroa FL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 21:364–370
Huertas E, Montero O, Lubián LM (2000) Effects of dissolved inorganic carbon availability on growth, nutrient uptake and chlorophyll fluorescence of two species of marine microalgae. Aquac Eng 22:181–197
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Hurtado AQ, Critchley AT, Trespoey A, Bleicher-Lhonneur G (2008) Growth and carrageenan quality of Kappaphycus striatum var. sacol grown at different stocking densities, duration of culture and depth. J Appl Phycol 20:551–555
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Oceanography 21:540–547
Jiang H, Zou DH, Chen BB (2016a) Effects of lowered carbon supplies on two farmed red seaweeds, Pyropia haitanensis (Bangiales) and Gracilaria lemaneiformis (Gracilariales), grown under different sunlight conditions. J Appl Phycol 28:3469–3477
Jiang H, Zou DH, Li XH (2016b) Growth, photosynthesis and nutrient uptake by Grateloupia livida (Halymeniales, Rhodophyta) in response to different carbon levels. Phycologia 55:462–468
Lillo C (1994) Light regulation of nitrate reductase in green leaves of higher plants. Plant Physiol 90:616–620
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equation for ascertaining chlorophylls and carotenoids. J Mar Res 21:155–163
Porra RJ (2005) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Richards DK, Hurd CL, Pritchard DW, Wing SR, Hepburn CD (2011) Photosynthetic response of monospecific macroalgal stands to density. Aquat Biol 13:41–49
Scrosati R (2005) Review of studies on biomass-density relationships (including self-thinning lines) in seaweeds: main contributions and persisting misconceptions. Phycol Res 53:224–233
Sültemeyer DF (1998) Carbonic anhydrase in eukaryotic algae: characteristics, regulation, and possible function during photosynthesis. Can J Bot 76:962–972
Viaroli P, Naldi M, Bondavalli C, Bencivelli S (1996) Growth of the seaweed Ulva rigida C. Agardh in relation to biomass densities, internal nutrient pools and external nutrient supply in the Sacca di Goro lagoon (northern Italy). Hydrobiologia 329:93–103
Zou DH (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou DH (2014) The effects of severe carbon limitation on the green seaweed, Ulva conglobata (Chlorophyta). J Appl Phycol 26:2417–2424
Zou DH, Gao KS (2002) Photosynthetic bicarbonate utilization in Porphyra haitanensis (Bangiales, Rhodophyta). Chin Sci Bull 47:1629–1633
Zou DH, Gao KS, Xia JR (2003) Photosynthetic utilization of inorganic carbon the economic brown algae, Hizikia Fusiforme (Sargassaceae) from the south China Sea. J Phycol 39:1095–1100
Acknowledgements
This study was supported by the Science and Technology Planning Project of Guangdong (2015A020216004) and the National Natural Science Foundation of China (No. 41276148 and 31370476). The authors would like to thank Yayun Deng and Jiejun Zhang for assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, H., Zou, D., Lou, W. et al. Effects of stocking density and decreased carbon supply on the growth and photosynthesis in the farmed seaweed, Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 29, 3057–3065 (2017). https://doi.org/10.1007/s10811-017-1174-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1174-7