Abstract
Growth and photosynthesis of cultivated seaweeds usually suffer carbon limitation during cultivation in the field. Pyropia haitanensis and Gracilaria lemaneiformis, collected from Nan’ao Island, Shantou, China, were cultured under ambient carbon and decreased carbon supply, with ambient sunlight and decreased sunlight conditions, aiming to investigate how the decreased carbon supply and sunlight conditions affect growth and photosynthesis in these two maricultured seaweed species. Decreased carbon supply significantly lowered the relative growth rate (RGR), quantum efficiency of open PS II (F v′/F m′), maximum photosynthetic electron transport rate (rETRm), and NO3 − uptake rate in both of the two seaweeds. Under ambient sunlight condition, the RGR of the P. haitanensis and G. lemaneiformis grown at decreased carbon supply was reduced about 83 and 95 %, respectively, compared with the algae grown at ambient carbon condition. The RGR, F v′/F m′, and NO3 − uptake rate were higher in P. haitanensis but were lower in G. lemaneiformis, with under decreased sunlight compared to ambient sunlight. The results indicated that decreased carbon supply reduced growth and PS II activity in both of the seaweeds, with the reduction being greater in G. lemaneiformis than in P. haitanensis. Additionally, G. lemaneiformis was adapted to grow at relative higher light conditions than P. haitanensis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentrations have been rising since the industrial revolution. It is anticipated that increasing atmospheric CO2 will lead to a linear proportional increase in the dissolved CO2 concentrations and a concomitant reduction of pH (seawater acidification) in near-shore areas, as a consequence of the continuous gas exchange between the air and seawater. Extensive attention has been paid to the influences of increasing atmospheric CO2 concentrations and/or seawater acidification on the physiology and ecology of seaweeds (e.g., Zou and Gao 2010; Harley et al. 2012; Koch et al. 2013). However, the realistic situation is that low dissolved inorganic carbon (DIC) concentrations in seawater would be of frequent occurrence, especially under the conditions of slow water exchanges, high standing stock, and large seaweed density (Friedlander and Levy 1995; Israel and Friedlander 1998; Richards et al. 2011; Zou 2014). Seaweed cultivation beds are often subject to low carbon as intense photosynthetic activity depletes DIC in the water. The highest difference in DIC content between a seaweed farm and natural seaweed vegetation bed was more than five times (Chung et al. 2013). In some seaweed species, it has been reported that as carbon level was decreased in seawater, pigment contents (García-Sânchez et al. 1994; Mercado et al. 1999; Andría et al. 2001; Zou 2014), Rubisco content, and carbonic anhydrase (CA) activity were increased (García-Sânchez et al. 1994; Andría et al. 2001), and photosynthetic activity was reduced (Mercado et al. 1999; Zou 2014).
Generally, there is usually an excess of light energy reaching the photosynthetic apparatus, and different mechanisms have evolved to protect the seaweeds from such excess energy (photoprotection). However, seaweed thalli also experience low irradiance as light is sharply attenuated by seawater and the sinking of thalli to deeper water and self-shading due to high stocking density. Seaweeds can improve the capability of acclimation to low-light growth condition through adjusting photosynthetic activity, biosynthetic processes, and absorption of nutrients (Lopes et al. 1997; Talarico and Maranzana 2000; Zou and Gao 2009). In general, the irradiance changes with the tide and weather, and the photosynthetic performance of seaweeds is related to these variations in light intensity. Diurnal variation of photosynthesis of algae has been extensively studied in the natural environment, with the main focus on diurnal variation of algal photosynthesis in natural light (Magnusson 1997; Beer et al. 2006; Serôdio et al. 2008), different light quality (Sagert et al. 1997; McMinn et al. 2003), and different growth depth (Sagert et al. 1997; Campbell et al. 2008). However, much less is understood on the diurnal variation of photosynthesis in seaweeds grown under different sunlight and carbon supply conditions.
Pyropia haitanensis (Bangiales, Rhodophyta) and Gracilaria lemaneiformis (Gracilariales, Rhodophyta) are the most two important species for seaweed cultivation in South China. Pyropia haitanensis is used for human food, and G. lemaneiformis is commonly used for high-quality raw material in the agar industry and also for human food. Moreover, the cultivation of those two species can be an effective bioremediation measure for eutrophication control in coastal waters (Fei 2004; Yang et al. 2005, 2015). The characteristics of photosynthetic inorganic carbon (Ci) utilization in these two algae have been investigated (Zou and Gao 2002; Zou et al. 2004). The results showed that P. haitanensis and G. lemaneiformis mainly used HCO3 − as its carbon source via external CA. Increasing CO2 concentration improved photosynthetic capacity of P. haitanensis when exposed to air during low tide. CO2 concentration had a weaker effect than light levels on the photosynthesis performance and growth of G. lemaneiformis (Zou and Gao 2009).
Pyropia haitanensis and G. lemaneiformis when cultivated in the bay usually experience decreased CO2 supply due to slow water exchange. Additionally, the two seaweed species also experience decreased irradiance due to self-shading and/or high tide. Therefore, it is interesting to examine how these two maricultured seaweeds respond to low carbon supply when growing at different sunlight levels. In the present study, we cultured P. haitanensis and G. lemaneiformis outdoor with different carbon supply and sunlight availability. We specially focused on (1) growth and biochemical composition, (2) photosynthetic characteristics, and (3) how N uptake respond to decreased carbon supplied on these two seaweeds under different sunlight conditions.
Materials and methods
Thalli of P. haitanensis and G. lemaneiformis were collected from a cultivation field at the Shen’ao Bay, Nan’ao Island, Shantou, China (23° 20′ N, 116° 40′ E) during December 2013 and March 2014, respectively. Thalli were artificially cultivated by means of the pole system. The thalli were gently rinsed to remove sediments and epiphytes. Only unwounded and healthy thalli were selected. Samples were transported to the laboratory in a plastic bucket with some seawater (temperature ca. 4 °C) and were maintained in plexiglass aquaria with filtered natural seawater (salinity ca. 33 PSU) at 20 ± 0.5 °C. The two seaweeds received an irradiance of about 100 μmol photons m−2 s−1 (PAR, LD cycle 12:12 h). The seawater was continuously aerated and renewed every day. The thalli were used for experimental treatments after 2 days of above laboratory maintenance (Zou 2014). The irradiance was quantified by means of a quantum sensor (QSL2100, USA).
Culture treatments
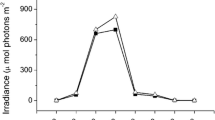
The thalli were cultured outdoors with two levels of inorganic carbon (Ci) availability and two levels of irradiance in the following four treatments: (1) ambient carbon supply + ambient sunlight, (2) decreased carbon supply + ambient sunlight, (3) ambient carbon supply + decreased sunlight, and (4) decreased carbon supply + decreased sunlight. For the ambient carbon (AC) treatment, the seawater was aerated continuously with ambient air (the CO2 concentration in the air was ca. 390 ppm; containing ca. 11.5 μM CO2, 1780 μM HCO3 −, 210 μM CO3 2−). For the low carbon (DC) treatment, the culture media had low Ci seawater (containing ca. 0.1 mM Ci) and were aerated continuously with air through a 5 M NaOH solution (the CO2 concentration in the air was ca. 50 ppm; Zou 2014; containing ca. 1.6 μM CO2, 800 μM HCO3 −, 300 μM CO3 2−). The low Ci seawater was prepared from Ci-free seawater as described below. Ci was removed from sterilized natural seawater by reducing pH to less than 4.0 with the addition of 0.5 M HCl and then sparging with high purity N2 gas for at least 5 h. Finally, the pH in the seawater was adjusted to pH 8.1 with freshly prepared 0.5 M NaOH solution. For the treatment of decreased sunlight (DL), the culture vessels were wrapped with sunshade net, and for the treatment of ambient sunlight (AL), nothing wrapped around the culture vessel. The irradiance of DL treatment was about 20 % of ambient sunlight in the culture vessel during daytime. The irradiance under the algal thalli were grown was measured at 2-h intervals between 06:30 and 20:30 in a typical day during the culture. The irradiances were at their maximum about 12:30, and the maximum value for P. haitanensis and G. lemaneiformis was about 1400 and 1563 μmol photons m−2 s−1, respectively (Fig. 1). For all of the treatments, the culture seawater was supplemented 40 μM H2PO4 − and 150 μM NO3 − to avoid the possible nutrient limitation. Three replicate cultures were maintained for each growth treatment.
Experimental treatments started when 20 g fresh weight (FW) algae were introduced into each of jars containing 10-L filtered seawater and cultured under outdoor conditions. The water motion resulting from the aeration allowed the algae to move gently without tumbling. The Ci concentrations of the culture media were examined periodically with a Total Organic Carbon Analyzer (TOC-5000A, Shimadzu, Japan), to ensure that the desired Ci level in the culture seawater for experimental treatment was right. The algal thalli were grown under these different carbon and irradiance regimes for 15 days and then were harvested to be used for experimental measurements.
Growth rates
Biomass was measured to estimate growth. The relative growth rate (RGR), expressed as percentage of increase in FW biomass per day (% day−1), was estimated assuming exponential growth during the culture period according to the formula: RGR = [(lnWt − lnW0) / t] × 100, where W 0 is the initial and W t the final FW of the algae, and t is the time of culture in days. The algal samples were softly blotted to remove excess water on filter papers before FW weighing.
Biochemical components
To determine pigment contents, about 0.1 g FW per sample was extracted in 100 % methanol. The concentration of chlorophyll a (Chl a) and carotenoids (Car) was calculated spectrophotometrically using the equations given by Porra (2005) and Parsons and Strickland (1963). To determine phycoerythrin (PE) and phycocyanin (PC), about 0.2 g FW per sample was extracted in 0.1 M phosphate buffer (pH 6.8). The extracts were then centrifuged at 10,000×g for 20 min. PE and PC contents in the supernatants were quantified according to Beer and Eshel (1985). For soluble protein (SP) determination, samples of about 0.2 g FW of algal biomass was homogenized with 0.1 M phosphate buffer (pH 6.8). The extracts were then centrifuged at 5000×g for 20 min. SP contents in the supernatants were quantified according to the method of Bradford (1976).
The diurnal variation of chlorophyll fluorescence
The chlorophyll fluorescence of the P. haitanensis and G. lemaneiformis was monitored at regular interval (2 h) during a day, using a portable pulse modulation fluorometer (Junior-PAM, Walz, Germany). The quantum efficiency of open PS II (F v′/F m′ = (F m′ − F o′) / F m′) of the algal thalli (dark-adapted for 10 s) was measured (Ralph et al. 1999). The rapid light curves (RLCs) consisted of the fluorescence response to eight different and increasing actinic irradiance levels as in our previous study (Zou 2014). The parameters of the RLCs were calculated following the model equation from Jassby and Platt (1976): rETR = rETRm × tanh (αE/rETRm), where rETR m is the saturated maximum rETR, tanh is the hyperbolic tangent function, α is the initial slope of the RLCs, and E is the incident irradiance.
Uptake rates of nitrate
The uptake rate of nitrate was determined by the disappearance of nitrate from the culture medium over a given time interval and expressed by the following equation: Nitrate uptake rate = (N 0 − N t) × V × W 0 −1 × t −1, where N 0 is the initial concentration of culture medium without incubated algae, N t the concentration of culture medium with incubated algae after t hours, V the volume of the culture medium, and W 0 the initial FW of the algae (Harrison 1988). NO3 − concentration was measured according to Strickland and Parsons (1972).
Statistical analyses
The data are expressed as the means ± standard deviation (SD, n ≥ 3). Statistical significance of the data was tested with analysis of variance (ANOVA) or t test using SPSS for Window version 19.0. The significance level was set at P < 0.05.
Results
Growth rates
Generally, G. lemaneiformis exhibited much higher (P < 0.01) rate of growth than P. haitanensis (Fig. 2). The growth of P. haitanensis required a relative low sunlight irradiance. It showed a negative RGR at ambient sunlight and a positive RGR in decreased sunlight. The RGR of P. haitanensis thalli grown with a decreased carbon supply was reduced about 83 % (under ambient sunlight) and 37 % (under decreased sunlight) compared with the thalli grown at ambient carbon supply (Fig. 2a).
The relative growth rate (RGR) of Pyropia haitanensis (a) and Gracilaria lemaneiformis (b) grown at different carbon and sunlight availability. Vertical bar represents ±SD of the means (n = 3). AC means the treatment of ambient carbon supply. DC means the treatment of decreased carbon supply. AL means the treatment of ambient sunlight. DL means the treatment of decreased sunlight
The mean RGR of G. lemaneiformis under the different conditions remained positive, with lower RGR (P < 0.01) of thalli grown under decreased carbon supply compared with ambient carbon availability. The RGR of the G. lemaneiformis thalli grown under decreased carbon supply was reduced about 95 % (under ambient sunlight) and 35 % (under decreased sunlight) compared with the thalli grown under ambient carbon supply (Fig. 2b).
Biochemical composition
Either decreased carbon supply or decreased sunlight increased the contents of chlorophyll a (Chl a; P < 0.05 for carbon levels; P < 0.01 for sunlight levels) and carotenoids (Car; P < 0.05 for carbon levels; P < 0.01 for sunlight levels) in P. haitanensis. However, the Chl a (P = 0.934) and Car (P = 0.278) contents were similar in the G. lemaneiformis thalli grown under different carbon availability. The Chl a (P < 0.01) and Car (P < 0.01) contents were greater in the G. lemaneiformis grown at decreased sunlight than the algae grown at ambient sunlight (Table 1).
The contents of PE (P = 0.195), PC (P = 0.490), and SP (P = 0.848) were similar between the P. haitanensis thalli cultured in the two different carbon concentrations. However, PE (P < 0.05), PC (P < 0.05), and SP (P < 0.01) contents were greater in G. lemaneiformis grown with decreased carbon supply compared to the thalli grown at ambient carbon supply. In both P. haitanensis and G. lemaneiformis, growth under decreased sunlight significantly increased the contents of PE (P < 0.01 for P. haitanensis; P < 0.01 for G. lemaneiformis), PC (P < 0.01 for P. haitanensis; P < 0.01 for G. lemaneiformis), and SP (P < 0.01 for P. haitanensis; P < 0.05 for G. lemaneiformis) compared with ambient sunlight (Table 1).
Chlorophyll fluorescence
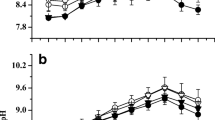
The daily variation of the quantum efficiency of open PS II (F v′/F m′) was contrary to change of daily irradiance. The F v′/F m′ values in both of P. haitanensis and G. lemaneiformis showed a decrease with gradually increasing sunlight, reached the lowest value at midday between 10:30 and 14:30, and thereafter increased with gradually decreasing sunlight (Figs. 3a, b and 4a, b). Values of the maximum photosynthetic electron transport rate (rETRm) showed clear diurnal trends. The values of both P. haitanensis thalli and G. lemaneiformis thalli were at their maximum at midday and at their minimum at morning and/or night (Figs. 3c, d and 4c, d). It was seen that both the F v′/F m′ (P < 0.01 for P. haitanensis; P < 0.01 for G. lemaneiformis) and rETRm (P < 0.01 for P. haitanensis; P < 0.01 for G. lemaneiformis) values in the two seaweeds grown under decreased carbon supply were lower than these grown with ambient carbon supply. The F v′/F m′ (P < 0.01) of P. haitanensis grown at ambient sunlight were lower than those grown with decreased sunlight, and for the rETRm (P = 0.951) in P. haitanensis thalli, there was no significant difference between the thalli grown under the two sunlight conditions. However, the F v′/F m′ (P < 0.05) and rETRm (P < 0.01) of G. lemaneiformis grown under ambient sunlight were higher than for the algae grown under decreased sunlight.
The diurnal variation of the quantum efficiency of open PS II (F v′/F m′) (a, b) and the maximum photosynthetic electron transport rate (rETRm) (c, d) in Pyropia haitanensis grown at different carbon and sunlight availability. Vertical bar represents ±SD of the means (n = 5). Significance levels are **P < 0.01 and *P < 0.05. AC means the treatment of ambient carbon supply. DC means the treatment of decreased carbon supply. AL means the treatment of ambient sunlight. DL means the treatment of decreased sunlight
The diurnal variation of the quantum efficiency of open PS II (F v′/F m′) (a, b) and the maximum photosynthetic electron transport rate (rETRm) (c, d) in Gracilaria lemaneiformis grown at different carbon and sunlight availability. Vertical bar represents ±SD of the means (n = 5). Significance levels are **P < 0.01 and *P < 0.05. AC means the treatment of ambient carbon supply. DC means the treatment of decreased carbon supply. AL means the treatment of ambient sunlight. DL means the treatment of decreased sunlight
Nitrogen uptake rate
Figure 5a illustrates that the NO3 − uptake rates were lower (P < 0.01) for P. haitanensis thalli cultured at decreased carbon supply compared to those grown at ambient carbon supply. Under ambient carbon growth condition, the uptake rate of nitrate in P. haitanensis thalli grown under decreased sunlight was higher (P < 0.01) than for the thalli grown at ambient sunlight. However, under decreased carbon growth condition, the NO3 − rates were similar (P = 0.687) between the thalli grown at the two sunlight conditions.
The nitrogen uptake rate during the sunlight period in Pyropia haitanensis (a) and Gracilaria lemaneiformis (b) grown at different carbon and sunlight availability. Vertical bar represents ±SD of the means (n = 3). AC means the treatment of ambient carbon supply. DC means the treatment of decreased carbon supply. AL means the treatment of ambient sunlight. DL means the treatment of decreased sunlight
Under ambient sunlight growth condition, the uptake rate of nitrate was decreased (P < 0.05) in the G. lemaneiformis thalli grown with decreased carbon supply than for the algae grown with ambient carbon supply. However, under decreased sunlight growth condition, the different carbon levels had no effect on the NO3 − uptake rates of the thalli (P = 0.996). Additionally, under ambient carbon growth condition, the uptake rate of nitrate of ambient sunlight-grown G. lemaneiformis thalli was increased (P < 0.05) relative to the decreased sunlight-grown thalli. However, under decreased carbon growth condition, the NO3 − rates showed no significant differences (P = 0.881) between the thalli grown under the different sunlight conditions (Fig. 5b).
Discussion
Growth and biochemical components
The RGR of P. haitanensis and G. lemaneiformis cultured under low carbon supply were lower than the algae grown under ambient carbon supply. Similar results have been described for Gracilaria tenuistipitata (García-Sânchez et al. 1994) and Ulva conglobata (Zou 2014). The effect of decreased carbon supply on the RGR of G. lemaneiformis was greater than on P. haitanensis. This implies that P. haitanensis has more effective strategies for decreased carbon supply compared to G. lemaneiformis. In our previous study (Zou and Gao 2002; Zou et al. 2004), we found that the pH compensation point of P. haitanensis (pH 9.9) was greater than G. lemaneiformis (pH 9.58). Such a relative high pH compensation point suggested that P. haitanensis was more capable of abstracting inorganic carbon from surrounding seawater to drive photosynthesis than G. lemaneiformis. However, under carbon starvation, seaweeds cannot gain sufficient photosynthetic carbon to compensate for respiratory Ci consumption, and this can result in negative growth of the seaweeds.
The cellular components were affected by the low carbon levels in seawater. The present results showed that the decreased carbon supply increased the Chl a and Car contents of P. haitanensis thalli and increased the phycoerythrin and phycocyanin contents of G. lemaneiformis thalli. These results agree with those shown in previous studies, where the pigment contents were increased by the low carbon growth conditions in Gracilaria sp., Ulva intestinalis (Andría et al. 2001, as Enteromorpha), and U. conglobata (Zou 2014). Contrasting results have been described for G. tenuistipitata (García-Sânchez et al. 1994) and Porphyra leucosticta (Mercado et al. 1999), in which the phycobiliproteins decreased in low carbon-grown thalli compared with high carbon-grown thalli. Consequently, it appears that the response to lowered carbon in seaweeds is species specific. The electron flow between PS II and PS I and carbon fixation pathway are related to the concentration of photosynthetic units and their minimal turnover time (Falkowski and Raven 1997). In this study, the increased pigment (Chl a, Car, PE, and PC) contents in the low carbon condition could compensate for the decreased electron transport rates. Thereby, both of these two economic red algae increased their capacity to acclimate low carbon. The SP content of G. lemaneiformis thalli grown at decreased carbon was higher than at ambient carbon, but low carbon growth conditions had no significant effect on SP content of P. haitanensis thalli. Additionally, the SP content was also increased by the low carbon growth conditions in G. tenuistipitata (García-Sânchez et al. 1994) and U. conglobata (Zou 2014). These results might imply a higher capacity of N assimilation in some species under low carbon supply.
The present results showed that the pigments and SP contents of P. haitanensis and G. lemaneiformis thalli grown at low sunlight were higher than in the algae grown at ambient sunlight. This would be conducive to light absorption and energy conduction as stated by Talarico and Maranzana (2000). Our results also showed that the carbon levels had no effect on the acclimation to low sunlight.
Photosynthetic characteristics
The present results showed a clear diurnal pattern of chlorophyll fluorescence. The maximum value of F v′/F m′ occurred close to morning and/or night, and minimum values occurred in the middle of the day, but the rETRm reached maximum values at the middle of the day and the rETRm increased with the weakening of illumination. The results agree with many other studies (e.g., Sagert et al. 1997; Beer et al. 2006; Campbell et al. 2008). With irradiance intensity increasing, photon flux is in excess of that required for photosynthesis and PS II activity is decreased reversibly to protect the algae from excess light energy. As irradiance decreased, F v′/F m′ and rETRm began to recover. The recovery might be related to the production of D 1 protein and non-photochemical quenching which dissipates excess energy as heat (Magnusson 1997; Serôdio et al. 2008). In this study, both of the two seaweeds grown at different culture conditions could adjust chlorophyll fluorescence with sunlight changes, implying that the photoprotective capacity of the two seaweeds has not been destroyed.
Both P. haitanensis and G. lemaneiformis thalli grown at decreased carbon supply had reduced F v′/F m′ and rETRm. These results are consistent with the observations of Zou (2014), where the F v/F m and rETRm were decreased under the decreased carbon growth conditions in U. conglobata. In the course of carbon starvation, the endogenous electron acceptors reduce and oxygen becomes the main available electron acceptor. At the same time, oxygen also can act as electron acceptor in photorespiration and Mehler reaction (Durchan et al. 2001); therefore, the activity of photorespiration and Mehler reaction may be enhanced or steady. However, the activity of PS II will be inhibited by active oxygen radicals (e.g., superoxide, hydroxyl radicals, and hydrogen peroxide) which are produced in the Mehler reaction. When carbon limitation occurs in seawater, more photosynthetic pigments absorb more light energy than can be utilized for carbon assimilation. The excess energy may result in photoinhibition. The non-photochemical quenching (NPQ) in algae can dissipate excess energy as heat, but this was not tested and requires further experimentation. Additionally, two processes can contribute to adjust the electron transport rate: the Mehler reaction and the rate of cyclic electron transport around PS I (Mercado et al. 1999). Therefore, the decreased electron transport rates might be associated with a decreased rate of cyclic electron transport around PS I.
Growth irradiance modulates photosynthetic activity of seaweeds to prevent possible photodamage. Furthermore, previous reports have indicated that irreversible photoinhibition was induced by photooxidative damage to PS II which may lead to significant decrease in plant productivity (Baker 1991). This could explain why F v′/F m′ and RGR of P. haitanensis grown at low irradiance were greater than in the thalli grown at ambient irradiance and why P. haitanensis cultured under ambient sunlight had a negative RGR. The high RGR of algae in low sunlight might be also related to the CCMs, which facilitate energy dissipation at high irradiance and downregulate energy consumption under low irradiance. Additionally, the F v′/F m′, rETRm, and RGR of G. lemaneiformis grown at low irradiance were lower than in the algae grown at ambient irradiance. These results indicate that short-term relative higher sunlight probably caused photodamage and decreased PS II capacity in P. haitanensis, whereas such photodamage did not occur in G. lemaneiformis. This suggests that G. lemaneiformis is more able to adapt to relatively higher growth sunlight conditions than P. haitanensis.
Nitrogen metabolism
The results showed that decreased carbon supply in culture reduced the NO3 − uptake, and this possibly explains the lower growth rate of the two algae under low carbon supply. The low NO3 − uptake rate may be caused by the decreased activity of nitrate reductase (NR) at low levels of CO2, as shown by Mercado et al. (1999). The opposite pattern was obtained regarding the high levels of CO2. For example, the activity of NR and NO3 − uptake were increased in P. leucosticta (Mercado et al. 1999), Ulva rigida (Gordillo et al. 2001), and Hizikia fusiforme (Zou 2005) grown at high levels of CO2 compared with the algae grown at normal CO2 level.
Under ambient carbon, the NO3 − uptake rate of P. haitanensis cultured at ambient sunlight was higher than for the algae cultured at decreased sunlight, whereas it was lower in G. lemaneiformis grown at ambient sunlight compared with the thalli grown under decreased sunlight. Light is an important factor involved in NR regulation (Lillo 1994). In general, light can stimulate nitrate reduction in algae (Lopes et al. 1997). Additionally, nitrate uptake demands carbon skeletons, reducing agent, and energy (Chow et al. 2004). Consequently, the response of NO3 − uptake rate to light in seaweeds is species-specific.
In conclusion, P. haitanensis and G. lemaneiformis grown at low carbon supplement condition showed lower RGR, nitrogen uptake rate, F v′/F m′, and rETRm compared with the algae grown with ambient carbon supply, and the effect of decreased carbon supply on the RGR of G. lemaneiformis was greater than for P. haitanensis. Low sunlight-grown P. haitanensis had increased RGR, nitrogen uptake rate, and F v′/F m′ compared with the algae grown at ambient sunlight. However, these above three physiological indicators of G. lemaneiformis reflected contrary results. We therefore suggest that the photosynthetic activity and aquaculture production of these economically important seaweeds would be lessened in seawater with low carbon supply, which usually occurs in cultivation sites with high biomass density and lowered seawater exchange. Additionally, the data suggest that P. haitanensis has a more effective strategy for decreased carbon supply compared with G. lemaneiformis, whereas G. lemaneiformis is better able to adapt to grow at higher sunlight conditions than P. haitanensis.
References
Andría JR, Brun FG, Pérez-Lloréns JL, Vergara JJ (2001) Acclimation responses of Gracilaria sp. (Rhodophyta) and Enteromorpha intestinalis (Chlorophyta) to changes in the external inorganic carbon concentration. Bot Mar 44:361–370
Baker NR (1991) A possible role for Photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81:563–570
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshwat Res 36:785–792
Beer S, Mtolera M, Lyimo T, Björk M (2006) The photosynthetic performance of the tropical seagrass Halophila ovalis in the upper intertidal. Aquat Bot 84:367–371
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campbell SJ, Kerville SP, Coles RG, Short F (2008) Photosynthetic responses of subtidal seagrasses to a daily light cycle in Torres Strait: a comparative study. Cont Shelf Res 28:2275–2281
Chow F, de Oliveira MC, Pedersén M (2004) In vitro assay and light regulation of nitrate reductase in red alga Gracilaria chilensis. J Plant Physiol 161:769–776
Chung IK, Oak JH, Lee JA, Shin JA, Kim GJ, Park KS (2013) Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J Mar Sci 11:1–7
Durchan M, Vacha F, Krieger-Liszkay A (2001) Effects of severe CO2 starvation on the photosynthetic electron transport chain in tobacco plants. Photosynth Res 68:203–213
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell Science, Malden, pp 128–135
Fei XG (2004) Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 512:145–151
Friedlander M, Levy I (1995) Cultivation of Gracilaria in outdoor tanks and ponds. J Appl Phycol 7:315–324
García-Sânchez MJ, Fernândez JA, Niell FX (1994) Effect of inorganic carbon supply on the photosynthetic physiology of Gracilaria tenuistipitata. Planta 194:55–61
Gordillo FJL, Niell FX, Figueroa FL (2001) Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 213:64–70
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Harrison PJ (1988) Determining phosphate uptake rates of phytoplankton. In: Lobban CS, Chapman DJ, Kremer BP (eds) Experimental phycology: a laboratory manual. Cambridge University Press, New York, pp 186–195
Israel A, Friedlander M (1998) Inorganic carbon utilisation and growth abilities in the marine red macroalga Gelidiopsis sp. Isr J Plant Sci 46:117–124
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Oceanography 21:540–547
Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol 19:103–132
Lillo C (1994) Light regulation of nitrate reductase in green leaves of higher plants. Physiol Plant 90:616–620
Lopes PF, Oliveira MC, Colepicolo P (1997) Diurnal fluctuation of nitrate reductase activity in the marine red alga Gracilaria tenuistpitata (Rhodophyta). J Phycol 33:225–231
Magnusson G (1997) Diurnal measurements of Fv/Fm used to improve productivity estimates in macroalgae. Mar Biol 130:203–208
McMinn A, Ryan K, Gademann R (2003) Diurnal changes in photosynthesis of Antarctic fast ice algal communities determined by pulse amplitude modulation fluorometry. Mar Biol 143:359–367
Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL (1999) Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J Appl Phycol 11:455–461
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equation for ascertaining chlorophylls and carotenoids. J Mar Res 21:155–163
Porra RJ (2005) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Ralph PJ, Gademann R, Larkum AWD, Schreiber U (1999) In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar Ecol Prog Ser 180:139–147
Richards DK, Hurd CL, Pritchard DW, Wing SR, Hepburn CD (2011) Photosynthetic response of monospecific macroalgal stands to density. Aquat Biol 13:41–49
Sagert S, Forster RM, Feuerpfeil P, Schubert H (1997) Daily course of photosynthesis and photoinhibition in Chondrus crispus (Rhodophyta) from different shore levels. Eur J Phycol 32:363–371
Serôdio J, Vieira S, Cruz S (2008) Photosynthetic activity, photoprotection and photoinhibition in intertidal microphytobenthos as studied in situ using variable chlorophyll fluorescence. Cont Shelf Res 28:1363–1375
Strickland JDH, Parsons TR (1972) A Practical Handbook of Seawater Analysis. Fisheries Research Board of Canada, Ottawa
Talarico L, Maranzana G (2000) Light and adaptive responses in red macroalgae: an overview. J Photochem Photobiol B 56:1–11
Yang H, Zhou Y, Mao Y, Li X, Liu Y, Zhang F (2005) Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. J Appl Phycol 17:199–206
Yang Y, Liu Q, Chai Z, Tang Y (2015) Inhibition of marine coastal bloom-forming phytoplankton by commercially cultivated Gracilaria lemaneiformis (Rhodophyta). J Appl Phycol 27:2341–2352
Zou DH (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735
Zou DH (2014) The effects of severe carbon limitation on the green seaweed, Ulva conglobata (Chlorophyta). J Appl Phycol 26:2417–2424
Zou DH, Gao KS (2002) Effects of desiccation and CO2 concentrations on emersed photosynthesis in Porphyra haitanensis (Bangiales, Rhodophyta), a species farmed in China. Eur J Phycol 37:587–592
Zou DH, Gao KS (2009) Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia 48:510–517
Zou DH, Gao KS (2010) Physiological responses of seaweeds to elevated atmospheric CO2 concentrations. In: Israel A, Einav R, Seckbach J (eds) Seaweeds and their role in globally changing environment. Springer, Dordrecht, pp 115–126
Zou DH, Xia JR, Yang YF (2004) Photosynthetic use of exogenous inorganic carbon in the agarophyte Gracilaria lemaneiformis (Rhodophyta). Aquaculture 237:421–431
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. U1301235 and No. 41276148) and Guangdong Science and Technology Bureau (2015A020216004). The authors would like to thank Yayun Deng and Jiejun Zhang for assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, H., Zou, D. & Chen, B. Effects of lowered carbon supplies on two farmed red seaweeds, Pyropia haitanensis (Bangiales) and Gracilaria lemaneiformis (Gracilariales), grown under different sunlight conditions. J Appl Phycol 28, 3469–3477 (2016). https://doi.org/10.1007/s10811-016-0882-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0882-8