Abstract
In order to achieve recognition as environmentally friendly production, flue gases should be used as a CO2 source for growing the microalgae Chlorella sorokiniana when used for hydrogen production. Flue gases from a waste incinerator and from a silicomanganese smelter were used. Before testing the flue gases, the algae were grown in a laboratory at 0.04, 1.3, 5.9, and 11.0 % (v/v) pure CO2 gas mixed with fresh air. After 5 days of growth, the dry biomass per liter algal culture reached its maximum at 6.1 % CO2. A second experiment was conducted in the laboratory at 6.2 % CO2 at photon flux densities (PFD) of 100, 230, and 320 μmol photons m−2 s−1. After 4 days of growth, increasing the PFD increased the biomass production by 67 and 108 % at the two highest PFD levels, as compared with the lowest PFD. A bioreactor system containing nine daylight-exposed tubes and nine artificial light-exposed tubes was installed on the roof of the waste incinerator. The effect of undiluted flue gas (10.7 % CO2, 35.8 ppm NO x , and 38.6 ppm SO2), flue gas diluted with fresh air to give 4.2 % CO2 concentration, and 5.0 % pure CO2 gas was studied in daylight (21.4 ± 9.6 mol photons m−2 day−1 PAR, day length 12.0 h) and at 135 μmol photons m−2 s−1 artificial light given 24 h day−1 (11.7 ± 0.0 mol photons m−2 day−1 PAR). After 4 days’ growth, the biomass production was the same in the two flue gas concentrations and the 5 % pure CO2 gas control. The biomass production was also the same in daylight and artificial light, which meant that, in artificial light, the light use efficiency was about twice that of daylight. The starch concentration of the algae was unaffected by the light level and CO2 concentration in the laboratory experiments (2.5–4.0 % of the dry weight). The flue gas concentration had no effect on starch concentration, while the starch concentration increased from about 1.5 % to about 6.0 % when the light source changed from artificial light to daylight. The flue gas from the silicomanganese smelter was characterized by a high CO2 concentration (about 17 % v/v), low oxygen concentration (about 4 %), about 100 ppm NO x , and 1 ppm SO2. The biomass production using flue gas significantly increased as compared with about 5 % pure CO2 gas, which was similar to the biomass produced at a CO2 concentration of 10–20 % mixed with N2. Thus, the enhanced biomass production seemed to be related to the low oxygen concentration rather than to the very high CO2 concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
We recently studied the effect of flue gases as a CO2 source for growing the microalgae Chlamydomonas reinhardtii (Mortensen and Gislerød 2014, 2015). This single-cell green algae is known to produce hydrogen when starved of sulfur under anaerobic conditions (Melis et al. 2000; Nguyen et al. 2011; Geier et al. 2012). If waste CO2 from industrial flue gas is used to grow the microalgae, hydrogen production will increase its reputation as an environmentally friendly energy source that contributes to reducing carbon dioxide emissions (IPCC 2013). Several studies have investigated the effect of flue gases on the growth of microalgae (Douskova et al. 2009; Kastanek et al. 2010; Borkenstein et al. 2011; Lara-Gil et al. 2014). Depending on the species and the content of different pollutants in the flue gas, the growth varied greatly compared with using pure CO2 gas. In the experiments with C. reinhardtii, it was shown that flue gases containing about 10 % CO2 or higher had a negative effect when the algae were grown in aerobic conditions (Mortensen and Gislerød 2014, 2015). This effect was not related to the accompanying air pollutants (NO x and SO2), but was connected to a negative effect of high CO2 concentrations. The very low oxygen concentration (down to about 1 %) in the flue gas from a silicomanganese smelter did not counteract this negative effect of the high CO2 concentration (Mortensen and Gislerød 2015). The combination of high CO2 and low O2 concentrations is of particular interest for plant production, since a combination of high CO2 and low O2 decreases the photorespiration and increases photosynthesis in C3-plants as well as in microalgae (Ramazanov and Cardenas 1992; Kliphuis et al. 2011). This was not the case for C. reinhardtii, however. Also other species, including Chlorella sorokiniana, are known to be able to produce hydrogen (Chader et al. 2009; Roy et al. 2013). The question was whether C. sorokiniana would respond to high CO2 concentrations and flue gases in a similar way to C. reinhardtii. It was therefore studied what effect high CO2 concentrations based on pure CO2 gas and on two different flue gases had on the growth of this algae. The flue gases included were from a waste incinerator and from a silicomanganese smelter. Since starch is an important component in the hydrogen-producing stage of C. sorokiniana, the starch content of the algae was analyzed in the different treatments (Melis et al. 2000; Jo et al. 2006; Skjånes et al. 2007; Branyikova et al. 2011).
Materials and methods
Chlorella sorokiniana strain SAG 211.8 k from SAG (Göttingen, Germany) was used in the experiments. The algae were stored on Petri dishes covered with TAP medium 1.5 % agar. The microalgae were grown in tubes or in bottles. The medium consisted of tap water with the addition of 2 g Kristalon Plus (www.yara.no) and 1.0 g urea per liter. Kristalon Plus is a popular fertilizer for tomatoes, cucumbers, and roses. It contains 7.9 % N, 3 % P, 26.5 % K, 3.9 % Mg, 5.6 % S, 0.027 % B, 0.004 % Cu, 0.2 % Fe, 0.06 % Mn, 0.004 % Mo, and 0.027 % Zn. The concentration of N in the nutrient medium was 628 mg L−1, which would be enough to produce about 7 g L−1 dry biomass of algae containing up to 60 % protein. Hence, the availability of nutrients should not limit the growth of the algae.
The light was measured using an LI-COR Model LI-250 instrument with a quantum sensor (400–700 nm) at the surface of the tubes. Inside the culture, the light decreased from the light-exposed side to the opposite side of the tubes, as well as with increasing cell concentration during growth. Typically, the light level decreased by about 40 % through the 8.0 cm diameter tube at the start of the experiment (about 0.03 g L−1 algae dry weight concentration) and by more than 99.5 % when the algae culture reached about 1.0 g L−1 dry weight. The CO2 concentration was measured using a Vaisala CO2 transmitter (Type GMT221, range 0–5 %) or a Vaisala GMP instrument with a sensor in the range 0–20 %. The CO2 concentration was recorded once an hour. The temperature was measured by copper-constantan thermocouples and recorded hourly using a Campbell AM25T multiplexer. The different CO2 concentrations with pure CO2 gas were established by mixing food-grade CO2 with fresh air. The CO2 gas flow was determined by capillaries with defined resistances. The gas pressure was defined by the height of a water column. In this way, a very accurate CO2 flow could be added to a constant rate of fresh air produced by air pumps (Resun ACO-001, ACO-004). The different gas mixtures were bubbled through plastic tubes with a 0.3-cm inner diameter at the bottom of the tubes or bottles at a rate of approximately 100 L h−1. All treatments in all experiments included three parallel tubes or bottles.
The dry weight was measured by vacuum filtering 10 or 20 mL of culture through a 90-mm filter (Whatman GF/B, cat. No. 1821–090) and drying it in an oven for 4 h at 80 °C. The turbidity was measured regularly using a Hanna instrument (HI 93703) in order to monitor the growth. The measurements were carried out in the range 0–50 FTU (the linear phase) by diluting the algal culture, if necessary. The FTU value calculated per 1.0 g L−1 dry biomass in the culture could vary, but it was usually around 160–180. These measurements are not presented, however, except for the experiment with flue gas from the silicomanganese smelter.
Laboratory experiments
The microalgae were grown in clear 50 cm acrylic tubes (80 mm inner and 90 mm outer diameter) filled with 1.5 L of growing medium (filled up to 30 cm). Up to 12 tubes could be placed in a row adjacent to each other in a temperature-controlled water bath made of clear acrylic. The light was supplied from one side by six cool white fluorescent tubes (Philips TL-D 58 W/840). Two experiments were carried out in the laboratory. First, the algae were grown at 0.040 ± 0.005, 1.34 ± 0.11, 5.9 ± 0.2, and 11.0 ± 0.5 % (v/v) CO2 for 5 days at a photon flux density (PFD) of 320 ± 20 μmol photons m−2 s−1 given 24 h day−1. The temperature the first day was a mean 22.5 °C, and it was then increased to 28.5 ± 0.5 °C. The start pH was 6.8, 6.3, 6.1, and 6.0 at the four CO2 concentrations, respectively. The algae concentration at the start of the experiment was 0.056 g L−1. A second experiment included PFD levels of 100 ± 10, 230 ± 10, and 330 ± 10 μmol photons m−2 s−1 given 24 h day−1. The CO2 concentration was 6.2 ± 0.8 % and the algae had been pre-grown at the same CO2 concentration. The mean temperatures of the algae culture were 25.1 ± 0.6, 26.6 ± 0.6, and 27.3 ± 0.6 °C at the three PFD levels, respectively. The algae concentration at start of the experiment was 0.021 g L−1 and the pH of the medium 6.2.
For algal growth, the concentration of dissolved CO2 in the nutrient medium is important and not the concentration of CO2 in the air bubbled into the culture, although a close relationship should be expected. In order to document this relationship, a test was carried out with different concentrations of pure CO2 mixed with air bubbled through the tubes filled with nutrient medium. The concentration of dissolved CO2 was measured using hand-held titration cells for titrimetric analysis (CHEMetrics Inc., USA, www.chemetrics.com). The results showed that the dissolved CO2 concentration increased from about 20 to about 180 mg L−1 with an increasing CO2 concentration from 0.04 % up to about 12 % accompanied by a decrease in pH from 7.0 to 5.7 (Fig. 1). The regression equation (order 2) was found to be y = 23.9 + 26.3x − 1.12x 2 (r 2 = 0.99).
Flue gas from a waste incinerator
Flue gas was provided by the waste incinerator at Forus Energigjenvinning (Forus Energy Recycling) (www.avfallnorge.no) located in Sandnes, Norway. This modern plant burns about 110,000 tonnes of waste yearly and delivers the energy for district heating.
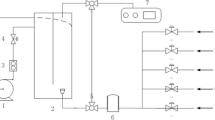
Flue gas from the chimney was sucked by pumps (Resun ACO-008A) with a capacity of 6.9 m3 h−1 through two 100-L plastic tubs in series for condensation of the water vapor. The flue gas was then diluted by mixing it with fresh air in order to establish an additional CO2 concentration based on flue gas. During the experimental period, the flue gas contained a mean of 10.7 ± 0.1 % CO2, 8.5 ± 0.1 % O2, 36 ± 26 ppm NO x (NO + NO2), 39 ± 13 ppm SO2, 8.0 ± 1.4 ppm HCl, 3.3 ± 4.4 ppm CO, and 0.25 ± 0.02 ppm HF (Fig. 2). The CO2, O2, NO x , and CO concentrations were measured using an Emerson MLT4/NGA2000 analyzer.
Nine transparent 80 cm PVC tubes (90 mm outer and 72 mm inner diameter) were arranged vertically in a row adjacent to each other on the front side of a plywood board and placed in a temperature-controlled water bath made of clear acrylic. An additional nine tubes were mounted on the back of the board. These tubes were exposed to artificial light by a 200 W white LED lamp (Cree LED 200 W/HLG-240H-42B) with 135 ± 15 μmol photons m−2 s−1 24 h day−1, corresponding to 11.7 mol photons m−2 day−1. While the tubes on the front panel were exposed to full daylight in a south-facing position, all daylight was excluded from the tubes on the back of the panel by means of black curtain. The equipment was placed on the roof of Forus Energigjenvinning, and the experiment was carried out from September 19 to 23. The mean (±SD) photosynthetic active radiation (PAR) of the daylight during the experiment was 21.4 ± 9.6 mol photons m−2 day−1 (Meteorological data from Særheim Research Centre, www.bioforsk.no). Three treatments were included in this experiment: flue gas containing 10.7 ± 0.1 % CO2, diluted flue gas containing 4.2 ± 0.1 % CO2, and 5 ± 1 % pure CO2. A volume of 3.0 L medium was filled in each of the 18 tubes, and algae pre-grown at 6 % CO2 were added, yielding a biomass concentration at the start of the experiment of 0.042 g L−1. The biomass production after 4 days was recorded. The temperature during this period varied between 19 and 21 °C.
Flue gas from a silicomanganese smelter
An experimental set-up with C. sorokiniana was established in a silicomanganese smelter (Eramet, Kvinesdal, Norway, www.eramet.no) in parallel with a recently reported study on C. reinhardtii (Mortensen and Gislerød 2015). Typical for the flue gas from this plant was its combination of high CO2 concentration (17.4 ± 2.9 %) and low O2 concentration (around 4 %). The NO x concentration was 102 ± 13 ppm, the SO2 concentration 1.1 ± 0.1, and the H2S concentration was 0.8 ± 0.1 ppm, as previously reported (Mortensen and Gislerød 2015). The microalgae were grown in 1.0 L clear plastic bottles (80 mm inner and 82 mm outer diameter) filled with 0.85 l of growing medium (filled up to 17 cm) indoors without daylight. A photon flux density of 300 ± 20 μmol photons m−2 s−1 was supplied 24 h day−1 from one side by fluorescent tubes, and the temperature was 28.7 ± 0.5 °C. The biomass concentration at the start of the experiment was 0.058 g L−1, corresponding to a turbidity (FTU) of 9.5. In addition to the flue gas treatment, a control treatment was established using pure CO2 gas at a concentration of 4.8 ± 0.8 % and a treatment combining 10–20 % CO2 gas mixed with pure N2 gas. The concentration of dissolved oxygen in the algae culture was measured by an Odeon OPTOD sensor.
Starch analysis
A sample of 50 mg dry algae was used to analyze the starch content using Megazyme protocol K-TSTA (http://secure.megazyme.com/Total-Starch-Assay-Kit) on the basis of released glucose. Corn starch was used for control of the analysis. The glucose content was analyzed by means of oxidase/peroxidase reagent and was used to adjust the starch values. The glucose content was analyzed separately. It was generally low (an average of 1.2 % of dry weight) and is not presented. Samples of dried foam were used in the laboratory experiments and frozen samples (thawed and centrifuged) in the experiments with flue gas from the waste incinerator.
The data were analyzed using the SAS-ANOVA procedure (SAS institute Inc., USA) based on tubes or bottles as replicates (n = 3).
Results
Laboratory experiments
The total dry biomass production after 5 days increased by 88 % when the CO2 concentration was increased from the natural concentration of 0.04 to 1.4 % CO2, and by 120 % at 5.9 % CO2 (Table 1). A further increase to 11.0 % CO2 decreased productivity. Typical for this algae culture was that the biomass production was a combination of increased concentration of the algae culture and foam floated on top of the culture. From the initial biomass concentration of 0.056 g L−1, the algae biomass had increased by about 40 times after 5 days. The starch concentration (2–4 % of the algae dry weight) as analyzed in the foam was not significantly affected by the CO2 concentration.
When the PFD increased from 100 to 230 μmol photons m−2 s−1, the dry biomass increased by 67 % after 4 days (Table 2). A further increase to 330 μmol photons m−2 s−1 increased the biomass by an additional 25 %. This meant that, over 4 days, the biomass had increased by 37 times at the lowest and by 77 times at the highest PFD from the initial concentration. The starch concentration of about 3 % of dry weight was unaffected by the light conditions.
Flue gas from a waste incinerator
The total algae dry weight production in the flue gas containing 10.7 % CO2 did not differ from that in diluted flue gas (4.2 % CO2) or 5 % pure CO2 (Table 3). This was the case when the algae were grown in daylight as well in artificial light only. During the 4 days, the dry weight had increased 28 times from the initial concentration of 0.042 g L−1. The production was the same in daylight (21.4 mol photons m−2 day−1 PAR) as in artificial light with 135 μmol photons m−2 s−1 PFD or 11.7 mol photons m−2 day−1 PAR. The photoperiod of the daylight was about 12 h, while artificial light was given continuously. The starch concentration was unaffected by the CO2 treatment, while the content was significantly higher in daylight (5–6 % of dry weight) as compared with artificial light (1–2 % of dry weight). After 4 days, the heating of the cultures was stopped and the temperature decreased to 10–15 °C throughout the following 5 days. After a sunny period, the algae in two of three tubes at 10.7 % CO2 in flue gas collapsed (the culture changed from green to brown). The conductivity of these cultures showed a marked increase, indicating cell leakage. The starch concentration in the intact cultures after the low temperature period was 9–10 % in daylight and 1–3 % of dry weight in artificial light (results not presented).
Flue gas from a silicomanganese smelter
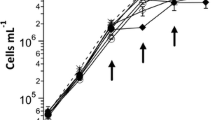
The dry weight production increased by 65 % in undiluted flue gas from a silicomanganese smelter containing 17.4 % CO2 as compared to pure 4.8 % CO2 (Table 4). Mixing 10–20 % pure CO2 gas in pure N2 gas produced the same results as in the flue gas. It should be noted that the oxygen concentration in the cultures with flue gas and pure N2 gas was much lower than in the pure 4.8 % CO2 control treatment. From the initial concentration of 0.056 g L−1, the algae production had increased by 35 times, similar to the increase in turbidity (Fig. 3).
Discussion
A CO2 concentration of about 6 % appeared to be optimal for growth of C. sorokiniana, while a concentration of 11 % only slightly decreased growth. Flue gas from the waste incinerator containing about 11 % CO2, 36 ppm NO x , and 39 ppm SO2 yielded the same productivity as diluted flue gas (4 % CO2) or 5 % pure CO2 gas mixed with air. This was the case in daylight as well as when the algae were grown in low-level artificial light. When the algae were grown in flue gas from a silicomanganese plant (17 % CO2, 100 ppm NO x , and 1 ppm SO2), however, the growth was significantly increased compared to 4.8 % pure CO2 gas. The low oxygen concentration in this flue gas from the silicomanganese smelter resulted in low concentrations of dissolved O2 (<5 mg L−1) in the algae culture (Mortensen and Gislerød 2015) compared to 5 % pure CO2 gas or 11 % flue gas from waste combustion (Mortensen and Gislerød 2014). Since mixing CO2 gas in pure N2 gas had the same stimulating effect as the flue gas from the silicomanganese smelter, this effect was most probably connected to a low O2 concentration in the algae culture. A positive effect of low O2 has previously been found in some studies with microalgae (Vance and Spalding 2005; Douskova et al. 2009; Kliphuis et al. 2011). This effect is usually related to a decrease in photorespiration and a reduction in the oxygenase activity of Rubisco. The microalga C. reinhardtii was tested on flue gas from the silicomanganese smelter at the same time as the present C. sorokiniana (Mortensen and Gislerød 2015). This algae responded negatively, however, to the undiluted flue gas in spite of a low O2 concentration. Thus, different microalgae appear to respond differently to low O2.
Two of the three C. sorokiniana cultures in the flue gas from the waste incinerator collapsed (took on a brownish color) after 3 days at low temperatures (down to 10 °C) after some hours of sunny weather with light levels of up to about 1500 μmol photons m−2 s−1 PFD at the tube surface. This observation is in line with Fischer et al. (2006) who showed that cells of C. reinhardtii were more susceptible to high-light stress under high CO2 concentrations than under low concentrations.
Compared with daylight, about half of the PAR level continuously supplied by low-level artificial light yielded the same algae growth. The algae were most probably not able to utilize the highest levels in the daylight (Mortensen and Gislerød 2014). In addition, a dark period is known to decrease algae growth much more than would be expected from the reduction in PAR (Jacob-Lopez et al. 2009).
A level of air pollutants in the two flue gases of up to 100 ppm NO x and 40 ppm SO2 seldom seems to affect the growth of microalgae (Brown 1996; Olaizola 2003; van den Hende et al. 2012; Farrelly et al. 2013; Jiang et al. 2013).
As more or less expected, the starch content always remained low irrespective of the light level and CO2 concentration. The sugars produced through photosynthesis were used for cell division and new growth as long as there were a surplus of nutrients and the temperature was high enough for cell division. Thus, decreasing the temperature to 10–15 °C (stopping the cell division) combined with some hours of sunny weather significantly increased the starch content. Nutrient depletion through low N or S in the medium is known to rapidly increase the starch content in parallel with a decrease in the protein content of algae cells (Ji et al. 2011; Markou et al. 2012; Yao et al. 2012; Guo et al. 2014). However, this depends on relatively good light conditions for the algae cells, and the culture should therefore not be too dense.
Based on the present results C. sorokiniana seemed to benefit from a flue gas that contained low O2 concentrations. Such a flue gas, however, will be accompanied by very high CO2 concentrations that can be injurious at low temperatures when the irradiance level is high. Temperatures below about 15 °C should therefore be avoided growing the algae in daylight in such a flue gas.
References
Borkenstein CG, Knoblechner J, Frühwirth H, Schagert M (2011) Cultivation of Chlorella emersonii with flue gas derived from cement plant. J Appl Phycol 23:131–135
Branyikova I, Marsalkova B, Doucha J, Branyik T, Bisova K, Zachleder V, Vitova M (2011) Microalgae—novel high efficient starch producer. Biotechnol Bioeng 108:766–776
Brown LM (1996) Uptake of carbon dioxide from flue gas by microalgae. Energy Convers Manag 37:1363–1367
Chader S, Hacene H, Agathos SN (2009) Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int J Hydrog Energy 34:4941–4946
Douskova I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185
Farrelly DJ, Everard CD, Fagan CC, McDonnell KP (2013) Carbon sequestration and the role of biological carbon mitigation: a review. Renew Sust Energ Rev 21:712–727
Fischer BB, Wiesendanger M, Eggen RL (2006) Growth condition-dependent sensitivity, photodamage and stress response of Chlamydomonas reinhardtii exposed to high light conditions. Plant Cell Physiol 47:1135–1145
Geier SC, Huyer S, Praebst K, Husmann M, Walter C, Buchholz R (2012) Outdoor cultivation of Chlamydomonas reinhardtii for photobiological hydrogen production. J Appl Phycol 24:319–327
Guo F, Wang H, Wang J, Zhou W, Gao L, Chen L, Dong Q, Zhang W, Liu T (2014) Special biochemical responses to nitrogen deprivation of filamentous oleaginous microalgae Tribonema sp. Bioresour Technol 158:19–24
IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds). Cambridge University Press, Cambridge, www.climatechange2013.org
Jacob-Lopez AJ, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycle (night/dark) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48:306–310
Ji CF, Yu XJ, Chen ZA, Xue S, Legrand J, Zhang W (2011) Effects of nutrient deprivation on biochemical compositions and photo-hydrogen production of Tetraselmis subcordiformis. Int J Hydrog Energy 36:5817–5821
Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T (2013) Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour Technol 128:359–364
Jo JH, Lee DS, Park JM (2006) Modeling and optimization of photosynthetic hydrogen gas production by green alga Chlamydomonas reinhardtii in sulfur-deprived circumstance. Biotechnol Prog 22:431–437
Kastanek F, Sabata S, Solcova O, Maleterova Y, Kastanek P, Branyikova I, Kuthan K, Zachleder V (2010) In-field experimental verification of cultivation of microalgae Chlorella sp. using flue gas from cogeneration unit as a source of carbon dioxide. Waste Manag Res 28:961–966
Kliphuis AMJ, Martens DE, Janssen M, Wijffels RH (2011) Effect of O2:CO2 ratio on the primary metabolism of Chlamydomonas reinhardtii. Biotechnol Bioeng 108:2390–2402
Lara-Gil JA, Alvarez MM, Pacheco A (2014) Toxicity of flue gas components from cement plants in microalgae mitigation systems. J Appl Phycol 26:357–368
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645
Melis A, Zhang L, Forestier M, Ghiradi M, Sebert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green algae Chlamydomonas reinhardtii. Plant Physiol 122:127–136
Mortensen LM, Gislerød HR (2014) The effect on growth of Chlamydomonas reinhardtii of flue gas from a power plant based on waste combustion. AMB Express 4:49
Mortensen LM, Gislerød HR (2015) The growth of Chlamydomonas reinhardtii as influenced by high CO2 and low O2 in flue gas from a silicomanganese smelter. J Appl Phycol 27:633–638
Nguyen AV, Toepel J, Burgess S, Uhmeyer A, Bilfernez O, Doebbe A, Hankamer B, Nixon P, Wobbe L, Kruse O (2011) Time-course global expression profiles of Chlamydomonas reinhardtii during photo-biological H2 production. PLoS One 6(12):e29364
Olaizola M (2003) Microalgal removal of CO2 from flue gases: changing in medium pH and flue gas composition do not appear to affect the photochemical yield of microalgal cultures. Biotechnol Bioprocess Eng 8:360–367
Ramazanov Z, Cardenas J (1992) Involvement of photorespiration and glycolate pathway in carbonic anhydrase induction and inorganic carbon concentration in Chlamydomonas reinhardtii. Physiol Plant 84:502–508
Roy S, Kumar K, Ghosh S, Das D (2013) Thermophilic biohydrogen production using pre-treated algal biomass as substrate. Biomass Bioenergy 61:157–166
Skjånes K, Lindblad P, Muller J (2007) BioCO2 — a multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol Eng 24:405–413
van den Hende S, Vervaeren H, Boon N (2012) Flue gas compounds and microalgae: (Bio-)chemical interactions leading to biotechnological opportunities. Biotechnol Adv 30:1405–1424
Vance P, Spalding MH (2005) Growth, photosynthesis, and gene expression in Chlamydomonas over a range of CO2 concentrations and CO2/O2 ratios: CO2 regulates multiple acclimation states. Can J Bot 83:796–809
Yao C, Ai J, Cao X, Xue S, Zhang W (2012) Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation. Bioresour Technol 118:438–444
Acknowledgments
The authors thank Forus Energigjenvinning and staff (in Sandnes) and Eramet Norway and staff (in Kvinesdal) for excellent cooperation. We also thank Dr. Kari Skjånes for providing the algae and Dr. Rune Slimestad (PlantChem, Norway) for technical assistance with the chemical analyses. This work was done as a part of the project “Use of solar energy for CO2 capture, algae cultivation and hydrogen production” headed by Dr. Stig Borgvang, Bioforsk Norway, and was financed by the Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mortensen, L.M., Gislerød, H.R. The growth of Chlorella sorokiniana as influenced by CO2, light, and flue gases. J Appl Phycol 28, 813–820 (2016). https://doi.org/10.1007/s10811-015-0649-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0649-7