Abstract
Algae have the potential to capture carbon dioxide (CO2) and nitric oxide (NO) from flue gases. However, the effects of high concentrations of these gases on the photophysiology of algae are poorly understood. To that end, we used the techniques of chlorophyll fluorescence to study the effect of industrially relevant levels of CO2 and NO on the photophysiology of two green microalgae, Asterarcys quadricellulare and Chlorella sorokiniana, that are tolerant to these gases. Measurements of maximum quantum yield (Fv/Fm) and maximum relative electron transport rate (rETRmax) show an enhanced performance of photosystem II (PSII) under high CO2 levels. In C. sorokiniana, high CO2 stimulated non-photochemical quenching (NPQ), while the opposite effect was observed in A. quadricellulare. Light-saturated photosynthetic rates (Pmax) of both species were highest at 10% CO2. Further, the tested levels of NO did not show adverse effect on the performance of PSII. OJIP chlorophyll fluorescence transients suggest that in C. sorokiniana, the energetic communication between PSII units declined at 15% CO2. However, in A. quadricellulare, this decline was visible even at 10% CO2 with complete inhibition of cell growth at 15% v/v. Overall, our results suggest that although photosynthesis was regulated differently in the two microalga, both species exhibited enhanced PSII performance under reasonably high levels of CO2 and NO. Thus, the two species are potential candidates for bio-fixation of CO2 and NO from flue gases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flue gas is an assortment of carbon dioxide (CO2), nitrogen oxides (NOx), sulfur oxides (SOx), carbon monoxide (CO), and particulate matter. Nitric oxide (NO) is the main component of NOx in the flue gas (> 90%). Both CO2 and NO are considered as major greenhouse components and are viewed as potential agents of global climate change. While CO2 is a source of carbon for algal mass culture, NO when dissolved in water forms nitrite and could be an effective N-source for algae (Zhang et al. 2015). Consequently, there has been significant interest in developing strategies for the photosynthetic capture of CO2 and NO from flue gases using microalgal cultures (Yoshihara et al. 1996; Chiu et al. 2011; Zhao et al. 2015; Ma et al. 2019). However, the high levels of these greenhouse components may result in stress to the microalgae (Chiu et al. 2008, 2009, 2011). The stress response of these photosynthetic microorganisms also depends upon the duration of exposure of the culture to the potentially toxic gases (Makino and Mae 1999; Nagase et al. 2001). The concentration of CO2 influences not only the growth rate, morphology, and activity of proteins involved in carbon acquisition by CO2-concentrating mechanisms (CCMs) but also the overall photosynthetic rates and capacity, as well as the organization of the photosynthetic apparatus and its performance (Spalding et al. 1984; Pierangelini et al. 2014a; Li et al. 2015). Therefore, besides understanding the growth and macromolecular composition of algae under elevated levels of CO2 and NO, it is also important to study the physiological effects of high concentrations of these gases in order to better exploit algal species for flue gas treatment and possible biofuel production.
Chlorophyll (Chl) a fluorescence analysis is a rapid, powerful, and non-invasive technique that is widely used in physiological and ecophysiological studies of plants and algae (Stirbet et al. 1998; Baker 2008). At room temperature, the fluorescence signal of plants and algae occurs in the spectral region of 680–740 nm and mainly originates from photosystem II (PSII) (Spalding et al. 1984; Maxwell and Johnson 2000; Strasser et al. 2004). The most pervasive use of Chl a fluorescence in microalgae is in examining the performance of PSII photochemistry, linear electron flow and CO2 assimilation (Baker 2008). Another application of Chl a fluorescence is in the monitoring of the structural and conformational changes in photosystems that accompany the photosynthetic reactions (Strasser et al. 2004). It is widely acknowledged that PSII in the chloroplasts of green algae, as well as in higher plants, is heterogeneous in relation to both the antenna (α and β heterogeneity) and reducing sites of P680 (QB and non-QB), and the reaction center chlorophylls of PSII (Guenther and Melis 1990). The antenna heterogeneity is defined by the biphasic Chl a fluorescence induction kinetics of PSII activity, which arises as the reaction centers (RC) progressively close upon photoreduction of the primary quinone electron acceptor of PSII (QA). The biphasic nature of the kinetics confirms the existence of two types of PSII centers in the chloroplast. The first phase, PSIIα (fast phase), is shown by the nonlinearity of a semi-logarithmic plot of fluorescence versus time and corresponds to sigmoidal induction kinetics. This sigmoidicity can be explained by the presence of α centers in a static pigment bed that allows energy transfer between PSII units. The second phase, PSIIβ (slow phase), is reflected by the linearity of the semi-logarithmic plot of fluorescence versus time and corresponds to exponential induction kinetics. The exponentiality of β centers can be explained by their existence as “separate packages” in which energy transfer from closed to open centers is not possible (Black et al. 1986). The rate difference between PSIIα and PSIIβ corresponds to their different light-harvesting antenna sizes. In mature chloroplasts of wild-type vascular plants, PSIIα accounts for 75–80% of all PSII centers and PSIIβ accounts for the remaining 20–25% (Guenther and Melis 1990).
Although the effects of CO2 and NO on the growth, biochemical composition, and CO2 concentrating mechanism (CCM) activity in algae have been widely studied (Nagase et al. 1997; Basu et al. 2013; Varshney et al. 2015, 2016, 2018), the effect of high levels of these gases on the performance of the photosynthetic apparatus and PSII heterogeneity in algae has not been adequately investigated (Spalding et al. 1984; Iwasaki et al. 1996; Bhola et al. 2016). Also, there can be large variability in the acclimation of photosynthetic characteristics and the defense strategies adopted by different algal species in response to certain environmental stress, and that may not always match the theoretical expectation (Vincent and Roy 1993; Ainsworth and Rogers 2007). Hence, in this study, we focussed on the changes in the functional and structural aspects of the photosynthetic apparatus of two high-CO2 and NO-tolerant freshwater green algae upon their prolonged exposure to industrial levels of CO2 and NO, by using in vivo Chl a fluorescence technique (PAM, FFI, and OJIP analysis). We also measured the light-saturated photosynthetic oxygen evolution and dark respiration rates (Pmax and Rd) of these species in response to elevated levels of CO2 and NO to better understand the effects of these gases on the photosynthetic performance of algae.
Materials and methods
Microorganisms and cultivation conditions
Two freshwater green algae species, Asterarcys quadricellulare and Chlorella sorokiniana, which were isolated from steel plant effluent water, were used in this study. These species were characterized phylogenetically and morphologically in a previous study (Varshney et al. 2018). The species were grown in Bold’s Basal medium (BB) at pH 6.8 in a Photo Multi-Cultivator MC 1000-OD (Photon System Instruments, Czech Republic) and during experiments the pH of the cultures was not controlled. The details of the photobioreactor setup have been specified in the previous study (Varshney et al. 2018). The BB media was prepared according to the medium recipe reported by the Culture Collection of Algae and Protozoa (CCAP) (https://www.ccap.ac.uk/media/documents/BB.pdf). The temperature of the cultivation vessels was maintained at 37 °C and a peak light intensity of 250 μmol photons m−2 s−1 was applied in a 14:10 light-dark (LD) cycle following a 14 h sine function. All the experiments were performed on triplicate-independent cultures and the initial cell concentration of 0.6 × 106 cells mL−1 was maintained in each vessel. Since the maximum working volume in each vessel was only 85 mL, the cultures were grown in semi-continuous culture mode, so that sufficient samples were available for different analysis. Initially, the sampling was performed in mid-exponential phase and the cultures were diluted to the previous day’s cell concentration. Once the sampling in mid-exponential phase was completed, the cultures were allowed to grow into stationary phase and the same sampling procedure was repeated.
The typical concentration of CO2 in the selected steel plant flue gas was 15–20% v/v, thus three gradually increasing concentrations of CO2, namely 5, 10, and 15% v/v, were prepared by mixing U.H.P. grade pure CO2 with compressed air. The filtered compressed air was used to grow cultures in ambient CO2 conditions (0.04% CO2). Since it has already been shown that A. quadricellulare does not grow at 15% CO2 (Varshney et al. 2018), this alga was tested only up to a CO2 level of 10% v/v. Further, since both the species exhibited maximum growth rate at 5% CO2, this level of CO2 was chosen for further experiments using two levels of simulated flue gas, namely 5% CO2 + 80 ppm NO and 5% CO2 + 160 ppm NO, obtained by blending gas from a cylinder that contained 500 ppm NO in N2, with CO2 and compressed air. While C. sorokiniana was tested for both blends of CO2 and NO, A. quadricellulare was only tested under 5% CO2 + 80 ppm NO. The gas mixtures were bubbled through the cultivation vessels at a fixed aeration rate of 0.45 vvm (gas volumetric flow rate per unit volumetric culture medium per minute). The initial inoculum was taken from log-phase stock cultures that were prepared by growing cells under the same conditions for at least 2 weeks; the cells underwent regular sub-culturing at every third or fourth day when they were in their late exponential phase.
Except for flash fluorescence induction (FFI) curves, all the other analyses presented here are based only on measurements performed on mid-exponential phase samples. The effect of NO was assessed through the phytoplankton pulse amplitude-modulation fluorometry (Phyto-PAM) analysis and photosynthetic oxygen evolution measurements.
Rapid light curve measurements
The photosynthetic performance of algae was examined by chlorophyll fluorescence measurements of PSII activity via rapid light curves (RLCs) generated using a pulse amplitude modulated fluorimeter (Phyto-PAM, Heinz Walz, Germany). Briefly, a 3.5 mL algal sample was carefully collected to minimize perturbation to the dissolved gases and dark acclimated for 15 min, which is usually sufficient in dense microalgal cultures (Masojídek et al. 2010). The sample was then exposed to a weak modulated measuring light beam at an intensity that did not excite the PSII reaction centers (i.e., low enough to avoid photochemistry) and resulted in a minimal level of fluorescence (Fo). Further, the sample was exposed to a short (0.2 s) saturating beam of very bright red light (655 nm), which resulted in the maximum reduction of QA, and the maximal fluorescence level Fm was attained. The difference between Fm and Fo was defined as variable fluorescence, Fv, and the ratio of Fv/Fm was used to calculate the maximum quantum yield of photosynthetic energy conversion in PSII. The RLCs were constructed by exposing culture samples to 20 increasing irradiance levels from 8 to 610 μmol photons m−2 s−1. Each light exposure was for 30 s and was followed by a saturating pulse to allow the determination of effective quantum yield under actinic light (non-modulated light that is absorbed by the photosynthetic apparatus and that will drive photosynthesis). The relative electron transport rate was calculated from the effective quantum yield and the incident photosynthetically active radiation (PAR), using Eq. (1):
where A is the absorptance that is assumed to be 0.84 for higher plant leaves, which means that 84% of the incident PAR is absorbed by the leaves. Similarly, FractionPSII for plant leaves is taken as 0.5, under the assumption that 50% of the absorbed PAR is distributed to PSII (that is, absorbed energy is evenly distributed between the two photosystems, PSI and PSII). Since the absorptance was not measured but was assumed, ETR is here expressed as relative values (rETR) (Baker 2008). RLCs are also used to calculate the light harvesting efficiency (α), light-saturated rETR (rETRmax), and the light intensity at which rETR saturates (Ik). The α represents the initial slope of the rETR versus light curve, and Ik corresponds to the PAR value that is located at the intersection of the initial slope (α) and a line that is parallel to the PAR axis and originates from rETRmax (Fig. 1, Supplementary Information). Numerically, Ik is derived from the expression rETRmax/α (Ralph and Gademann 2005). All the above parameters were calculated by the in-built PhytoWin software that provides routines for fitting the RLC parameters using mechanistic model of Eilers and Peeters (1988). The light intensity at which rETR saturated was then used to obtain the maximum photosynthetic rate (Pmax) from the oxygen evolution experiments.
Non-photochemical quenching analysis
The Phyto-PAM fluorimeter was also used to perform analysis of non-photochemical quenching (NPQ), which is an estimation of the capacity of cells to dissipate excess light energy as heat (Kalaji et al. 2014). A 15 min dark-adapted sample was exposed to a weak measuring light beam and then to a saturating light pulse to determine the values of Fo and Fm, respectively. The measurement was continued by switching on an actinic light source of an intensity of 480 μmol photons m−2 s−1 (chosen from RLCs to be supra-saturating for electron transport) for 10 min to induce photosynthesis, during which a 20 s cycle of saturating red pulses was applied until a stable value of maximum fluorescence yield in the light (Fm′) was attained. We used the Stern-Volmer approach (Eq. 2) as the NPQ values generated by the fluorometer software as part of the data generation during RLC can sometime be less reliable as they are taken over a short time frame rather than at a steady state.
Photosynthetic oxygen evolution measurements
The rates of light-saturated photosynthetic oxygen evolution (Pmax) and dark respiration (Rd) were measured using a Clark-type oxygen electrode (Hansatech, UK) at 37 °C. For A. quadricellulare, 15 × 106 cells, and for C. sorokiniana, 30 × 106 cells were harvested from the culture and resuspended in 2.0 mL of fresh culture medium that was buffered with 20 mM HEPES at pH 6.8; the medium contained 2.0 mM inorganic carbon (NaHCO3). Samples were placed into the O2 electrode chamber and were continuously stirred at 75 rpm by a magnetic flea that was placed within the electrode chamber. The cell suspension was bubbled with N2 gas to reduce the O2 concentration to 20% of air saturation (Yang and Gao 2003). The cells were then dark acclimated for 20 min or until the oxygen concentration decreased at a stable rate and the ultimate 3 min of this incubation was used to measure the Rd. The saturating light intensity, which provided rETRmax, as determined from PAM RLC measurements was then applied to the samples to obtain Pmax. The rates of oxygen evolved or consumed by the cells were calculated by the Oxygraph Plus software. The gross photosynthetic rate (Gross P) was calculated by correcting the net exchange rate of O2 (Pmax) for the absolute value of dark respiration rate (Rd) by using Eq. (3) (Henley 1995).
where Rd is expressed as (positive) oxygen consumption.
Flash fluorescence induction curves
Flash fluorescence induction (FFI) curves were generated using a double-modulation kinetic fluorimeter FL-3000 (Photon Systems Instruments, Czech Republic). Briefly, samples containing 10 × 106 cells of A. quadricellulare or 40 × 106 cells of C. sorokiniana were harvested and dark acclimated for 15 min. A single turnover saturating flash of 100% light intensity (equivalent to 208,000 μmol photons m−2 s−1) (Nedbal et al. 1999; Dao and Beardall 2016a) was applied for 50 μs, with the detector sensitivity set at 5%. Based on the equations of Lavergne and Trissl (1995), the rise of fluorescence after applying the saturating flash was numerically fitted to a theoretical fluorescence induction curve. This was done in order to calculate minimum fluorescence (Fo), maximum fluorescence (Fm), functional absorption cross-section (σPSII), sigmoidicity (J), PSIIα/PSIIβ stoichiometry, and reaction center excitation rates (Lavergne and Trissl 1995; Nedbal et al. 1999; Vinet and Zhedanov 2011).
Chl a fluorescence transients (OJIP test)
The progressive reduction of the electron transport chain (ETC) leads to the polyphasic rise of Chl a fluorescence from Fo to Fm, in four major steps, namely O, J, I, and P, which are known as OJIP transient (Fig. 2, Supplementary Information). These transients yield information about the quantitative characteristics of PSII and the heterogeneity that is involved in the reduction of the PQ pool (Antal and Rubin 2008; Markou et al. 2017). Their shape is a sensitive indicator of changes in different environmental conditions such as light intensity, temperature, CO2 concentration, and chemical influences etc. (Strasser et al. 2004). For our experiments, we recorded these transients by using the same double-modulation kinetic fluorimeter FL-3000 as described in the previous section. To generate the curves, a multiple turnover flash at 100% light intensity was applied to the sample that had been dark acclimated for 15 min. Measurements were conducted for 1 s and were recorded at every 10 μs up to 2 ms and then at every 1 ms until 1 s. The fluorescence intensity was observed to increase from a minimum value Fo = F50μs (O-step), via two intermediate steps, FJ = F2ms (J-step) and FI = F30ms (I-step) to a maximum value, Fm (P-step). It has been reported that under stress conditions when the oxygen-evolving complex (OEC) of PSII is completely destroyed, an earlier step at approximately 300 μs develops in the fluorescence rise from O-P and is labeled as the K-step (Schmitz and Maldonado-Rodriguez 2001; Strasser et al. 2004; Zhao et al. 2008). To standardize the results, all the fluorescence values were normalized to Fo. By using Fo, FJ, FI, and Fm values, the key JIP-test parameters were derived by following the equations of Strasser et al. (2004).
The relative variable fluorescence values at step K and J were calculated according to Eq. (4) and Eq. (5).
The maximum quantum yield of primary photochemistry(φP0) was calculated using Eq. (6). It is considered as a synonym of efficiency and is the ratio of the total energy flux that is trapped by the PSII RCs (when all the RCs are open) and is used for photochemistry at time zero (TRo) to the total energy influx that is absorbed by the antenna pigments (ABS) (Fig. 3, Supplementary Information).
Furthermore, the probability (Ψo) that a trapped exciton moves an electron into the ETC beyond QA− was calculated by Eq. (7). It is the ratio of trapped excitation energy that goes beyond QA oxidation (ETo) to the energy flux that was trapped by the PSII RC (TRo).
The net rate of closure of RCs was calculated from the initial slope of fluorescence transient (Mo) by using Eq. (8), where trapping flux (TRo) increases the number of closed RCs and electron flux (ETo) decreases it.
The performance index (PI, on absorption basis), which is a product of the terms that represent the energy bifurcations in PSII, was calculated using Eq. (9)
The hyperbola constant or curvature constant (CHYP) that is equivalent to the sigmoidicity parameter “J”, and the overall grouping probability (p2G) were calculated according to Eq. (10) and Eq. (11). CHYP is a product of energy fluxes at two extremes (with all RCs open or closed) and the probabilities of all types of energetic communication among the PSII pools, which is commonly known as overall grouping probability (p2G).
where,
Finally, the specific energy flux (per fully active, that is, QA reducing PSII RC) of absorption (ABS), was calculated using Eq. (12). It is an estimation of the average amount of absorbing chlorophylls per (active) RC, that is, the apparent antenna size (Strasser et al. 2004; Zhang et al. 2010a).
Statistical analysis
Comparison of parameters (obtained during log phase) with respect to changing gas concentrations was tested by one-way ANOVA, which was then followed by a Tukey multiple-comparison test. Parameters involving the additional variable of changing growth phase were compared by using two-way RM ANOVA, which was followed by a Sidek multiple-comparison test. All the analyses were carried out by using the statistical software GraphPad Prism version 7.02 for Windows, GraphPad Software, USA, www.graphpad.com. A P value ≤ 0.05 was considered statistically significant.
Results
RLC measurements
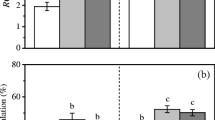
We find the Fv/Fm values for both the species to be in the range of 0.68–0.77 under the conditions tested (Fig. 1a, b). The high-CO2-grown cells of C. sorokiniana exhibited higher yields of PSII than the ambient-grown cells with the highest Fv/Fm of 0.77 under 5% CO2. The Fv/Fm values did not change significantly between 5% and 10% CO2 and 5% CO2 + 80 ppm NO-grown cells. Whereas, the Fv/Fm values of the 15% CO2 and 5% CO2 + 160 ppm NO-grown cells were found to be lower than that of 5% CO2-grown cells. On the other hand, the Fv/Fm values of A. quadricellulare remained unchanged at around 0.70 under all the conditions tested. Further, similar to the pattern observed for Fv/Fm, the rETRmax of C. sorokiniana appeared to be more responsive to CO2 concentration than A. quadricellulare. When the CO2 level for growth was increased from 0.04 to 5% v/v, the rETRmax in the former species increased by 55.8%, while in the latter it only increased by 8.6% (Fig. 1c, d). For both the species, the maximum rETRmax was attained at 5% CO2, and no significant difference was observed between the rETRmax of 5% and 10% CO2 cultivated cells. In C. sorokiniana, the rETRmax declined as the CO2 level was raised from 10 to 15%; however, blending 80 ppm NO with 5% CO2 did not affect this parameter, and it remained in the range of 5% and 10% CO2 cultivated cells. A further increment in NO from 80 to 160 ppm led to a decline in rETRmax to the level of 15% CO2 cultivated cells. In the case of A. quadricellulare, although Fv/Fm remained unaffected, a marginal decline of 7% in rETRmax was observed when cells that were grown under 10% CO2 were compared with cells that were cultivated at 5% CO2 + 80 ppm NO gas concentration.
Effect of CO2 and NO levels on the a, b maximum quantum yield of PSII (Fv/Fm); and c, d maximum relative electron transport rate (rETRmax) of C. sorokiniana and A. quadricellulare, respectively. Experiments were conducted in a semi-continuous culture mode at 37 °C and 14:10 LD cycle that had a light regime of 14 h sine function at an amplitude of 250 μmol photons m−2 s−1. Air that contained specific concentrations of CO2 and NO was bubbled through the cultivation vessels at an aeration rate of 0.45 vvm. The bars that are marked with different letters are significantly different (P < 0.05). (Data are shown as mean ± SEM, n = 3)
For C. sorokiniana, the value of α remained unchanged up to 10% CO2 but declined marginally at 15% CO2. However, in A. quadricellulare, the α value at 10% CO2 was slightly higher than that observed at ambient CO2 but was in the range of α values at 5% CO2 and 5% CO2 + 80 ppm NO (Fig. 2a, b). In C. sorokiniana, a higher Ik was observed under elevated CO2 concentrations than at ambient conditions and Ik in A. quadricellulare remained constant across all the tested gas levels (Fig. 2c, d).
Effect of CO2 and NO levels on the a, b light harvesting efficiency (α) and c, d light saturation parameter (Ik) of C. sorokiniana and A. quadricellulare, respectively. Refer to the legend to Fig. 1 for additional details. Since we did not measure true ETR, α values are only relative
NPQ analysis
In the exponential growth phase, ambient-grown cells of C. sorokiniana had the lowest NPQ capacity, which increased as the CO2 concentration was raised to 5% v/v. No significant difference between the NPQ capacity of 5% and 10% CO2-grown cells was noted. However, the NPQ declined as the CO2 level was further increased to 15% v/v. During the stationary phase, the ambient-grown cells showed an increase in NPQ, while the cells that were cultivated under high-CO2 levels exhibited negligible NPQ (Fig. 3a). In the case of A. quadricellulare, during the log phase, the ambient-grown cells exhibited maximum NPQ capacity, and NPQ declined with increasing CO2 concentration. However, except for the ambient condition, NPQ increased as the cells moved from log to stationary phase (Fig. 3b).
Effect of CO2 levels on the NPQ capacity of aC. sorokiniana and bA. quadricellulare, respectively. The bars that are marked with different letters are significantly different. The asterisks indicate significant differences between the log and stationary phases of cells at each gas concentration (P < 0.05). Refer to the legend of Fig. 1 for additional details
Overall, the photosynthetic parameters that were obtained from the Phyto-PAM measurements reveal that C. sorokiniana was more responsive to changing gas concentrations and had a higher tolerance to CO2 and NO than A. quadricellulare.
Dark respiration and photosynthesis
Both Pmax and Rd were stimulated under elevated levels of CO2 compared to values under ambient conditions. The saturating light intensity that was used to obtain Pmax was 505 and 435 μmol photons m−2 s−1 for C. sorokiniana and A. quadricellulare, respectively. In general, the magnitudes of Pmax and Rd of A. quadricellulare were always higher than those of C. sorokiniana. For both species, Pmax increased linearly from ambient to 10% CO2 concentration. Further, in C. sorokiniana, Pmax declined by ~ 31% as the CO2 concentration increased from 10 to 15% v/v. For both species, NO-supplemented cells exhibited lower Pmax values than those obtained for cells that were cultivated in mixtures of only air + CO2 (Fig. 4a, b).
Effect of CO2 and NO levels on the a, b maximum photosynthetic rates (Pmax.), c, d dark respiration rates (Rd), and e, f gross photosynthetic rates (Gross P) of C. sorokiniana and A. quadricellulare, respectively. Refer to the legend of Fig. 1 for additional details
We observed that C. sorokiniana exhibited the highest Rd at 5% CO2-level, and this declined as the CO2 concentration for growth was increased further to 10% v/v. No significant difference between the Rd of 10% and 15% CO2-grown cells was observed; however, Rd declined further when CO2 was blended with NO. On the other hand, in A. quadricellulare, Rd followed the same pattern as that of Pmax (Fig. 4c, d).
FFI curve analysis
The Fv/Fm values obtained from the FFI technique exhibited a pattern that was similar to the values that were obtained from the PAM-RLCs. The stationary phase measurements of FFI curves show that Fv/Fm declined as the cultures moved from the exponential to the stationary phase (Fig. 5a, b). The antenna connectivity parameter JconPSIIα is dependent on both the connectivity of photosynthetic units as well as the reaction center parameters. In both the species, the JconPSIIα was higher during the log phase than that was observed during the stationary phase. Moreover, in C. sorokiniana, the JconPSIIα of elevated-CO2-grown cells was higher than that of ambient-grown cells, and a good correlation was observed between the antenna connectivity and NPQ. On the contrary, despite the lower NPQ capacity of 5% CO2-grown cells of A. quadricellulare, their JconPSIIα was higher than that of ambient and 10% CO2-grown cells, which represent a weaker correlation between JconPSIIα and NPQ (Fig. 5c, d).
Effect of CO2 concentration and the changing growth stage on the a, b maximum quantum yield of PSII (Fv/Fm) and c, d antenna connectivity parameter of PSIIα (JconPSIIα) of C. sorokiniana and A. quadricellulare, respectively. The statistical representation is as described in the legend to Fig. 3. Refer to the legend of Fig. 1 for additional details
During the log phase, there was no alteration in the antenna heterogeneity, whereas during the stationary phase elevated CO2 grown cells exhibited a higher percentage of PSIIα and a lower percentage of PSIIβ than that which was seen in ambient-grown cells. Thus, overall, as the cells moved from the exponential to stationary phase, there was a significant rise in the percentage of PSIIα centers and a drop in the percentage of PSIIβ centers of high-CO2-grown cells while the heterogeneity remained unaltered in ambient-grown cells (Fig. 6a–d).
Effect of CO2 levels and the changing growth stage on the a, b proportion of α centers of PSII, PSIIα and c, d proportion of β centers of PSII, PSIIβ of C. sorokiniana and A. quadricellulare, respectively. The statistical representation is as described in the legend to Fig. 3. Refer to legend of Fig. 1 for additional details
In C. sorokiniana, the log phase σPSIIα of ambient-grown cells was marginally higher than that of high-CO2-grown cells. As the cells moved to stationary phase, a remarkable increase in the values of σPSIIα was observed; however, this increment was more pronounced under high CO2 than under an ambient condition (Fig. 7a). On the contrary, in A. quadricellulare, the log phase value of σPSIIα remained unchanged under all the tested levels of CO2, and no significant change was observed even when the cells progressed to the stationary phase (Fig. 7b). Further, in C. sorokiniana, varying CO2 concentration did not have any significant effect on the log phase values of σPSIIβ (Fig. 7c). However, the log phase values of σPSIIβ in A. quadricellulare were higher for elevated CO2-grown cells than for ambient-grown cells. Though an increase in the σPSIIβ value of ambient-grown cells was observed with the changing growth stage, this parameter remained unchanged in high-CO2-grown cells (Fig. 7d).
Effect of CO2 levels and the changing growth stage on the a, b effective absorption cross-sectional area of PSIIα centers, σPSIIα and c, d effective absorption cross-sectional area of PSIIβ centers, σPSIIβ of C. sorokiniana and A. quadricellulare, respectively. The statistical representation is as described in the legend to Fig. 3. Refer to the legend of Fig. 1 for additional details
OJIP transients
Fluorescence induction (FI) curves of A. quadricellulare cells that were cultivated under ambient air showed lower overall fluorescence intensity than those that were cultivated under high-CO2 (Fig. 8a). On the contrary, for C. sorokiniana, the fluorescence intensity remained unaltered up to 10% CO2; however, significant changes were observed as the CO2 level was raised to 15% v/v (Fig. 8b). Furthermore, despite the decline in the fluorescence intensity, the shape of the OJIP curve was not greatly affected. Based on these fluorescence signals, selected parameters were calculated for A. quadricellulare and C. sorokiniana (Tables 1 and 2, respectively).
OJIP transients of cells of aA. quadricellulare and bC. sorokiniana cultivated under different CO2 concentrations. Refer to the legend of Fig. 1 for details on operating conditions
In C. sorokiniana, the elevated concentrations of CO2 led to lower values of VJ than those that were obtained under an ambient CO2 level. However, in the case of A. quadricellulare, the value of VJ remained unchanged under all tested CO2 levels. Further, in C. sorokiniana, the φP0 of ambient-grown cells was lower than that of cells that were cultivated under elevated CO2. However, no significant difference between the φP0 values of 5%, 10%, and 15% of CO2-grown cells was observed. On the other hand, in A. quadricellulare, φP0 remained unchanged under all the tested CO2 levels. These results agree well with the Fv/Fm values that were obtained from FFI measurements. A similar pattern was also obtained for the yield of electron movement into the ETC per trapped exciton (Ψo) (Tables 1 and 2).
In C. sorokiniana, the absorption flux per reaction center (ABS/RC) of ambient-grown cells was more than that of 5% and 15% CO2-grown cells even though the Chl a content of the former was lower than that of the latter (Table 1, Supplementary information). Also, despite having a smaller antenna size than those of ambient and 10% CO2-treated cells, the highest Chl a content of 5% CO2-grown cells indicates that the largest number of active RCs occur at this gas concentration. Similarly, regardless of the lower Chl a content in 10% CO2-grown cells (Table 2, Supplementary information), the comparable ABS/RC of ambient and 10% CO2-grown cells in A. quadricellulare is an indicator of the presence of a larger number of inactive PSII centers at 10% CO2. Since we could not find any specific pattern in other flux values such as trapping flux (TRo/RC), electron transport flux (ETo/RC), and dissipated energy flux (DIo/RC), they have not been discussed further here.
The JIP analysis shows that the performance index on absorption basis (PIABS) of the high CO2-grown cells of C. sorokiniana was significantly higher than that of the ambient-grown cells, and the PIABS values of 5%, 10%, and 15% CO2-grown cells were comparable to each other. This is in agreement with the Fv/Fm values that were obtained from FFI measurements. In contrast, in A. quadricellulare, even though Fv/Fm remained unchanged under different CO2 levels, PIABS went up when the CO2 concentration was increased from ambient to 5% but decreased at 10% CO2.
Similar to the sigmoidicity parameter J (obtained from the FFI measurements), a few other parameters such as the CHYP and p2G, obtained from OJIP transients, are typically used to compare the connectivity between the PSII units. In C. sorokiniana, growth under 5% and 10% CO2 led to better energetic connectivity (higher CHYP and p2G) between the PSII units than that was observed under ambient conditions. However, further increase in CO2 levels to 15% v/v significantly reduced the connectivity between the PSII units and the numerical values of CHYP and p2G were even smaller than those that were obtained under ambient conditions. Likewise, in A. quadricellulare, 5% CO2-grown cells exhibited better energetic connectivity between the PSII units over ambient cells, while 10% of CO2 grown cells exhibited the lowest connectivity among all the three CO2 treatments.
Discussion
Our results show that statistically significant differences in the performance and composition of the photosynthetic physiology of C. sorokiniana and A. quadricellulare were observed under elevated concentrations of CO2 and NO. The Fv/Fm is often used as a sensitive indicator of stress in organisms that perform oxygenic photosynthesis. For healthy and non-stressed cells, this value is approximately 0.83 for most vascular plants, and between 0.6 and 0.65 for green algae; the yield declines as stress to PSII increases (Maxwell and Johnson 2000; Dao and Beardall 2016b). The PAM results show that under the tested gas levels, the Fv/Fm values of both the species were within the range for healthy green algae, which implies that the tested concentrations of CO2 and NO did not cause any stress to the overall functioning of PSII. The lower Fv/Fm that was obtained for ambient-grown cells of C. sorokiniana could be due to an increase in the backward flow of electrons from QA- to the water side in PSII. This reduces the normal transport of electrons from PSII to PSI, which leads to a reduction in the apparent concentration of reduced QA, thus resulting in a lower fluorescence yield. A similar phenomenon has been reported previously when air-adapted cells of Chlamydomonas reinhardii exhibited lower fluorescence yield than high CO2-cultivated cells (Mende 1980; Spalding et al. 1984). Further, the rETR is a measure of the number of electrons that pass through PSII per unit time; therefore, rETRmax can be used to estimate the capacity of the photosynthetic apparatus at saturating light (Ralph and Gademann 2005). The rETRmax of high CO2 grown cells was higher than that of ambient-grown cells. Reports in the literature suggest that under current levels of CO2 (~ 400 ppm), photosynthesis, in higher plants at least, can be limited by the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) (Bernacchi et al. 2005; Ainsworth and Rogers 2007). While microalgae possess CCMs to improve Rubisco carboxylation efficiency (Giordano et al. 2005), these will be repressed under the elevated CO2 concentrations used here. As the concentration of CO2 rises, the carbon fixation rate increases, which implies that the demand for ATP that is required for the regeneration of ribulose-1,5-bisphosphate (RuBP) also goes up. As a result, the control of photosynthesis shifts from RuBisCo to RuBP regeneration, and resource investment from carboxylation to electron transport takes place, which results in higher rETRmax at elevated levels of CO2 (Bernacchi et al. 2005; Ainsworth and Rogers 2007; Li et al. 2015). Under enhanced electron transport, the stimulated photosynthetic uptake of CO2 also led to improved growth rates (Varshney et al. 2018). Simulated growth rates have also been observed in a number of previous studies (Qiu and Gao 2002; Yang and Gao 2003). The parameter α is a function of both the light harvesting efficiency and the photosynthetic energy conversion efficiency (Henley 1993). Previous studies have also correlated changes in α with the changes in cell pigment composition or content and the associated proteins that are present in the light-harvesting complex (LHCs) (Pierangelini et al. 2014a; Kamalanathan et al. 2016), both of which can be affected by CO2. In C. sorokiniana, the lower value of α at 15% CO2 (compared to 5% CO2) can be correlated to the lower pigment content/cell at this CO2 level, which would further indicate a lower light harvesting ability at this gas concentration. However, in both the species, though the chlorophyll content was lower at 10% CO2, α at this CO2 level was comparable with values obtained for cells grown at 5% CO2 and 5% CO2 + 80 ppm NO, which suggests that the algae had alternate ways to compensate for the lower chlorophyll values up to 10% v/v CO2 (Table 1 and 2, Supplementary information). This result is in line with previous reports (Dao and Beardall 2016a). Ik is commonly used to compare the photoacclimation status of algae, both between as well as within species (Henley 1993). For C. sorokiniana, the higher Ik observed under elevated CO2 concentrations when compared to ambient-grown condition could be correlated to simulated photosynthetic capacity (rETR) of cells under high CO2. In contrast, the constant Ik for A. quadricellulare under all the tested gas levels imply that the ratio of rETRmax and α remained the same, despite the fluctuations in their individual values. Similar effects were also reported, for example, in the red macroalga Palmaria palmata and the cyanobacterium Cylindrospermopsis raciborskii, where the former did not show a significant increase in Ik, but the latter showed an enhanced Ik value under elevated CO2 levels (Pierangelini et al. 2014b; Sebök et al. 2017).
NPQ is a mechanism of regulating and protecting the photosynthetic apparatus when the absorption of light energy exceeds its utilization (Müller et al. 2001). The NPQ capacity of C. sorokiniana increased in going from ambient to 5% CO2 level. Iwasaki et al. (1996) also reported a marginal increase in the NPQ capacity of the marine green microalga Chlorococcum littorale, when its cells were shifted from ambient to a high-CO2 level (40% v/v). However, these researchers could not provide a rationalization for this change (Iwasaki et al. 1996). It is evident that under CO2-limited conditions (ambient-grown cells), the CCM is activated, which increases the uptake of inorganic carbon (Ci) into the cell, due to which the photosynthetic CO2-utilization efficiency also increases. However, the CCM is a highly energy-intensive process (Raven et al. 2014), and this could be a mechanism adopted by ambient-grown cells to dissipate excess excitation energy (Tchernov et al. 1998). In contrast, high-CO2-grown cells do not express CCMs; thus, an alternative dissipation process, NPQ, is used. Further, the lower value of NPQ at the 15% CO2 level might reflect the reduced performance of photoprotective mechanisms under this condition, which may lead to photodamage. Alternatively, A. quadricellulare exhibited maximum NPQ capacity under ambient conditions. It has been reported that during CCM induction, organisms shift their photosynthetic linear ETC to cyclic electron transport flow around PSI by means of a state transition (Falk and Palmqvist 1992; Pierangelini et al. 2014b). This satisfies the higher ATP requirements for active Ci transport. However, if energy dissipation via CCM activity and C assimilation is inadequate, then cells need to engage NPQ, as appears to be happening with the log phase cells of A. quadricellulare. Further, suppression of CCMs (under high CO2) results in high NADPH requirements, which stimulate NADP+ reduction by linear electron transport. Hence, NPQ decreases as the CO2 level goes up. A similar phenomenon has also been reported by Li et al. (2016), in which ambient-grown cells of C. sorokiniana exhibited greater NPQ capacity than 1% CO2-grown cells. Likewise, Sebök et al. (2017) also reported that in the red macroalga P. palmata, the need for energy dissipation via NPQ declined as the CO2 levels increased.
The higher magnitudes of Pmax and Rd for A. quadricellulare than those for C. sorokiniana could be due to bigger cells of the former than those of latter. The details on the growth rates (μmax) and cell size of these species have been provided previously (Varshney et al. 2018). In C. sorokiniana, despite the comparable μmax and rETRmax of 5% and 10% CO2-grown cells, the Pmax of the latter was marginally higher than that of the former. This indicates that along with the downregulation of CCM, the increase in CO2 concentration also enhanced the capacity of cells to carry out light-saturated photosynthesis and incorporate carbon into carbohydrates (Pierangelini et al. 2014b; Varshney et al. 2018). A further increment in CO2 level from 10 to 15% led to a decline in Pmax, which was also supported by the lower μmax, rETRmax, and the smaller cell size of the latter as reported previously (Varshney et al. 2018). Likewise, the lower Pmax of NO-supplemented cells can also be attributed to the smaller biovolume of these cells (Varshney et al. 2018).
In the present study, a decrease in Fv/Fm was observed when the culture moved from log to stationary phase. Previous reports suggest that this could be an indicator of PSII impairment and can be attributed to either a decline in the proportion of PSII RCs that are capable of performing photochemistry and/or an increase in NPQ (Maxwell and Johnson 2000; Dao and Beardall 2016a). In our case, under elevated CO2, a decline in the stationary phase Fv/Fm values of A. quadricellulare can be correlated to both the increased inactive RCs as well as to the increased NPQ. However, in C. sorokiniana, except for ambient-grown cells that did not show any difference between the log and stationary phase values of Fv/Fm, a decline in the stationary phase values obtained at all other gas levels can be attributed only to increased inactive RCs as NPQ was negligible during the stationary phase. Further, JconPSIIα describes the migration of excitation energy from closed to open PSII centers and the extent to which the PSII antenna can be described as a lake where PSII centers are energetically connected by a shared antenna, as opposed to an isolated unit where each PSII reaction center only receives energy from its neighboring antenna (Lavergne and Trissl 1995). The higher values of JconPSIIα observed for high-CO2-grown cells support the lake model and confirms the presence of more energetically connected antennae than that was observed for ambient grown cells. In addition, log phase cells exhibited better connectivity between RCs than the stationary phase cells.
It has been reported that the antenna heterogeneity varies both with alteration in growth conditions as well as in growth stage (Marchin et al. 2014). Further, the PSIIα centers are distinguished from PSIIβ centers by their bigger antenna size (2–3 times bigger than that of PSIIβ), higher degree of excitation transfer between themselves and the plastoquinone pool (high degree of connectivity), and their inability in the transferring of energy to PSI (Marchin et al. 2014; Pierangelini et al. 2014b). Previous reports suggest that under CO2-enriched conditions when the CCM is downregulated, a higher percentage of PSIIα centers and a lower percentage of PSIIβ centers are attained in comparison to the proportions that are attained under CO2-limited conditions (Pierangelini et al. 2014b). Also, when the cells are stressed, the number of inactive RCs rises, and the contribution of PSIIβ centers and QB non-reducing centers goes up (Markou et al. 2017). However, in the present study, a decline in PSIIβ reaction centers during the stationary phase could be correlated to a transition from state 2 to state 1, which leads to conversion of PSIIβ to PSIIα and either of PSIIα or PSIIβ can be active or inactive (Pierangelini et al. 2014b; Dao and Beardall 2016a).
Further, one of the fundamental properties that are responsible for the efficient functioning of the photosynthetic light-harvesting antenna (open PSII units) is its functional absorption cross-sectional area (σPSII), which is expressed in terms of area (Å2) (Ihnken et al. 2011; Ruban et al. 2011). It defines the size of the antenna pigments transferring excitation to the functional PSII RC and therefore, the rate at which photons are delivered to PSII RCs for a given photon flux to the antenna (Cosgrove and Borowitzka 2010). Similar to the present study, Pierangelini et al. also reported that the σPSIIα of high-CO2-cultivated cells of C. raciborskii CS506 was lower than that of low-CO2-cultivated cells (Pierangelini et al. 2014b). The smaller antenna size can increase the photon use efficiency of algae as it minimizes the wasteful dissipation of absorbed light energy and allows better light penetration (Melis et al. 1998; Seth and Wangikar 2015). This could be the cause of higher light-saturated photosynthetic rates (Pmax) under high-CO2 levels. In contrast, a significant increase in the antenna size during the stationary phase could be a way to compensate for the damage (lower Fv/Fm in the stationary phase, see Fig. 5a) that is caused by prolonged exposure to such high concentrations of CO2.
A number of reports suggest that when the algal cells are cultivated under stress conditions such as high temperature (Zhao et al. 2008), high salt concentration (Zhang et al. 2010b), high metal concentration (Zhang et al. 2010a), and ammonia toxicity (Markou et al. 2016), the Fm value decreases and the shape of the FI curve becomes almost flat. This decrease in Fm and fluorescence levels can be attributed to the inhibition of electron transport after QA, and the inability of the OEC to provide electrons to PSII and to reduce PQ (Strasser and Srivastava 1995; Markou et al. 2017). In the present study, despite the decline in the fluorescence intensity, the relatively unaltered shape of the OJIP curve suggests that the growth inhibition was not severe. Among the calculated parameters, VJ is used as an estimation of QB non-reducing RCs, that is, the number of reaction centers that cannot transfer electrons from QA to QB (Jianrong and Qiran 2009; Markou et al. 2016). In the case of C. sorokiniana, the lower values of VJ under elevated concentrations of CO2 indicate the stronger limitation of electron transfer from QA to QB under ambient conditions. However, the constant value of VJ for A. quadricellulare under all the tested CO2 levels implies that the capacity for reoxidation of QA− remained unaltered with changing CO2 concentrations. The higher ABS/RC of ambient-grown cells than that of 5% and 15% CO2-grown cells could be correlated to either the presence of a larger number of inactive PSII centers or an increase in the accumulation of PSIIβ centers. In our case, the accumulation of PSIIβ did not change with changing concentrations of CO2 (from results of FFI analysis); therefore, the observed higher ABS/RC is likely to be associated with an increase in the inactive PSII centers. In addition, the highest Chl a content of 5% CO2-grown cells indicate the presence of the largest number of active RCs that occur at this gas concentration. The parameter, ABS/RC, represents the incident photon flux that was absorbed by the antenna pigments, thus forming the excited chlorophyll, Chl* (Strasser et al. 2004; Dao and Beardall 2016a). Similarly, regardless of the lower Chl a content in 10% CO2-grown cells, the comparable ABS/RC of ambient and 10% CO2-grown cells in A. quadricellulare is an indicator of the presence of larger number of inactive PSII centers at 10% CO2. As evident from Eq. 9, the PIABS combines three different functional steps of photosynthesis such as the (i) absorption of light energy, (ii) trapping of excitation energy, and (iii) conversion of trapped energy to electron transport, into one parameter and computes the PSII photosynthetic activity (Kalaji et al. 2014; Dao and Beardall 2016a). The PIABS of C. sorokiniana, obtained from OJIP analysis, was consistent with the Fv/Fm obtained from FFI measurements. However, in A. quadricellulare, despite the constant Fv/Fm for all the tested CO2 levels, the overall photosynthetic activity of 5% of CO2 grown cells was more than that of ambient-grown cells. This is because the PIABS is a more sensitive indicator of stress in photosynthesis than Fv/Fm alone and the effect of varying concentrations of CO2 on the other steps, which have been discussed in “OJIP transients” section, was more profound than that on the primary photochemistry alone.
Other parameters such as p2G and CHYP, which have been recommended for evaluating the connectivity between the PSII units, were also derived from the OJIP transients. The lower values of p2G and CHYP signify lower connectivity between PSII units, which also implies that only a smaller fraction of excitation energy is used for photochemistry and the larger portion is dissipated as heat and fluorescence. On the contrary, higher p2G and CHYP reflect the clear sigmoidal shape of the fluorescence transient and higher energetic connectivity between the PSII units. Although it has been reported previously that a decline in connectivity between PSII centers is correlated with the decrease in the percentage of PSIIα centers (Kyle et al. 1982), we did not observe any change in the proportion of PSIIα centers with changing connectivity.
Overall, although there was a drastic effect on the photosynthetic apparatus and performance of C. sorokiniana at 15% CO2, A. quadricellulare showed almost consistent performance across all tested CO2 concentrations.
Conclusion
This study demonstrated the long-term (spanning several weeks) effect of elevated levels of CO2 and NO, which are typically present in the industrial flue gases, on the photosynthetic mechanism of two freshwater green microalgal isolates. Under different gas concentrations, the photosynthetic regulatory patterns in the selected species were significantly different from each other. However, both the species maintained their photosynthetic capacity by using simple, low biological cost mechanisms of antenna size and changes in the proportion of PSII centres, in parallel with the alterations in the energetic connectivity between PSII units. The photosynthetic efficiency for carbon acquisition was significantly higher at elevated CO2 levels than that was observed under ambient conditions. The optimal CO2 concentration for both the species was 5% v/v; however, the photosynthetic apparatus was not under much stress up to a CO2 level of even 10% v/v. Moreover, the blending of NO (with CO2 and air) up to the tested levels did not have any severe impact on the quantum yield and photosynthetic capacity of algal cells. Although, the photosynthetic performance observed under laboratory conditions cannot fully predict the behavior of algae in the field because diurnal changes in the solar photon flux density, cloud cover, temperature cues, and vertical migrations are just a few of the other factors that also induce considerable changes in the photochemical properties of algae. Nonetheless, this bench-scale study on the selected novel algal species is the first of many steps that ensures that the selected candidates are suitable for further investigations in large-scale photobioreactors and ponds for CO2 and NO mitigation from flue gases.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Antal T, Rubin A (2008) In vivo analysis of chlorophyll a fluorescence induction. Photosynth Res 96:217–226
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Basu S, Roy AS, Mohanty K, Ghoshal AK (2013) Enhanced CO2 sequestration by a novel microalga: Scenedesmus obliquus SA1 isolated from bio-diversity hotspot region of Assam, India. Bioresour Technol 143:369–377
Bernacchi CJ, Morgan PB, Ort DR, Long SP (2005) The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220:434–446
Bhola VK, Swalaha FM, Nasr M, Kumari S, Bux F (2016) Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. Eur J Phycol 51:401–412
Black MT, Brearley TH, Horton P (1986) Heterogeneity in chloroplast photosystem II. Photosynth Res 8:193–207
Chiu SY, Kao CY, Chen CH, Kuan TC, Ong SC, Lin CS (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Chiu SY, Kao CY, Huang TT, Lin CJ, Ong SC, Chen CD, Chang JS, Lin CS (2011) Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour Technol 102:9135–9142
Cosgrove J, Borowitzka MA (2010) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Springer, Dordrecht, pp 1–17
Dao LHT, Beardall J (2016a) Effects of lead on two green microalgae Chlorella and Scenedesmus: photosystem II activity and heterogeneity. Algal Res 16:150–159
Dao LHT, Beardall J (2016b) Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 147:420–429
De Marchin T, Ghysels B, Nicolay S, Franck F (2014) Analysis of PSII antenna size heterogeneity of Chlamydomonas reinhardtii during state transitions. BBA-Bioenergetics 1837:121–130
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Falk S, Palmqvist K (1992) Photosynthetic light utilization efficiency, photosystem II heterogeneity, and fluorescence quenching in Chlamydomonas reinhardtii during the induction of the CO2-concentrating mechanism. Plant Physiol 100:685–691
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Guenther JE, Melis A (1990) The physiological significance of photosystem II heterogeneity in chloroplasts. Photosynth Res 23:105–109
Henley WJ (1993) Measurement and interpretatiom of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Henley WJ (1995) On the measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 31:674
Ihnken S, Kromkamp JC, Beardall J (2011) Photoacclimation in Dunaliella tertiolecta reveals a unique NPQ pattern upon exposure to irradiance. Photosynth Res 110:123–137
Iwasaki I, Kurano N, Miyachi S (1996) Effects of high-CO2 stress on photosystem II in a green alga, Chlorococcum littorale, which has a tolerance to high CO2. J Photochem Photobiol B Biol 36:327–332
Jianrong XIA, Qiran T (2009) Early stage toxicity of excess copper to photosystem II of Chlorella pyrenoidosa–OJIP chlorophyll a fluorescence analysis. J Environ Sci 21:1569–1574
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, Poli D, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Serôdio J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014) Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth Res 122:121–158
Kamalanathan M, Pierangelini M, Shearman LA, Gleadow R, Beardall J (2016) Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J Appl Phycol 28:1509–1520
Kyle DJ, Haworth P, Arntzen CJ (1982) Thylakoid membrane protein phosphorylation leads to a decrease in connectivity between photosystem II reaction centers. Biochim Biophys Acta Bioenerg 680:336–342
Lavergne J, Trissl HW (1995) Theory of fluorescence induction in photosystem II: derivation of analytical expressions in a model including exciton-radical-pair equilibrium and restricted energy transfer between photosynthetic units. Biophys J 68:2474–2492
Li T, Tao Q, Di Z, Lu F, Yang X (2015) Effect of elevated CO2 concentration on photosynthetic characteristics of hyperaccumulator Sedum alfredii under cadmium stress. J Integr Plant Biol 57:653–660
Li T, Kirchhoff H, Gargouri M, Feng J, Cousins AB, Pienkos PT, Gang DR, Chen S (2016) Assessment of photosynthesis regulation in mixotrophically cultured microalga Chlorella sorokiniana. Algal Res 19:30–38
Ma S, Li D, Yu Y, Li D, Yadav RS, Feng Y (2019) Application of a microalga, Scenedesmus obliquus PF3, for the biological removal of nitric oxide (NO) and carbon dioxide. Environ Pollut 252:344–351
Makino A, Mae T (1999) Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol 40:999–1006
Markou G, Depraetere O, Muylaert K (2016) Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: a study on chlorophyll fluorescence and electron transport. Algal Res 16:449–457
Markou G, Dao LHT, Muylaert K, Beardall J (2017) Influence of different degrees of N limitation on photosystem II performance and heterogeneity of Chlorella vulgaris. Algal Res 26:84–92
Masojídek J, Vonshak A, Torzillo G (2010) Chlorophyll a fluorescence applications in microalgal mass cultures. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications. Springer, Dordrecht, pp 277–292
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
Melis A, Neidhardt J, Benemann JR (1998) Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol 10:515–525
Mende D (1980) Evidence for a cyclic PS-II-electron transport in vivo. Plant Sci Lett 17:215–220
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nagase H, Yoshihara KI, Eguchi K, Yokota Y, Matsui R, Hirata K, Miyamoto K (1997) Characteristics of biological NOx removal from flue gas in a Dunaliella tertiolecta culture system. J Ferment Bioeng 83:461–465
Nagase H, Yoshihara K, Eguchi K, Okamoto Y, Murasaki S, Yamashita R, Hirata K, Miyamoto K (2001) Uptake pathway and continuous removal of nitric oxide from flue gas using microalgae. Biochem Eng J 7:241–246
Nedbal L, Trtílek M, Kaftan D (1999) Flash fluorescence induction: a novel method to study regulation of photosystem II. J Photochem Photobiol B Biol 48:154–157
Pierangelini M, Stojkovic S, Orr PT, Beardall J (2014a) Photosynthetic characteristics of two Cylindrospermopsis raciborskii strains differing in their toxicity. J Phycol 50:292–302
Pierangelini M, Stojkovic S, Orr PT, Beardall J (2014b) Elevated CO2 causes changes in the photosynthetic apparatus of a toxic cyanobacterium, Cylindrospermopsis raciborskii. J Plant Physiol 171:1091–1098
Qiu B, Gao K (2002) Effects of CO2 enrichment on the bloom-forming cyanobacterium Microcystis Aeruginosa (Cyanophyceae): Physiological responses and relationships with the availability of dissolved inorganic carbon. J Phycol 38:721–729
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Raven JA, Beardall J, Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res 121:111–124
Ruban AV, Johnson MP, Duffy CDP (2011) Natural light harvesting: principles and environmental trends. Energy Environ Sci 4:1643–1650
Schmitz P, Maldonado-Rodriguez R (2001) Evaluation of the nodulated status of Vigna unguiculata probed by the JIP test based on the chlorophyll a fluorescence rise. In: 12th International Photosynthesis Congress. Brisbane, Australia,
Sebök S, Herppich WB, Hanelt D (2017) Red alga Palmaria palmata—growth rate and photosynthetic performance under elevated CO2 treatment. J Appl Phycol 29:381–393
Seth JR, Wangikar PP (2015) Challenges and opportunities for microalgae-mediated CO2 capture and biorefinery. Biotechnol Bioeng 112:1281–1296
Spalding MH, Critchley C, Govindjee, Orgren WLO (1984) Influence of carbon dioxide concentration during growth on fluorescence induction characteristics of the green alga Chlamydomonas reinhardii. Photosynth Res 5:169–176
Stirbet A, Govindjee SBJ, Strasser RJ (1998) Chlorophyll a fluorescence induction in higher plants: modelling and numerical simulation. J Theor Biol 193:131–151
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plant and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgio GC, Govindjee (eds) Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, pp 321–362
Tchernov D, Hassidim M, Vardi A, Luz B, Sukenik A, Reinhold L, Kaplan A (1998) Photosynthesizing marine microorganisms can constitute a source of CO2 rather than a sink. Can J Bot 76:949–953
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Varshney P, Sohoni S, Wangikar PP, Beardall J (2016) Effect of high CO2 concentrations on the growth and macromolecular composition of a heat- and high-light-tolerant microalga. J Appl Phycol 28:2631–2640
Varshney P, Beardall J, Bhattacharya S, Wangikar PP (2018) Isolation and biochemical characterisation of two thermophilic green algal species- Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res 30:28–37
Vincent WF, Roy S (1993) Solar ultraviolet-B radiation and aquatic primary production: Damage, protection, and recovery. Environ Rev 1:1–12
Vinet L, Zhedanov A (2011) A ‘missing’ family of classical orthogonal polynomials. J Phys A Math Theor 44:085201
Yang Y, Gao K (2003) Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J Appl Phycol 15:379–389
Yoshihara K-I, Hiroyasu N, Eguchi K, Hirata K (1996) Biological elimination of nitric oxide and carbon dioxide from flue gas by marine microalga NOA-13 cultivated in a long tubular photobioreactor. J Ferment Bioeng 82:351–354
Zhang D, Pan X, Mu G, Wang J (2010a) Toxic effects of antimony on photosystem II of Synechocystis sp. as probed by in vivo chlorophyll fluorescence. J Appl Phycol 22:479–488
Zhang T, Gong H, Wen X, Lu C (2010b) Salt stress induces a decrease in excitation energy transfer from phycobilisomes to photosystem II but an increase to photosystem I in the cyanobacterium Spirulina platensis. J Plant Physiol 167:951–958
Zhang S, Pei H, Hu W, Qi F, Han L, Song M, Han F (2015) Biomass and lipid accumulation of three new screened microalgae with high concentration of carbon dioxide and nitric oxide. Environ Technol 36:2278–2284
Zhao B, Wang J, Gong H, Wen X, Ren H, Lu C (2008) Effects of heat stress on PSII photochemistry in a cyanobacterium Spirulina platensis. Plant Sci 175:556–564
Zhao B, Su Y, Zhang Y, Cui G (2015) Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae. Energy 89:347–357
Acknowledgments
This work was supported by the JSW Foundation, India, Wadhwani Research Centre for Bioengineering, IIT Bombay, India and the Department of Biotechnology, Ministry of Science and Technology, Government of India (Grant No: BT/EB/PAN IIT/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 166 kb)
Rights and permissions
About this article
Cite this article
Varshney, P., Beardall, J., Bhattacharya, S. et al. Effect of elevated carbon dioxide and nitric oxide on the physiological responses of two green algae, Asterarcys quadricellulare and Chlorella sorokiniana. J Appl Phycol 32, 189–204 (2020). https://doi.org/10.1007/s10811-019-01950-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01950-2