Abstract
We investigated the extent to which nitrogenous and phosphorus nutrients from liquid anaerobic digestates could be recycled for photosynthetic growth of a microalga, Scenedesmus sp. AMDD. Digestates recovered from the anaerobic digestion of cow manure and swine manure and a co-digestion of swine manure and algal biomass were diluted in distilled water and used for algal growth with and without supplemental CO2 addition. Nutrient assimilation and final biomass yield were retarded in all but the swine manure/algae co-digestate cultures supplemented with high CO2. Swine manure digestate cultures supplemented with the typical complement of micronutrients normally added with a commonly used growth medium or with Fe/EDTA failed to grow any better than unamended controls. When the culture medium was prepared by blending swine manure digestate with 25 or 50 % algal biomass digestate, diluting it with lake water or by supplementing with magnesium, nutrient assimilation and final algal biomass yields were maximized, indicating that magnesium was critically limiting for algal growth in swine manure digestates. Magnesium amendment thus appears to be essential if nutrients from swine manure digestates are recycled for algal growth. No such requirement is necessary for recycling nutrients from digestates generated wholly or in part from algal biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been a resurgence of interest in the alternative energy potential of microalgae. Most of the renewed research focus has involved the manipulation of physiological parameters to increase the intracellular lipid content of microalgae followed by the extraction of the oils and subsequent conversion to biodiesel. Economics is considered a key barrier to full-scale algal biodiesel production as a drop-in fuel, energy source, and commodity (Chisti 2007). The developing consensus is that there is a need to couple microalgal oil production with another revenue stream or with another form of energy production, such as anaerobic digestion (AD), to become a viable alternative energy production pathway (Chisti 2007; Sialve et al. 2009; Collet et al. 2011).
Anaerobic digestion of biomass is a relatively mature industrial process which utilizes bacterial populations in a controlled, anoxic environment to produce biogas, which can be further upgraded to methane for combustion to generate energy or compressed to a liquid fuel. Anaerobic digestion of biomass creates a nutrient-rich waste stream, called digestate, which can be spread as crop fertilizer but has the potential to be used as a nutrient source for microalgal growth. Recycling the nutrients from AD and assimilating them into algal biomass can result in further feedstock for the process without incurring the monetary or environmental costs of using nitrogenous or phosphorus fertilizers while simultaneously remediating the liquid waste stream from the process. Studies assessing the integration of algal growth and AD were performed as far back as the 1950s (Golueke et al. 1957), and the topic has once again gained momentum in the bioenergy sector. Recently, Ras et al. (2011) performed experiments with Chlorella vulgaris in an integrated algal cultivation and digestion system, Vergara-Fernández et al. (2008) digested a variety of marine algae to assess their biogas generating potential, and Wang et al. (2010) observed efficient nitrogen and phosphorus remediation from anaerobic digestates of dairy manure using Chlorella sp.
We were interested in investigating the degree to which remineralized nutrients found in anaerobic digestates from different organic substrates, such as animal manures and microalgal biomass itself, could be reused for microalgae cultivation. In our view, linking the energy production pathway to the supply of nutrients could improve the overall sustainability of biofuel production from microalgae by lessening its dependence on commercial fertilizers.
Material and methods
Liquid anaerobic digestates were provided by the Centre for Agricultural Renewable Energy and Sustainability located at the University of Guelph, Ridgetown Campus (Ridgetown, Ontario, Canada). Various digestates were supplied from different agricultural feedstocks including vegetable wastes, cow manure, and swine manure and from a large-scale co-digestion of swine manure with dried Nannochloropsis granulata, a marine microalga. Liquid digestates from the AD of Scenedesmus sp. AMDD, a favorite model freshwater alga in our lab (McGinn et al. 2011, 2012), were provided by B. Tartakovsky (NRC Montreal). For one experiment, water from a nearby lake (Kearney Lake, Halifax, Nova Scotia, Canada) was sampled and filter-sterilized for use in algal growth.
Flask-level growth
Scenedesmus sp. AMDD was grown in an environmentally controlled plant growth chamber set at 22 °C in 250-mL Erlenmeyer flasks which contained approximately 100 mL of algal culture. Growth light was supplied from fluorescent bulbs (Philips 65 W F72T8 warm white) fixed at the top of the chamber to give an irradiance of 85–90 μmol photon m−2 s−1 measured with a flat quantum sensor at the surface of the algal cultures. To promote more efficient gas exchange with the headspace and ensure that the algae remained suspended, the cultures were mixed with PTFE magnetic stir bars. Digestates from three different AD feedstocks were used to prepare algal growth media: a co-digestion of algal biomass and swine manure, cow manure, and vegetable wastes. The digestates were diluted in deionized water (DI H2O) to obtain a final nitrogen (N) concentration of approximately 1.5 × 10−3 mol L−1 (measured as NH3-N) and various phosphorus (PO4 3−) concentrations depending on the digestate source, passed through a 0.45-μm nominal-pore-size glass fiber filter, and followed by filtration through a 0.22-μm polycarbonate membrane filter. The NH3 concentration of 1.5 mM obtained after dilution was chosen based on previous work which showed it to be appropriate for growth of the same algal strain in municipal wastewater (McGinn et al. 2012). The strength of the required dilution varied between 1 and 6.5 % (balance DI H2O) depending on the strength of the undiluted digestate. One hundred mL of diluted, filtered digestate was transferred into a sterile flask and inoculated (1 % (v/v)) from an exponentially growing culture of Scenedesmus sp. AMDD. The cell density of the cultures was determined daily using a particle enumerator (Multisizer III Counter, Beckman Coulter, USA). A spectrophotometric nitrate (NO3 −) assay indicated that the digestates were NO3 −-depleted to levels below the assay detection limit. Ammonia and phosphate assays were performed using a benchtop spectrophotometer and commercially available assay kits (Hach, USA).
Bottle experiments under CO2 supplementation
Culture experiments were performed in 1-L glass bottles which allowed for the provision of supplemental CO2 through a pH-stat system. All other growth conditions were the same as for the flask-level experiments. Cultures of Scenedesmus sp. AMDD were inoculated (1 % (v/v)) into digestates from algal biomass and swine manure co-digestion, swine manure, cow manure, and digestion of algal biomass alone, diluted to approximately 1.5 × 10−3 mol L−1 NH3-N in DI H2O. Further digestate treatments for growth experiments included swine manure digestate blended with algal biomass digestate (25 and 50 %), swine manure digestate supplemented with MgSO4 and with MgCl (3.04 × 10−4 mol L−1), swine manure digestate with Fe/EDTA addition (1.17 × 10−5), and swine manure digestate with trace metal and vitamin addition (Guillard 1975). The microalgal cultures were aerated through a 0.22-μm in-line PTFE filter, and pH was monitored and controlled using Omega brand pH controllers and automated carbon dioxide (CO2) injections. In order to prevent carbon limitation of algal growth, CO2 was periodically injected into the culture through the air line in response to a transient rise in pH triggered by algal demand for CO2. Cell density and growth rates, nutrient levels, and final biomass were monitored and determined as described for the flask-level growth experiments. Upon reaching the stationary growth phase, a 5–15-mL aliquot of algal culture was filtered onto a precombusted glass fiber filter (550 °C), oven-dried at 120 °C, weighed, and combusted in a Vario MICRO Cube elemental analyzer (Elementar, USA) to determine the C and N content of the biomass. All culture experiments were performed in triplicate.
Results
Deionized water dilutions
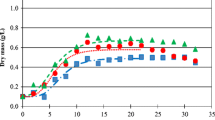
The maximum yields attained in our flask cultures ranged from 0.21 g dw L−1 in diluted vegetable waste digestate to a maximum of 0.27 g dw L−1 in diluted swine/algae digestate and cow digestate (Fig. 1a, n = 3 ± SD). In all three sources of digestate, the growth rate of the culture was greatest between inoculation and day 1, slowing considerably between days 1 and 3 (Fig. 1a). In all treatments, growth ceased by the third day of culture before the onset of N and P limitations. By the end of the sixth day, relatively high levels of residual N and P remained in the culture media (Fig. 1b, c). These results suggested that the cultures had excess macronutrients for growth and that some other factor was limiting algal growth in these diluted digestate cultures.
a Growth curves of Scenedesmus sp. AMDD in anaerobic digestates from algae/swine manure (SM/AB), cow manure (CM), and vegetable waste (Veg). Digestates were diluted to an initial NH3-N concentration of 1.5 × 10−3 mol L−1. b Residual NH3-N and c PO4 3− concentrations (μM) in cultures from a supporting the growth of Scenedesmus sp. AMDD over 6 days of algal cultivation (n = 3 ± SD)
One-liter culture experiments were performed at pH 7 with CO2 supplementation in diluted swine/algae digestate. We observed an increase in final biomass yield to 0.42 g L−1 relative to the initial flask-level growth experiments (Table 1, treatment 1). As in our flask experiments, exponential growth had ceased by day 3 of the experiment; however, NH3-N and PO4 3− continued to be drawn down to near depletion. By the sixth day of cell growth, the levels of NH3-N and PO4 3− in the culture media had been reduced to 6.89 × 10−6 mol L−1 NH3-N and 1.56 × 10−6 mol L−1 PO4 3−. Additional 1-L culture experiments with diluted swine and cow manure digestates supplemented with CO2 resulted in final biomass yields of 0.14 and 0.11 g dw L−1, respectively (Table 1, treatments 2 and 3). In both experiments, neither NH3-N nor PO4 3− was depleted by algal growth.
To investigate the lower biomass yields that we observed in swine digestates compared to the co-digested algae/swine digestates, Scenedesmus sp. AMDD was grown in diluted swine manure digestate amended with algae digestate obtained from separate experiments where algal biomass was the only substrate for the anaerobic digestor. Swine manure and algae digestates were blended at ratios of 3:1 and 1:1 swine/algae. In parallel experiments, swine manure digestates were amended either with the full complement of trace metals and vitamins typically used in f/2 media (Guillard 1975), with Fe/EDTA, or with Mg2+, added as either MgSO4 or MgCl. Scenedesmus sp. AMDD grown in algal biomass digestates achieved a final biomass yield of 0.45 g dw L−1 with complete N drawdown (Table 1, treatment 4). The algal cultures grown in mixed digestates (3:1 and 1:1 swine/algae digestate) obtained final cell densities of 0.37 and 0.42 g dw L−1, respectively, coincident with near total removal of NH3-N from the media (Table 1, treatments 5 and 6). Algal cultures grown in swine digestates amended with trace metals and vitamins or with Fe/EDTA supported final biomass yields of 0.09 and 0.07 g dw L−1 respectively, and NH3-N drawdown was incomplete in both (Table 1, treatments 7 and 8). Cultures grown in swine manure digestates amended with Mg2+ (added as either MgSO4 or MgCl) assimilated virtually all of the N in the growth medium and obtained final biomass yields of 0.33 and 0.40 g dw L−1, respectively (Table 1, treatments 9 and 10).
Freshwater experiments
Scenedesmus sp. AMDD was cultivated in swine manure digestates diluted to 1.5 × 10−3 mol L−1 NH3-N in 0.22 μm of filtered lake water in 1-L bottles with CO2 supplementation. Final biomass yield was 0.55 g dw L−1, and nearly all of the NH3-N and PO4 3− were removed from the growth medium (Table 1, treatment 11).
Particulate nitrogen analysis
Elemental analysis of filtered dried algal biomass from our batch culture experiments indicated a high fraction of nitrogen uptake by Scendesmus sp. AMDD. In biomass from cultures where NH3-N appeared to be drawn down from the growth medium, the fraction of measured particulate N in the biomass compared to the N provided in the growth medium was as high as 1.08 (mol/mol; Table 1, treatment 9), with the additional N likely the result of residual transfer from the inoculum culture. In cultures where we measured a significant level of residual NH3-N in the culture medium at the cessation of growth, the fraction of N in the biomass relative to the N provided in the growth medium ranged from 0.33 to 0.38 (Table 1, treatments 2, 7, and 8).
Discussion
Growth and nutrient drawdown by Scenedesmus sp. AMDD was greatest when cultivated in swine manure digestates diluted in lake water. Growth in digestates that contained a fraction of digested algal biomass resulted in good nutrient assimilation and high biomass yields. Animal manure-only digestates in DI H2O did not support complete NH3-N drawdown; however, when supplemented with Mg2+, algal growth and nutrient drawdown were increased in these media.
Research has been conducted on a variety of microalgae grown on digestates from various sources. Golueke and Oswald (1959) were successful in cultivating Scenedesmus sp. in digestate in an integrated system of digestion and algal growth. Our trials focused more on the growth and nutrient drawdown characteristics of Scenedesmus sp. AMDD and attempted to use a ‘cleaner’ source of digestate, by diluting in DI H2O followed by filtration to reduce the bacterial load, to investigate the potential of the digestates alone as a source of nutrients for sterile algal cultures. Olguin et al. (1994) demonstrated efficient uptake of both NH4 + and PO4 3− by Spirulina maxima in swine manure digestates that had been diluted with seawater. Similar to our study, they observed an increase in cell yields upon supplementation with CO2, and they concluded that a coupled system of AD and algal cultivation is a viable means of treating effluent and recycling nutrients. Blier et al. (1995) observed complete inorganic nutrient removal in separate batch cultures of the cyanobacterium Phormidium bohneri and the microalga Micractinium pusillum grown in anaerobic effluent from a cheese factory. Ammonium was drawn down to insignificant levels by the fourth day of algal growth, which is a similar rate to what we observed in our most productive cultures.
The results from initial experiments with digestates diluted in DI H2O suggested limitation by a factor other than the macronutrients N and P. Supplementation with CO2 allowed for complete drawdown of NH3-N and PO4 3+ in cultures grown in algae/swine manure co-digestates and in blended algae/swine manure digestates but not in cultures grown in either swine or cow manure digestates as the only nutrient sources. This suggested that for optimal growth, there was at least one other factor in addition to carbon required which was apparently available in algae digestates but missing in animal manure digestates. Supplementation of swine manure digestate with trace metals and vitamin stocks or with Fe/EDTA did not improve growth rates or biomass yields (in fact, both decreased slightly), which suggested that micronutrients were not the missing requirement. Further experiments indicated that cell yields were limited when cultures of Scenedesmus sp. AMDD were grown in Bold’s Basal Medium (Bold 1949) in which the Mg2+ had been omitted (results not shown). Subsequent experiments showed that algal growth rates and biomass yields in swine manure digestates approached those achieved in optimal treatments only when supplemented with Mg2+, indicating that this element was likely the key nutrient required for high biomass yields and which was present in adequate quantities in algae digestates. Dilution of swine manure digestate in lake water resulted in the highest biomass yields of all of the treatments, suggesting that Mg2+ concentrations were likewise sufficient to meet the growth requirements of the algae. Although we did not conduct elemental analysis on the lake water used in our experiments, the concentration of Mg2+ in this lake has averaged 0.03 ± 0.02 mM since 2006 (n = 13; Stantec 2012), which suggested that this level of Mg2+ is adequate for optimal growth. Park et al. (2010) also observed an increase in cell growth following the addition of Mg2+ to semi-continuous cultures of Scenedesmus acuminatus grown in piggery effluent. Magnesium is an essential macronutrient for algal growth and is an essential constituent of the chlorophyll molecule. A deficiency of Mg2+ could prevent the algal culture from accumulating sufficient chlorophyll to sustain photosynthesis and growth and could therefore limit overall productivity. Complete N drawdown by Scenedesmus obliquus in AD wastes from swine manure at different dilutions in tap water has been observed (de la Noüe and Bassères 1989), and it is possible that municipal tap water contained sufficient Mg2+ for algal growth if, as in our digestate, Mg2+ levels were not sufficiently high. Swine manure digestates can support substantial algal growth and nutrient recycling, with minor supplementation of Mg2+ or dilution with algae-derived digestates if required, and could be a viable source of nutrients to support industrial-scale growth of algal biomass. In addition, if AD wastes were to be used as a supplement to municipal wastewater as a medium for algal growth, there would likely be no need to add trace elements such as Mg2+ to achieve high biomass and efficient nutrient drawdown (McGinn et al. 2012). This further supports the idea that AD wastes could be useful in a closed loop system of algal biomass and energy production, and wastewater remediation.
The modest enhancement in growth rate and biomass yield in cultures grown in lake water compared to the most productive cultures prepared by dilution in DI H2O may have been due to a form of mixotrophic growth via the assimilation of dissolved organic carbon compounds, like tannins and small DOCs, which were shown to be significant in samples of the lake water (Stantec 2012). In a separate study, mixotrophic growth with acetate and glycerol has been shown to boost productivity in Scenedesmus sp. AMDD compared to autotrophic media (Park et al. 2011).
This study is unique in that it examined the potential of a variety of agriculturally derived digestates along with digestate from microalgal biomass subjected to AD. It allowed us to investigate suitable substrates for algal cultivation for a biorefinery system and supports the idea that co-digestion can not only provide energy production in the form of methane gas, but also provides evidence that algal-derived digestates may enhance the remediation potential of animal waste-derived digestates through enhanced algal productivity and nutrient assimilation.
References
Blier R, Laliberté G, de la Noüe J (1995) Tertiary treatment of cheese factory anaerobic effluent with Phormidium bohneri and Micractinium pusillum. Bioresour Technol 52:151–155
Bold HC (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull Torrey Bot Club 76:101–108
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Collet P, Hélias A, Lardon L, Ras M, Goy R-A, Steyer J-P (2011) Life cycle assessment of microalgae culture coupled to biogas production. Bioresour Technol 102:207–214
de la Noüe J, Bassères A (1989) Biotreatment of anaerobically digested swine manure with microalgae. Biol Wastes 29:17–31
Golueke CG, Oswald WJ (1959) Biological conversion of light energy to the chemical energy of methane. Appl Microbiol 7:219–227
Golueke CG, Oswald WJ, Gotaas HB (1957) Anaerobic digestion of algae. Appl Microbiol 5:47–55
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 26–60
McGinn PJ, Dickinson KE, Bhatti S, Frigon J-C, Guiot SR, O’Leary SJB (2011) Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: opportunities and limitations. Photosynth Res 109:231–247
McGinn PJ, Dickinson KE, Park KC, Whitney CG, MacQuarrie SP, Black FJ, Frigon J, Guiot SR, O’Leary SJB (2012) Assessment of the bioenergy and bioremediation potentials of the microalga Scenedesmus sp. AMDD cultivated in municipal wastewater effluent in batch and continuous mode. Algal Res 2:155–165
Olguin EJ, Hernández B, Araus A, Camacho R, González R, Ramírez ME, Galicia S, Mercado G (1994) Simultaneous high-biomass protein production and nutrient removal using Spirulina maxima in sea water supplemented with anaerobic effluents. World J Microbiol Biotechnol 10:576–578
Park J, Jin H-F, Lim B-R, Park K-Y, Lee K (2010) Ammonia removal from anaerobic digestion effluent of livestock waste using green algal Scenedesmus sp. Bioresour Technol 101:8649–8657
Park KC, Whitney C, McNichol J, Dickinson KE, MacQuarrie S, Skrupski BP, Zhou J, Wilson K, O’Leary SJB, McGinn PJ (2011) Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: potential applications for wastewater remediation for biofuel production. J Appl Phycol 24:339–348
Ras M, Lardon L, Sialve B, Bernet N, Steyer J-P (2011) Experimental study on a coupled process of production an anaerobic digestion of Chlorella vulgaris. Bioresour Technol 102:200–206
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27:409–416
Stantec (2012) An analysis of the HRM lakes water quality monitoring program data (2006–2011). File no. 121510918. Stantec, Dartmouth
Vergara-Fernández A, Vargas G, Alarcón N, Velasco A (2008) Evaluation of marine algae as a source of biogas in a two-stage anaerobic reactor system. Biomass Bioenergy 32:338–344
Wang L, Yecong L, Chen P, Min M, Chen Y, Zhu J, Ruan R (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628
Acknowledgments
Thanks to the technical assistance of Mac MacAlpine and Lucas McNeal (University of Guelph, Ridgetown) and to Laura Garrison (NRC, Ketch Harbour). This is NRC publication no. 50506.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjornsson, W.J., Nicol, R.W., Dickinson, K.E. et al. Anaerobic digestates are useful nutrient sources for microalgae cultivation: functional coupling of energy and biomass production. J Appl Phycol 25, 1523–1528 (2013). https://doi.org/10.1007/s10811-012-9968-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-012-9968-0