Abstract
Integration of algae production with livestock waste management has the potential to recover energy and nutrients from animal manure, while reducing discharges of organic matter, pathogens, and nutrients to the environment. In this study, microalgae Chlorella sp. were grown on centrate from anaerobically digested swine manure. The algae were harvested for mesophilic anaerobic digestion (AD) with swine manure for bioenergy production. Low biogas yields were observed in batch AD studies with algae alone, or when algae were co-digested with swine manure at ≥43 % algae (based on volatile solids [VS]). However, co-digestion of 6–16 % algae with swine manure produced similar biogas yields as digestion of swine manure alone. An average methane yield of 190 mL/g VSfed was achieved in long-term semi-continuous co-digestion studies with 10 ± 3 % algae with swine manure. Data from the experimental studies were used in an energy analysis assuming the process was scaled up to a concentrated animal feeding operation (CAFO) with 7000 pigs with integrated algae-based treatment of centrate and co-digestion of manure and the harvested algae. The average net energy production for the system was estimated at 1027 kWh per day. A mass balance indicated that 58 % of nitrogen (N) and 98 % of phosphorus (P) in the system were removed in the biosolids. A major advantage of the proposed process is the reduction in nutrient discharges compared with AD of swine waste without algae production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many environmental problems result from increased use of concentrated animal feeding operations (CAFOs) for livestock production. Swine manure, which contains high concentrations of organic matter, nitrogen (N), phosphorus (P), pathogens, and other compounds of concern, such as pharmaceuticals, accounts for approximately 40–60 % of livestock wastewater worldwide [1]. With the increase in livestock production in CAFOs, over-application of manure to land results in soil and water pollution. For example, nitrate concentrations in surface waters in Brittany (France) increased from 5 to 35 mg/L after intensive CAFOs were constructed in the region [2]. The US Environmental Protection Agency encourages CAFOs to apply advanced technologies to mitigate nutrient, BOD5, and total suspended solids (TSS) and green house gases (GHG) emissions to the environment associated with land application and open anaerobic lagoons [3]. A number of biological treatment processes have been studied to treat livestock effluent, including aerated lagoons, activated sludge reactors with anoxic zones, and sequencing batch reactors [2]. However, the high energy costs associated with aeration of these processes hinder their widespread application. Anaerobic digestion (AD) is currently being promoted as a way to generate methane from livestock wastes for use as an energy source, while reducing odorous emissions and producing stabilized biosolids that can be used as a soil amendment [4]. However, AD effluents contain high concentrations of N and P. Therefore, an additional nutrient removal process may be required before discharge or reuse of the effluent.
The use of microalgae as part of municipal wastewater treatment in open ponds (e.g., stabilization ponds, facultative ponds, and lagoons) is well established [5, 6], as algae can provide dissolved oxygen (DO) for bacterial biodegradation of organics and nitrification. Recently, a novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo sequencing batch reactor (PSBR) was developed in our laboratory to treat AD effluent [7]. These systems can provide a low cost method of completely removing nitrogen from high nutrient strength wastewaters [7]. Algae grown in high N wastewater have low lipid contents (in the range of 0.6 to 10.9 % w/w), which are too low for profitable biodiesel production [8, 9]. Therefore anaerobic co-digestion of algal biomass harvested from PSBRs with the existing waste stream can be more economically sustainable. The integration of on-site energy production, solids stabilization, and nutrient removal in CAFOs could be achieved by applying anaerobic co-digestion of algae and swine manure with algae-based nutrient removal in a PSBR.
A number of studies have investigated algae production for bioenergy uses coupled with treatment of high nutrient strength wastewaters, such as centrate from dewatering AD sludges [8, 10–12]. These wastewaters have the potential to support high algal biomass densities, resulting in reduced downstream harvesting and dewatering costs [13]. Several prior studies have carried out batch anaerobic co-digestion experiments with swine manure and algae. Astals et al. [14] studied the anaerobic co-digestion of swine manure and algae (Scenedesmus sp.), with and without extraction of intracellular algal products. They found that 30 and 50 % (VS based) of raw algae co-digested with swine manure resulted in a synergetic effect, with methane yields of algae increasing from 163 to 245 mL CH4/g VS. González-Fernández et al. [15], however, found methane yields decreased with increasing percentages of algae during anaerobic co-digestion of swine manure with 14.6–85.4 % of algae (by chemical oxygen demand (COD)). Additional batch studies are needed to resolve the conflicting results and to find the optimal percentage of algae for co-digestion with swine manure. In addition, no prior continuous anaerobic co-digestion studies have been carried out with algae and swine manure, which are necessary to evaluate the long-term performance so that realistic energy production rates for full-scale CAFOs can be estimated.
The objective of this research was to investigate the energy production potential of anaerobic co-digestion of swine manure with algae. Batch AD studies were initially used to determine the optimal ratio of algae and swine manure for co-digestion. Algae were grown in a laboratory-scale PSBR and co-digested in a semi-continuous reactor with swine manure. Data from PSBR and semi-continuous reactor studies were used in an energy analysis conducted for a case study of a large swine CAFO that applies the proposed integrated process.
Materials and Methods
Algae Cultivation
The wild-type algal consortium used in this study consisted primarily of Chlorella sp. Enrichment and characterization of the consortium has been described elsewhere [10]. Due to limited availability of AD swine manure centrate during the batch AD experiments, algae were cultivated in 1 L batch glass photo-bioreactors in a 50/50 (V/V) mixture of synthetic and real AD swine manure effluent. During the semi-continuous AD experiments, algae were cultivated in 100 % real centrate in a PSBR, as described by Wang et al. [7]. For both cultivation methods, reactors were maintained in a 21 ± 2 °C constant temperature room and illuminated using cool white fluorescent tubes. Algae were harvested by gravity settling or centrifugation to reach the target VS concentration.
Synthetic AD swine manure centrate consisted of (mg/L) the following: NH4HCO3 (4000), KHCO3 (1000), NaHCO3 (4000), CaCl2·2H2O (25), MgSO4·7H2O (75), K2HPO4 (108), K2SO4 (20), and NaCl (25). Real AD swine manure centrate was obtained from a 24-L pilot-scale mesophilic (35 °C) AD operated in our laboratories at a solids residence time (SRT) of 21 days. Swine manure was collected from Twenty Four Rivers Farm (Plant City, FL) on a weekly basis, mixed with urea and local groundwater, and fed to the digester three times per week. Urea was added to make up for the loss of urine due to the farm operation. The same manure was used in the batch and semi-continuous digestion experiments. Centrate was separated from biosolids by centrifugation at 3500×g for 15 min.
Batch Anaerobic Digestion Experiments
Batch mesophilic ADs were set up in a constant temperature room at 35 °C to evaluate the effect of varying algae and swine manure ratios on digestion performance. Digestions were performed in duplicate in 200 mL glass serum bottles with a working volume of 130 mL for 31 days. Algae and swine manure sources are described above. Inoculum was collected from a full-scale mesophilic AD treating waste activated sludge at the Clearwater, FL wastewater treatment plant and was added to each reactor at 37 ± 6 % based on VS. Characteristics of swine manure, algae, and inoculum are shown in Table 1. Proportions of algae (A) and swine manure (SM) by VS in the six treatments (T1 to T6) were as follows: 100 % SM (T1), 6 % A + 94 % SM (T2); 16 % A + 84 % SM (T3); 43 % A + 57 % SM (T4); 75 % A + 25 % SM (T5); 100 % A (T6). Duplicate sets of anaerobic inoculum were digested under the same conditions as controls. Each bottle was purged with N2 for 1 min before sealing to remove oxygen. Intermittent mixing was provided by shaking the bottles twice each day. Biogas volume was measured by inserting a needle attached to a 50-mL glass syringe through the septa of each serum bottle. The volume of the ejected plunger was recorded as the gas volume and converted to standard temperature and pressure conditions (STP, 0 °C and 1 atm).
Semi-continuous Anaerobic Digestion Experiments
Two bench-scale semi-continuous mesophilic ADs (R1 and R2) were set up in 1 L glass bottles in a constant temperature room at 35 °C. Each reactor had working volume of 0.8 L. The reactors were seeded with sludge from the pilot-scale AD described above. The reactors were fed and effluent was wasted three times per week at a rate required to maintain the SRT of 21 days. R1 was fed with 100 % swine manure. R2 was fed with a mixture of 90 % swine manure and 10 ± 3 % algae by VS. R2 was covered with aluminum foil to avoid algal photosynthesis. Thermolyne Cimarec 2 magnetic stirrers (Dubuque, Iowa) at low speed (300 rpm) were used to maintain the biomass in suspension. Biogas was collected at the top of the reactor and measured by the water displacement method using a Wet Tip Gas Meter (Nashville, TN). The volume of biogas was converted to STP. Effluent samples were collected weekly for chemical analysis after the reactors had been operated for three SRTs.
Analytical Methods
Biogas composition was analyzed using a gas chromatograph (GC) equipped with a thermal conductivity detector (Gow-Mac instrument Co. Series 400). Helium was used as the carrier gas. The injector, detector, and column temperatures were 80, 120, and 80 °C, respectively. Measurements of total solids (TS) and VS were performed according to Method 2540 in Standard Methods [16]. Soluble samples were prepared by filtration through 0.45 μm filter paper. COD was measured according to Standard Methods (5200B) using Orbeco-Hellige mid-range (0–1500 mg/L) COD kits. NH4 +-N and PO4 3−-P concentrations were measured using a Metrohm Peak 850 Professional AnCat ion chromatography system (Metrohm Inc., Switzerland). Total N concentrations were measured using Hach TNT plus 827 test kits and total P was measured using Hach TNT plus 845 test kits. Volatile fatty acid (VFA) concentrations were measured by the esterification method using Hach TNT plus 872 test kits. The results were reported as the equivalent concentration of acetic acid. Fecal indictor bacteria (Escherichia coli) were monitored using an IDEXX Colilert® and Quanti-Tray®/2000 method. The lipid content of the algae used in the batch experiment was quantified by the modified method of Bligh and Dyer [17], as described in Mandal and Mallick [18].
Data Analysis
The solubilization efficiency (S) and anaerobic biodegradability (%) of each substrate from the batch AD studies were calculated based on the following [19]:
Where sCOD is the soluble COD from the digested substrates, tCOD refers to the initial total COD of the substrates, \( {V}_{{\mathrm{CH}}_4} \) is the cumulative methane production at STP, and \( {\mathrm{COD}}_{{\mathrm{CH}}_4} \) is theoretical conversion coefficient from methane to COD (350 L CH4/kg COD).
Tukey’s test is designed for the pairwise comparisons. Statistical analysis of biogas production from batch anaerobic digestion was performed using single factor analysis of variance (ANOVA) along with Tukey’s test. When the calculated p value was less than α (α = 5 %), a significant difference was reported. The methane production and VS reduction from semi-continuous digestion (R1 and R2) were analyzed by ANOVA. The ANOVA assume that residuals are normally distributed. Thus, data from semi-continuous digestion studies were also tested for normality assumption using Aderson-Darling methodology embedded in Mintab 16, which is based on the empirical distribution function. All the statistical analyses were conducted using Minitab 16 (PA, USA). The statistical analysis of the distribution of the VS and methane yields data of R1 and R2 were shown in the supplementary information (Fig. S1).
System Description for Energy Analysis
To evaluate the feasibility of the co-digestion system, this study analyzed energy consumption and production for integrated anaerobic co-digestion and algae cultivation systems. This process refers to a hypothetical system based on data from our laboratory-scale studies. The combined system is shown in Fig. 1, including anaerobic co-digestion, dewatering, algae cultivation, algae harvesting, and power generation processes. Operational parameters for the system are shown in Table 2. Published data from a large-size CAFO located in Dubuque, IA [20], which has 7000 pigs with an average weight of 140 lbs was used as a basis for energy analysis for the integrated system. However, since the published data did not include information about daily waste production from the CAFO, the waste production was calculated based on Hamilton et al. [21]. Anaerobic co-digestion was assumed to be performed under mesophilic conditions in a digester with a 21-day SRT. Stabilized biosolids from anaerobic co-digestion were assumed to be dewatered to a solids content of 25 % using a centrifuge, with polymer added as a coagulant to increase the dewatering efficiency. Centrate from the dewatering process was assumed to be sent to a PSBR for treatment. The PSBR was based on the system described by Wang et al. [7], which uses an algal-bacterial consortium to improve organic carbon, N, and P removal (see “Semi-continuous Anaerobic Digestion Performance” for a summary of results). Based on the laboratory study, no CO2 addition was required and algae could be harvested by gravity sedimentation and centrifugation; however, an external electron donor (acetate) was required for denitritation. Note that the laboratory-scale PSBR was fed with diluted centrate [7]. In practice, full-strength centrate will be fed to the PSBR; therefore, an SRT of 24 days and HRT of 12 days were used in the model, resulting in a similar N loading rate as the laboratory-scale PSBR. Nitrogen and phosphorus data obtained from semi-continuous anaerobic co-digestion studies were used for the system mass balance analysis. It was assumed that 0.0036 m3 water evaporates per m2 of PSBR area per day [22]. Biogas produced from anaerobic co-digestion was assumed to be used to generate electricity and heat using an on-site combined heat and power (CHP) system. A biogas boiler was assumed to be used if additional heat is required.
Energy Analysis
The energy analysis was carried out on the integrated system shown in Fig. 1. A conventional system, with just AD of swine waste, was used as a comparison. Energy inputs include electricity and heat for AD, pumping, dewatering, algae cultivation, and algae harvesting. The total heat requirement for AD was estimated by a theoretical method, by assuming the heat requirement consists of energy needed for heating the incoming slurry and heat loss from the digester. It was reported that the heat loss accounts for only 2–8 % of the heat requirement for the incoming slurry [23] and is highly dependent on the digester materials and insulation [24]. In this analysis, the digester was assumed to be well insulated (heat transfer coefficient of 0.7 W/m2-°C). The theoretical heat requirement was calculated from:
Where E Heat is heat requirement (kWh/day), ρ is density of water (kg/m3), γ is specific heat of the water (kWh/kg-°C), T R is temperature in the digester (°C), T In is temperature of influent (°C), k is heat transfer coefficient (W/m2-°C), and A is surface area of anaerobic digester wall (m2). Since most of the slurry consists of water, it was assumed that the specific heat is the same as the water. The range of heat requirements was calculated using the theoretical method based on ambient temperature ranging from 0 to 25 °C. An empirical method was used to calculate the power requirements for mixing in the co-digestion system. The normalized power requirement factor for mixing (0.13 kWh/m3-day) was estimated using daily power requirement values per working volume from the literature [25–27]. It was assumed that the energy source for pumping was electricity. The power requirement for the pump was estimated using the following [28]:
Where P is power requirement (W), Q is flow rate (m3/s), ρ is density of water (kg/m3), H is head loss (m), g is acceleration of gravity (m/s2), and E is efficiency of the pump. We assumed that the head losses for the influent of the AD and centrifuge were 5 and 1 m, respectively.
The energy requirements for dewatering and microalgae harvesting were calculated according to [29]:
Where P centrifuge is the power required by centrifuge (kWh), S is the volume of slurry (m3) that goes through the centrifuge (daily basis), and CentThru (m3/h) and CentPower (kW) are constants depending on the centrifuge capacity.
The PSBR was assumed to be in an open pond and the major energy input is electricity used for mixing using a paddle wheel. The energy requirement for mixing was estimated using the following [22]:
Where P is the power (W) required to operate the paddlewheel, g is the acceleration of gravity (m/s2), ρ culture is the density of the algae culture (kg/m3), v mixing is the mixing velocity (m/s), n is Manning’s number (s/m0.33), δ water is the depth of the water (m), and ε mixing is the overall efficiency of the mixing system.
The energy production was estimated based on the methane production from our laboratory-scale results (see “Semi-continuous Anaerobic Digestion Performance”). The energy output was calculated from:
Where P production is the energy production (kWh/day), Y CH4 is the methane yield (m3/gVS), ξ is the average heating value of methane (kWh/m3), η Heat is the heat energy conversion efficiency, and η Electricity is the electricity energy conversion efficiency. All parameters used for the energy analysis are summarized in Table 3.
Results and Discussion
Batch Anaerobic Digestion Performance
The lipid content of algae harvested for the batch AD was only 12 % of the total biomass, which is similar to the value reported for algae grown in anaerobically digested municipal sludge centrate [10]. The accumulation of lipids in algae require nutrient limited conditions [30]. However, the high nutrient concentrations in AD centrate do not promote lipid accumulation in microalgae. Sialve et al. suggested that when using algae as a biofuel source, AD would be the optimal option if the lipid content in the biomass is less than 40 % [31]. Therefore, anaerobic co-digestion of algae with other biomass is appropriate to produce energy from these algae.
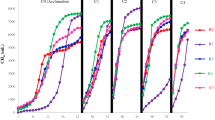
Cumulative biogas yields during the batch AD experiments are shown in Fig. 2a. The biogas yield from algae alone was significantly lower than from swine manure (p < 0.05). The cellulose or hemicellulose structure of the algal cell wall, which is difficult to degrade under anaerobic conditions, mostly likely inhibited biogas production [32, 33]. This was also observed in the volatile solids removal (VSR) data (Fig. 2b), which was 39 % for 100 % swine manure and 33 % for algae alone. Co-digestion of algae with swine manure improved the biogas yield of the algae. Statistical analysis indicated that up to 16 % algae addition resulted in a similar biogas yield as swine manure alone. Differences between biogas yields from digesters with 16 % algae addition, 6 % algae addition, and 100 % swine manure were not significant (p > 0.05) as shown in Fig. 2a. However, there was a significant difference in biogas yields with 6 % algae addition and more than 43 % algae addition.
As shown in Table 1, the COD/TN ratio was 17 for algae and 35 for swine manure. The optimum C/N ratio for AD has been reported in the range of 20–30 [34], indicating that the low COD/N ratio in the algal biomass was also not favorable for AD. The biogas composition and methane yields of the different treatments are shown in Table 4. The methane composition of the biogas ranged from 69 to 73 %. Digestion sets with 6 % A + 94 % SM and 75 % A + 25 % SM resulted in the highest methane contents of 73 %. The methane yield from algae alone was 250 mL/g VSfed, which is slightly higher than previously reported for Chlorella (230 mL/g VSfed) [26]. Considering that the digestion time in this study (31 days) was longer than the previous study (20 days), the methane production was comparable.
The final methane yield of swine manure alone was 317 mL/g VSfed. Co-digestion of 6 % A + 94 % SM resulted in the highest methane yield of 348 mL/g VSfed. The results show that a small amount of algae addition will not impair the methane yield of swine manure; however, addition of more than 43 % algae decreases the methane yield.
An analysis of the solubilization efficiency and biodegradability of each substrate is shown in Table 4. The algae alone sets had the lowest solubilization efficiency (47 %) and biodegradability (41 %) compared with swine manure, which are consistent with the biogas and methane yield results. In general, the anaerobic degradability of algae is poor. Future research should include the methods to improve the solubilization efficiency and the biogas yield of algae, such as thermal pre-treatment.
Semi-continuous Anaerobic Digestion Performance
Semi-continuous anaerobic co-digestion of algae and swine manure was carried out to evaluate the long-term performance of this process. A reactor operated with swine manure by itself was used as a control and to provide data for the energy analysis for the conventional system. Algae harvested from the PSBR were fed to the semi-continuous reactor for co-digestion. The operation and performance of PSBR are described in Wang et al. [7]. Briefly, centrate from a pilot-scale anaerobic digester treating swine manure was diluted three times to feed the PSBR. The average NH4 +-N concentration of the PSBR influent was 297 ± 29 mg/L. The PSBR was operated under alternating 12 h light/12 h dark periods with an average and SRT of 8 days. Sodium acetate was added as an external carbon source during the dark period. The PSBR removed over 90 % of the total nitrogen (TN) from the centrate, of which 80 % was removed through nitritation/denitritation and the rest was due to biomass uptake. About 45 % of TP was removed from the centrate. The areal algal productivity was 4.55 g/m2/day, assuming 80 % of the surface area of the photoreactor described in Wang et al. [7] was illuminated, which is within the range of published values (2.6–17.9 g/m2/day) for algal systems that applied wastewater as a growth medium [35]. Harvested algae were fed to the co-digestion reactor and accounted for 10 ± 3 % (by VS) of the feedstock. This proportion of algae was less than 16 % (by VS), which resulted in no significant differences in biogas yield compared with digestion of swine manure alone in the batch experiments described above. Trends in influent and effluent VS concentrations in R1 were similar to R2, as shown in Fig. 3. The variations in influent VS were due to the rainy season in Florida. The average VS of the R1 and R2 influents were 40.9 ± 7.0 and 37.4 ± 5.5 g/L, respectively. The average effluent VS concentrations in R1 and R2 were 24.6 ± 1.9 and 22.1 ± 1.7 g/L, resulting a VSR of 40 and 41 %, respectively. Despite the high variability in the influent VS, the effluent VS concentrations were stable over the long term of operation. Statistical analysis indicated that no significant differences in VS reduction were observed between R1 and R2 over six SRTs operation (p = 0.41).
Chemical properties of the influent and effluent from R1 and R2 are shown in Table 5. Total N and total P were conserved during digestion. The P concentration in the influent of R1 and R2 were 1.7 ± 0.58 and 1.5 ± 0.38 g/L, respectively; however, little soluble P was detected in the influent or effluent. The soluble portion of P only accounted 2–8 % of the total P in the influents and effluents.
The NH4 +-N concentration in the influent of R1 ranged from 422 to 681 mg/L. The NH4 +-N concentrations in the influent of R2 was slightly lower (407 to 577 mg/L). NH4 + is released during the hydrolysis of proteins and leads to an increase in NH4 + in AD effluents. Therefore, higher NH4 +-N concentrations were detected in the effluents of R1 and R2 than the influents (Table 5). High NH4 + concentrations may inhibit biogas production in AD due to the toxicity of free ammonia nitrogen (FAN) to methanogens. FAN increases with increasing pH, total ammonia nitrogen, and temperature. The highest calculated FAN concentration in both reactors was less than 25 mg/L, which is well below the methanogenic toxicity of 80–100 mg/L reported by [36].
Total VFA concentrations as acetic acid in the influent of R1 and R2 were high, and values decreased after digestion. Acetic acid is the major precursor of methanogenesis. The low acetic acid concentrations in the effluent of both digesters indicated stable digester performance and good synergistic activities of hydrolysis and methanogenic microorganisms. The long-term operation of co-digestion of algae and swine manure did not cause the accumulation of VFA, which can decrease reactor pH. Based on the average pH of 7.32 ± 0.19, VFA levels were not inhibitory to the co-digestion process.
The average biogas yields for R1 and R2 over the entire experimental period were 340 ± 49 and 300 ± 46 mL/g VSfed, respectively. Although average the biogas yield from co-digestion was slightly lower than when swine manure was digested alone, these differences were not significant. The average methane composition of R1 (56 ± 3 %) and R2 (57 ± 2 %) was also not significantly different. The average methane yields of R1 and R2 were 212 ± 31 and 190 ± 30 mL/g VSfed, respectively. The methane yields ranged from 175 to 242 and 161 to 234 mL/g VSfed, for R1 and R2, respectively. As discussed previously, there was high variability in the organic loading rates (OLR g/L/day), resulting in variations in biogas yield. To investigate the effect of acclimation on AD performance, methane production was normalized by the total VS fed into the digesters for each SRT period (21 days; Fig. 4). The average OLR for each SRT of R1 varied from 1.20 to 1.63 g VS/L/day, and the OLR of R2 ranged from 1.16 to 1.68 g VS/L/day. The methane production over six SRTs for both reactors followed a normal distribution. Significant changes in methane production were not observed over six SRTs based on the statistical analysis (p = 0.22), which is consistent with the VS reduction results. The methane production and VS reduction of the R1 and R2 indicated that when adding small portions of algae to existing digesters additional acclimation time would not be necessary.
E. coli Reduction During Anaerobic Digestion
One of the purposes of AD is to reduce the pathogen content of the swine manure and decrease the health risk of the effluent to humans and animals. The bacterial pathogenic indicator organism, E. coli, was monitored after three SRTs of operation. Both digesters achieved 2.1–2.3 log10 reduction of E. coli (Fig. 5), which is typical of prior studies (1.5–2 log10 reduction) for mesophilic AD [37–39]. Factors affecting E. coli removal include temperature, reactor configuration, pH, VFA and FAN content, microbial competition, and SRT [39–41]. Because E. coli remaining in the effluent were above 6 log10 CFU/g TS, further processes to reduce pathogens may be required to reduce health risks depending on the discharge of reuse of the effluent.
System Analysis
Mass Balance on N and P in the Integrated System
A nutrient mass balance (Fig. 6) was conducted based on experimental N and P concentrations from this study and Wang et al. [7]. The mass of N and P produced from a CAFO with 7000 pigs are approximately 93.4 and 79.4 kg/day, respectively. Accounting for harvested algal biomass, the anaerobic digesters received 94.2 kg N/day and 81.2 kg P/day. A loss of nitrogen during anaerobic digestion of approximately 6.6 kg N/day occurs due to outgassing of NH3. N and P recovered in the biosolids are 53.8 kg N/das (58 %) and 77.7 kg P/day (98 %), respectively. Approximately 33 kg N (35 %) are removed in the PSBR system by denitritation. Only 1.1 kg N/day and 1.7 kg P/day are released with the treated water. Based on the mass balance of N and P, there is little risk of N and P accumulation in the proposed system. Note that a carbon balance on the system is not presented because of the existence of heterotrophic bacteria in the open algae photo-bioreactor, which will contribute to organic carbon removal and CO2 emissions, and the production of CO2 in the open photo-bioreactor system was not measured. A general mass balance of COD based on experimental data indicated that about 26 % of COD was removed during anaerobic digestion; 7 % of COD was removed in the PSBR; 4 % of COD remained in the effluent of PSBR due to the poor biodegradability of centrate after anaerobic digestion; and approximately 67 % of the COD was contained in the biosolids for final disposal.
Energy Analysis of the Integrated System
The AD of swine manure alone and proposed integrated systems were evaluated from an energy perspective. As stated previously, the energy analysis was based on data from the laboratory-scale studies. Energy inputs and outputs for AD of swine manure alone and for the proposed system are summarized in Table 6. The proposed system requires approximately 1331 kWh total energy per day at an ambient temperature of 20 °C, while the system with AD of swine manure alone needs approximately 1302 kWh total energy per day. The higher electricity demand for the proposed system is mainly due to the additional equipment needed for algae cultivation (paddlewheel) and harvesting (centrifuge), while more heat is required because of the greater reactor size required compared with AD alone. For both systems, the highest energy demand comes from heating the digester, which accounts for about 83.1 % of total energy in the proposed system and 84.3 % of total energy in the AD alone system. The heat produced from the CHP would be sufficient to meet the heat requirements of the digester during most months of the year in Dubuque, IA. During the winter; however, the heat produced is lower than the amount required (2340 kWh/day for the proposed system, 2324 kWh/day for the system with AD alone) and a biogas boiler might be needed for both systems. In this case, about 72 and 48 % of the methane gas will be diverted to biogas boiler to supplement the heat requirements for the proposed and AD alone systems, respectively.
The proposed system produced an average 304 m3 methane per day, and the CHP can produce an average amount of energy of 2358 kWh/day. On the other hand, the AD alone system produced average 341 m3 methane per day, and about 2645 kWh/day total energy can be generated from the system. Both systems are able to gain a positive net energy balance in the form of heat and electricity, shown in Table 7.
The proposed system produced average 1501 kg/day stabilized biosolids and 36.4 m3/day treated water with N concentration of 52 mg/L and P concentration of 47 mg/L, shown in Table 7 and Fig. 1. The proposed system has a net energy gain of approximately 1027 kWh/day, which is higher than a reported value of 875 kWh/day from an AD system using dairy manure [26]. Although the proposed system requires a higher energy input than the AD alone system, as discussed previously, the amount of biogas produced from the proposed system is comparable to the system with AD alone.
In addition to net energy output, the proposed system is an effective way to reduce nutrients from the livestock wastewater [7, 11]. The effluent from the system with AD alone has a high nutrient concentration and requires additional treatment (energy requirements for this treatment are not included in this analysis); however, the proposed system is able to provide improved effluent quality (Fig. 1). The amount of stabilized biosolids produced from the proposed system is also comparable to the system with AD alone (Table 7) and can be used as soil amendment to recover its nutrient content. Therefore, the proposed system is attractive due to its ability to generate energy, recover nutrients, and improve effluent water quality.
Conclusions
This research investigated batch and semi-continuous anaerobic co-digestion of algae and swine manure. The batch AD study showed that the anaerobic digestibility of algae alone is poor compared with swine manure, while co-digestion of 6–16 % algae with swine manure resulted in similar biogas production as swine manure alone. Addition of more than 43 % algae will deteriorate the biogas production of swine manure. Semi-continuous digestion of 10 ± 3 % of algae by VS with swine manure resulted in a similar biogas production rate and VSR as digestion of swine manure alone. An energy analysis for a CAFO with 7000 pigs showed that the proposed system integrating algae production and AD will be energy positive, with an average net energy production of approximately 1027 kWh per day. The proposed system is comparable to a conventional system where swine manure is digested alone in terms of net energy; however, it provides better effluent quality and produce comparable amount of stabilized biosolid to the system with AD alone that can be used as a soil amendment. Therefore, the proposed system is attractive due to its ability to generate energy, recover nutrients, and improve effluent water quality.
References
Han MJ, Behera SK, Park HS (2012) Anaerobic co-digestion of food waste leachate and piggery wastewater for methane production: statistical optimization of key process parameters. J Chem Technol Biotechnol 87(11):1541–1550
Bernet N, Béline F (2009) Challenges and innovations on biological treatment of livestock effluents. Bioresour Technol 100(22):5431–5436
U.S. Environmental Protection Agency (2004) A manual for developing biogas systems at commercial farms in the United States: AgSTAR Handbook (EPA-430-B-97-015). Chapter 1 Overview of biogas technology. Retrieved from http://www2.epa.gov/sites/production/files/2014-12/documents/agstar-handbook.pdf
Holm-Nielsen JB, Ai Seadi T, Oleskowicz-Popielc P (2009) The future of anaerobic digestion and biogas utilization. Bioresour Technol 100(22):5478–5484
Oswald JW, Gotaas HB, Golueke CG, Kellen WR (1957) Algae in waste treatment. Sewage Ind Waste 29(4):437–457
WEF (1990) Natural systems for wastewater treatment: manual of practice FD-16. Water Environment Federation, Alexandria
Wang M, Yang H, Ergas S, van der Steen P (2015) A novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo-sequencing batch reactor (PSBR). Water Res 87:38–48
Mulbry W, Kondrad S, Buyer J (2008) Treatment of dairy and swine manure effluents using freshwater algae: fatty acid content and composition of algal biomass at different manure loading rates. J Appl Phycol 20(6):1079–1085
Hu B, Min M, Zhou W, Du Z, Mohr M, Chen P, Zhu J, Cheng Y, Liu Y, Ruan R (2012) Enhanced mixotrophic growth of microalga Chlorella sp. on pretreated swine manure for simultaneous biofuel feedstock production and nutrient removal. Bioresour Technol 126:71–79
Halfhide T, Dalrymple O, Wilkie A, Trimmer J, Gillie B, Udom I, Zhang Q, Ergas S (2014) Growth of an indigenous algal consortium on anaerobically digested municipal sludge centrate: photobioreactor performance and modeling. BioEnerg Res 8(1):249–258
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101(8):2623–2628
Yuan X, Wang M, Park C, Sahu AK, Ergas SJ (2012) Microalgae growth using high-strength wastewater followed by anaerobic co-digestion. Water Environ Res 84(5):396–404
Udom I, Zaribaf BH, Halfhide T, Gillie B, Dalrymple O, Zhang Q, Ergas SJ (2013) Harvesting microalgae grown on wastewater. Bioresour Technol 139:101–106
Astals S, Musenze RS, Bai X, Tannock S, Tait S, Pratt S, Jensen PD (2015) Anaerobic co-digestion of pig manure and algae: impact of intracellular algal products recovery on co-digestion performance. Bioresour Technol 181:97–104
González-Fernández C, Molinuevo-Salces B, García-González MC (2011) Evaluation of anaerobic codigestion of microalgal biomass and swine manure via response surface methodology. Appl Energy 88(10):3448–3453
APHA (American Public Health Association) (2012) Standard methods for the examination of water and wastewater 20th ed. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Bligh EG, Dyer WJ (1959) A rapid method pf tpta; lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84(2):281–291
Wang M, Park C (2015) Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 80:30–37
U.S. Environmental Protection Agency (2004) Managing manure nutrients at concentrated animal feeding operations, EPA-821-B-04-006, Chapter 5 Voluntary Performance Standards for CAFOs., pp 5-13–5-18
Hamilton DW, Luce WG, Heald AD (1997) Production and characteristics of swine manure. Oklahoma Cooperative Extension Service No. F-1735, Stillwater
Murphy CF, Allen DT (2011) Energy-water nexus for mass cultivation of algae. Environ Sci Technol 45(13):5861–5868
Zupancic GD, Ros M (2003) Heat and energy requirements in thermophilic anaerobic sludge digestion. Renew Energy 28:2255–2267
Tchobanoglous G, Burton FL, Stensel HD (2003) Treatment, reuse, and disposal of solids and biosolids. In: Wastewater engineering: treatment and reuse, 4th edn. McGraw-Hill, Boston
Collet P, Hélias A, Lardon L, Ras M, Goy RA, Steyer JP (2011) Life-cycle assessment of microalgae culture coupled to biogas production. Bioresour Technol 102(1):207–214
Frost P, Gilkinson S (2010) Interim technical report: first 18 month performance summary for anaerobic digestion of dairy cow slurry at AFBI Hillsborough., Agri-Food and Biosciences Institute, Retrieved May 28, 2014, from http://www.afbini.gov.uk/afbi_ad_18_months-web.pdf
Pronto J, Gooch C (2012) Anaerobic digestion at Patterson Farms, Inc.: case study. Department of Biological and Environmental Engineering, Cornell University. Retrieved May 28, 2014, from http://www.manuremanagement.cornell.edu/Pages/General_Docs/Case_Studies/Patterson_case_study_revision_3.pdf
Crittenden JC, Trussell RR, Hand DW, Howe KJ, Tchobanoglous G (2012) Air stripping and aeration. In: MWH’s water treatment: principles and design. Wiley, Hoboken, NJ, pp. 1163-1244
Sazdanoff N (2006) Modeling and simulation of the algae to biodiesel fuel cycle. (Undergraduate thesis) Retrieved from https://kb.osu.edu/dspace/bitstream/handle/1811/5981/Modeling_and_Simulation_of_the_Algae_to_Biodiesel_Fuel_Cycle-Sazdanoff_undergrad_thesis.pdf;jsessionid=B72D030B040E323EB11ED103EB15E2E3?sequence=1
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30(6):972–979
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27(4):409–416
Dębowski M, Zieliński M, Grala A, Dudek M (2013) Algae biomass as an alternative substrate in biogas production technologies—review. Renew Sust Energ Rev 27:596–604
Wang M, Sahu AK, Rusten B, Park C (2013) Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour Technol 142:585–590
Sakar S, Yetilmezsoy K, Kocak E (2009) Anaerobic digestion technology in poultry and livestock waste treatment—a literature review. Waste Manag Res 27(1):3–18
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102(1):17–25
Nielsen HB, Angelidaki I (2008) Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour Technol 99(17):7995–8001
Lansing S, Martin JF, Botero RB, Nogueira da Silva T, Dias da Silva E (2010) Wastewater transformations and fertilizer value when co-digesting differing ratios of swine manure and used cooking grease in low-cost digesters. Biomass Bioenergy 34(12):1711–1720
Manser ND, Mihelcic JR, Ergas SJ (2015) Semi-continuous mesophilic anaerobic digester performance under variations in solids retention time and feeding frequency. Bioresour Technol 190:359–366
Smith SR, Lang NL, Cheung KHM, Spanoudaki K (2005) Factors controlling pathogen destruction during anaerobic digestion of biowastes. Waste Manag 25(4):417–425
Kim JK, Oh BR, Chun YN, Kim SW (2006) Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J Biosci Bioeng 102(4):328–332
Sahlstrom L (2003) A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour Technol 87(2):161–166
Acknowledgments
The authors want to acknowledge the assistance of Merrill P. Dilbeck, an undergraduate student at the University of South Florida. This material is based upon work supported by the National Science Foundation under Grant No. 1243510. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 409 kb)
Rights and permissions
About this article
Cite this article
Wang, M., Lee, E., Zhang, Q. et al. Anaerobic Co-digestion of Swine Manure and Microalgae Chlorella sp.: Experimental Studies and Energy Analysis. Bioenerg. Res. 9, 1204–1215 (2016). https://doi.org/10.1007/s12155-016-9769-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9769-4