Abstract
Twelve salts were tested for their ability to coagulate microalgae cells in cultures of Chlorella minutissima. The final aim was to develop an easy and efficient approach for harvesting microalgae biomass in dense cultures. Aluminum, ferric, and zinc salts coagulated C. minutissima cultures, while optimum concentration was 0.75 and 0.5 g L−1 for sulfate and chloride salts, respectively. Aluminum salts were most efficient, but caused some cell lysis, which may render this approach inappropriate in some cases. Ferric and zinc salts were ranked second and third, respectively, according to their culture cell-coagulation efficiency. Ferric salts caused a change in the color of the cells, mainly at concentrations higher than 1 g L−1. Zinc salts were less harmful for the microalgal cells, but an additional problem was observed with cell aggregates adhering to the walls of the glass test tubes. Selection of the appropriate coagulant is related to the purpose of the coagulation process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microalgae are cultured for several purposes, such as use in aquaculture, production of food for humans and livestock, cosmetics, biodiesel, pigments or bioactive compounds (Lebeau and Robert 2003; Lee et al. 1998; Ponis et al. 2003). Culture of microalgae results in suspensions of microalgae cells in growth medium. In the case of some microalgae, such as Arthrospira sp., the cells are large, and it is easy to separate the biomass from the water by filtration, while in other cases, as during the production of microalgae for use in aquaculture, microalgae are used directly from the cultures together with the growth medium. In other instances, however, such as extraction of pigments and biodiesel production, there is a need for separation of the cell biomass, which can be accomplished mainly by centrifugation (Price et al. 1974). Centrifugation is a method that has been applied successfully for harvesting microalgae, but shows some major disadvantages. At first, the process involves exposure of microalgae cells to high gravitational and shear forces which may damage the cell structure. Secondly, processing large culture volumes can be time- and energy consuming and requires costly equipment (Knuckey et al. 2006). Alternative processes have been developed including foam fractionation (Gsordas and Wang 2004), filtration (Millamena et al. 1990; Poelman et al. 1997), and coagulation–flocculation (Rossingol et al. 1999).
Harvesting of algal cells by coagulation involves pH adjustment or electrolyte addition, whereas flocculation involves addition of cationic polymers. Such approaches are quite convenient, because they allow rapid treatment of large quantities of microalgae cultures (Oh et al. 2001). Coagulation–flocculation is the coalescence of finally divided suspended cells into larger loosely attached conglomerates, which slowly sink to the bottom of the container. In general, the first stage of coagulation–flocculation is the aggregation of suspended cells into larger particles, resulting from the interaction of the coagulant–flocculant with the surface charge of the cells. Two major forces are involved: electrostatic repulsion forces dominate at large distances (negative-charged cell surfaces repel each other), while intermolecular or Van der Waals attraction forces dominate at very short distances. Intermolecular forces are stronger than electrostatic forces, but act over very short distances. The second stage involves the coalescing of aggregates into large flocs that settle out of suspension (Knuckey et al. 2006). A large number of chemical products have been tested as coagulants or flocculants. The most efficient was aluminum sulfate followed by some cationic polyelectrolytes (Pushparaj et al. 1993; Yu and Tse 1997). The properties of cellular surface, pH of the growth medium, concentration of the coagulant–flocculant, ionic strength of the culture solution, and the number of cells per unit volume are the major factors that influence coagulating–flocculating reactions in microalgae cultures and thereby harvesting of algal biomass (Bilanovic et al. 1998; Lee et al. 1998).
This study aimed at screening of 12 salts as coagulants for harvesting cultures of the microalga C. minutissima, and determination of the optimal concentration of each efficient coagulant. This study aimed as well to evaluate the influence of the coagulants on the function of the photosynthetic apparatus, on the viability of coagulated algal cells, and to determine the correlation between coagulant dose and algal concentration.
Materials and methods
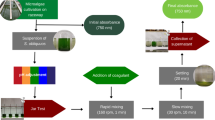
The green microalga Chlorella minutissima, strain Foti and Novak isolated from Heraklion Bay, was used in this study (Kotzabasis et al. 1999). The culture medium was fertilized with 0.1 g L−1 Cell-hi F2P (Cellpharm Ltd, UK). The ability to coagulate cells of C. minutissima was initially tested in cultures with optical density OD750 = 2.4 (approximately 220 × 106 cells mL−1). Coagulation experiments were run with small volumes of culture (20 mL) distributed in cylindrical glass tubes (40 mL).
A first series of experiments was run to examine coagulation efficiency of twelve salts: Al2(SO4)3, AlCl3, Fe2(SO4)3, FeCl3, ZnSO4, ZnCl2, CaSO4, CaCl2, MgSO4, MgCl2, (NH4)2SO4, and NH4Cl. Ten different doses of each salt were used, which ranged from 0–5 g L−1 with a step of 0.5 g L−1. The coagulation efficiency was measured 0, 1, 2, 3, 4, 5, 6, 18, and 24 h after start. This experiment was run three times with two replicates each time.
A second series of experiments was run, with the salts that showed the best coagulation efficiency in the first series of experiments, to determine with higher precision the optimal concentration of each efficient salt for the culture cell coagulation of C. minutissima. The doses tested in this experiment were 0.25, 0.5, 0.75, and 1 g L−1. This experiment was run three times with two replicates each time.
A third series of experiments was run, with the three efficient chloride salts, in order to determine their coagulation efficiency with higher precision. The doses tested were 0–1.5 g L−1, with a step of 0.1 g L−1. This experiment was run two times with two replicates each time.
A fourth series of experiments was run, with the six efficient coagulants that were determined in the first series of experiments and the optimal doses that were found in the second series of experiments in the incubation time of 3 h, to examine the cell viability and the molecular function and structure of the photosynthetic apparatus. This experiment was run two times with two replicates each time.
A fifth series of experiment was run, with AlCl3 in order to examine if there is any correlation between algal concentration and coagulant dose. During these tests, three cell concentrations were used (25, 50, and 100% of the cell concentration used in the previous experiments). This experiment was run two times with two replicates each time.
Determination of coagulation efficiency
After addition of the coagulant, each tube was stirred for about 10 s in a vortex, and 2 mL of the culture were used to follow the kinetic of coagulation activity by measuring OD750 (Buelna et al. 1990;Makridis and Vadstein 1999) in a spectrophotometer using a specific-absorbance cuvette (10 × 4 × 45 mm). Following this approach, it was possible to measure each time at the same point level of the culture. A reference culture (control) was used, where no coagulant was added. Different blanks (culture medium and appropriate quantity of each salt) were used for the measurement of absorbance at each experimental salt concentration, in order to take into account the influence of diluted salts on the absorbance measurements. In other words, we had as many blanks as the number of the experimental coagulant concentrations. Coagulation efficiency was calculated by use of the following equation (Buelna et al. 1990):
where A: OD750 of sample, and B: OD750 of control.
Pictures of all tubes were taken at each sampling point against white background.
Fluorescence induction measurements
Maximum yield of primary photochemistry (F v/F m) was measured according to the JIP method of Strasser and Strasser (1995) by use of Handy Plant Efficiency Analyser, PEA (Hansatech, UK). This method is based on the measurement of a fast fluorescence transient with a 10 μs resolution in a time span of 40 μs to 1 s. Fluorescence was measured at 12-bit resolution and excited by three light-emitting diodes providing a saturated light intensity of 3,000 μmol photons m−2 s−1 of red (650 nm) light. This method allowed the dynamic measurement of a photosynthetic sample at a given physiological state.
Viability of cells
Cell viability was determined by the Evans blue method (Widholm 1972). Briefly, 1 mL samples of each culture were centrifuged at 600 g, the supernatant was discarded, added 100 µL of 1% Evans blue solution, and incubated for 10 min at room temperature. The cells were then washed twice in deionized water. Finally, fresh preparations of the centrifuged samples were examined for the viability by light microscopy. Cells with broken cell walls appeared blue, as Evans blue solution diffused in the protoplasm region and stained the cells blue.
Statistical analysis
One-way ANOVA was used to compare coagulation efficiency in experimental treatments with the control treatment. Statistical analyses were run using the software program STATGRAPHICS Plus 5.0.
Results
Efficiency of various salts as cell coagulants
Only six among the 12 tested salts, (Al2(SO4)3, AlCl3, Fe2(SO4)3, FeCl3, ZnSO4, and ZnCl2), succeeded in coagulating totally the microalgae cells (P < 0.05; Fig. 1). The other six salts (CaSO4, CaCl2, MgSO4, MgCl2, (NH4)2SO4, and NH4Cl) showed no difference from the control treatment in relation to the formation of cell aggregates (P > 0.05; only results with MgSO4 and MgCl2 are shown as an example in Fig. 1).
The measurement of optical density was sufficient for the cases where the salts did not coagulate the cells. In the cases, however, where the salts coagulated the microalgae cells, it was shown that some cell aggregates settled in the bottom of the tubes, whereas in some other cases cell aggregates floated in the air–water interface. The optimum salt concentration was therefore determined after observation of the pictures taken, where it was shown the minimum concentration at which the majority coagulants settled at the bottom of the tube.
Chloride salts (AlCl3, FeCl3, and ZnCl2) were more efficient in comparison with sulfate salts (Al2(SO4)3, Fe2(SO4)3, and ZnSO4). After addition of the appropriate quantity of each chloride coagulant, formation of cell aggregates was immediately observed. While in the case of sulfate salts, formation of cells was observed 2–5 h after addition of coagulants, and about 0.25 g L−1 additional salt dose was required to coagulate totally the same quantity of microalgae compared with the corresponding chloride salts. Chloride coagulants appeared to be efficient in a wider range of concentrations, while sulfate salts (with the exception of ZnSO4) were efficient in a more limited range of concentrations (Fig. 1). Among the six coagulants, aluminum salts were more efficient than ferric salts, and these more efficient than zinc salts. Coagulation time when using aluminum salts was one and a half, and two times shorter, respectively, than what was the case for ferric and zinc salts. After addition of zinc salts, cell aggregates adhered to the walls of the glass tubes and did not settle at the bottom of them. This problem was not shown after addition of aluminum and ferric coagulants (Fig. 1). Addition of FeCl3 at a concentration higher than 1 g L−1 changed the color of the culture from green to brown.
In the second series of experiments, the optimal salt concentration for the cell coagulation of the cultures of C. minutissima was determined with a higher precision, and four doses of each efficient coagulant (Al2(SO4)3, AlCl3, Fe2(SO4)3, FeCl3, ZnSO4, ZnCl2) were used. The most efficient concentration for both aluminum and ferric sulfate salts was 0.75 g L−1 (Fig. 2).There was however a difference in the time needed to obtain 80% coagulation activity, which was 2 and 4 h for aluminum and ferric sulfate, respectively. The most efficient concentration for coagulation of C. minutissima when using aluminum and ferric chloride salts was 0.5 g L−1, and the time needed to obtain 80% coagulation was 1 and 3 h, respectively. It was thus shown that chloride salts were more efficient than sulfate ones, as they needed a lower dose and shorter time to obtain the same, as sulfate salts, coagulation of C. minutissima.
It was difficult to determine the optimal concentration of zinc salts for an efficient culture cell coagulation from the results that are presented in Fig. 2. The problem was the adherence of cells on the walls of the glass tubes, which did not permit a confident measurement of the optical density. However, when we examined the photographs from this kinetic experiment (results not shown), it was determined that the optimal concentrations of ZnSO4 and ZnCl2 salts were 0.75 g L−1 and 0.5 g L−1, respectively.
The first two series of experiments showed that chloride salts were more efficient than sulfate salts. A third series of experiments was run with more detailed gradient in the concentration of chloride coagulants (from 0 to 1.5 g L−1, with a step of 0.1 g L−1). The results 3 h after the incubation time are presented in Fig. 3. AlCl3 was more efficient at a concentration range of 0.4 to 0.8 g L−1, where 0.5 g L−1 was the optimal one, and most of the aggregates were accumulated at the bottom of the tubes. Similar results were obtained with FeCl3, although longer incubation time was demanded in comparison with AlCl3. Zinc chloride caused a slower coagulation than ferric chloride, but the optimal concentration was the same (0.5 g L−1). However, the above-mentioned problem with cells adhering to the walls of the test tubes was apparent.
In conclusion, the ranking of optimal to less optimal coagulant—in reference to the culture cell-coagulation time—for cultures of C. minutissima was: AlCl3, FeCl3, and ZnCl2.
Cell viability in the presence of coagulants
Viability of the cells was tested with Evans blue solution, for all six efficient cell coagulants at their optimal concentrations (0.75 and 0.5 g∙L−1 for sulfate and chloride salts, respectively). Aluminum salts caused cell lysis (Fig. 4), and this phenomenon was more evident with AlCl3 than with Al2(SO4)3. Addition of AlCl3 and Al2(SO4)3 resulted in cells lysis of about 25%, and 10% of total number of cells, respectively. It was therefore observed that there was a correlation between the velocity of coagulation and the percentage of damaged cells: rapid coagulation resulted in higher percent of cell lysis. In contrast to aluminum salts, no cell lysis was observed when ferric and zinc salts were used as cell coagulants for microalgae cultures.
Precipitated cells were examined with fluorescence induction measurements for changes that had taken place in the molecular structure and function of their photosynthetic apparatus. Measurements were made only when using the optimal doses for coagulation. No differences were observed in JIP-parameters between control (without any coagulant) and treatments added coagulant (data not shown). F v/F m in the control culture (without coagulant) was 0.685, while the range of this parameter in the presence of culture cell coagulants was between 0.633 (for Fe2(SO4)3) and 0.659 (for ZnCl2). Thus, the molecular function and structure of the photosynthetic apparatus were not affected by the addition of the coagulants at the optimal concentration of 0.5 g L−1 for chloride salts and 0.75 g L−1 for sulfate salts in the experimental incubation time up to 24 h.
Correlation between the dose of the culture cell coagulant and the algal concentration
The concentration of the cells is a very important parameter for the determination of the optimal dose of culture cell coagulants. An experiment was run at three different cell concentrations (OD750 = 0.6, 1.2, and 2.4) using AlCl3 as a culture cell coagulant to determine the correlation between cell concentration and dose of coagulant (Fig. 5).
a Best dose of the culture cell coagulant of AlCl3 using the half (OD750 = 1.2) of the standard initial cell concentration of OD750 = 2.4. b Best dose of the culture cell coagulant of AlCl3 using the 25% (OD750 = 0.6) of the standard initial cell concentration of OD750 = 2.4. c Linear correlation between culture cell coagulant dose and culture cell concentration (filled diamond 0.063 g L−1, filled square 0.125 g L−1, filled upright triangle 0.25 g L−1, empty square 0.5 g L−1)
The best dose of culture cell coagulant for the initial cell concentration of OD750 = 2.4 was 0.5 g L−1. For the concentration of OD750 = 1.2, we tested three different doses 0.125, 0.25, and 0.5 g L−1, and the optimal one was 0.25 g L−1, while for OD750 = 0.6, the tested doses were 0.063, 0.125, and 0.25 g L−1 and the optimal was 0.125 g L−1 (Fig. 5). A linear correlation between cell concentration and coagulant dose was thus observed because when using half of the initial cell concentration, the demanded dose was exactly half of the initial dose. Similar results were shown when using 25% of the initial cell concentration, where the demanded dose was 25% of the initial dose.
A linear correlation was determined between the cell concentration and the coagulant dose, for the specific tested range of concentrations (OD750 = 0.6–2.4), which was expressed by the following equation:
Discussion
Among the 12 tested salts, only six (Al2(SO4)3, AlCl3, Fe2(SO4)3, FeCl3, ZnSO4, ZnCl2) succeeded in coagulating totally the culture cells of the microalga C. minutissima within 24 h. The other six salts (CaSO4, CaCl2, MgSO4, MgCl2, (NH4)2SO4, and NH4Cl) showed no difference from the control treatment in relation to the generation of cell aggregates. In culture medium, salts are dissociated creating ions, which interact with the negatively charged algal cell wall and cell aggregates are formed. A higher activity of the formed cations results in higher efficiency of the coagulation process. The activity of cations is determined to a great extent by their electronegativity. Calcium, magnesium, and ammonium ions have lower electronegativity than aluminum, ferric, and zinc ions. This could explain why aluminum, ferric, and zinc salts were more efficient in coagulating microalgae cultures, while calcium, magnesium, and ammonium could not succeed as coagulants in a time interval of 24 h.
Among the six efficient coagulants, it was observed that chloride salts were more efficient than the corresponding sulfate ones (Figs. 1 and 2). This could be explained by the fact that chloride anions are more soluble in water than sulfate anions. Additionally, chloride salts showed a wider concentration range, in which a quite good solubility (near optimal) could be obtained (Maeda et al. 2002). In this study, chloride coagulants appeared to be efficient in a wider concentration range, on the contrary to sulfate salts, which were efficient in a more limited range, because of their very limited solubility range.
Among both chlorides and sulfates salts, it was observed that aluminum salts were more efficient than ferric salts, and these in turn more efficient than zinc salts (Fig. 2 and 3), based on the demanded coagulation time. These results could be explained by the molecular weight and the charge of the formed cations. Aluminum ions have the highest charge density, which led to extended molecular conformation and bridging between cells, and improved charge neutralization of microalgae cell surfaces. In addition, the higher the molecular weight, the lower the solubility. These two facts can be combined and the observed coagulation efficiency in the tested salts could be explained. Aluminum salts have a lower molecular weight than ferric salts and zinc salts. In parallel, the charge of aluminum and ferric cations is +3, higher than the corresponding charge of zinc cations (+2). Aluminum salts (lower molecular weight and higher charge) showed better behavior as coagulant. Ferric salts were not as efficient as aluminum salts because, independent of the high charge of ferric ions (equal to aluminum), they have higher molecular weight that decreased their solubility and as a result, their efficiency as coagulants. Finally, zinc salts were the least efficient coagulants, because they have higher molecular weight and lower charge, compared with aluminum and ferric salts.
Independent of the time of coagulation and the dose of coagulants, the above salts exhibited some problems. A small percentage of damaged cells was observed when using aluminum salts as coagulants (about 10–25%), which could be caused by rapid aggregation of microalgae (Fig. 4), or by destabilization of cell membrane of microalgae. However, the remaining alive cells did not show any stress effect in the molecular structure and function of the photosynthetic apparatus after 24 h, as determined by fluorescence induction measurements.
When using ferric salts, the color of culture changed from green to brown–yellow, mainly at concentrations over 1 g L−1. It seemed that ferric salts over a specific concentration influenced the pigments of the microalgae, and especially chlorophylls. However, there was no effect on the photosynthetic apparatus and cell viability, as shown in Fig. 4.
When using zinc salts, aggregates adhering on the walls of the glass tubes were observed (Fig. 1). This attachment was quite weak, as cell aggregates easily detached from the tube walls, after a slight knock of the tube. In an industrial scale, this problem could be easily avoided by selection of the appropriate material for inner surface of the container where cell coagulation takes place.
The correlation of cell concentration and coagulant concentration permitted us to assume that the predominant destabilization mechanism is charge neutralization and not sweep flocculation, which is an inefficient use of coagulation. In the case of sweep flocculation, there is no stoichiometric relationship between the particle concentration and the optimum coagulant dose (Gregory and Duan 2001), whereas such a relationship was established for the ranges of microalgae cell concentration and coagulant concentration used in this study.
The selection of the appropriate coagulant is directly correlated to the target of the process. For example, if the purpose of coagulation is the production of pigments, then ferric salts are undesirable. Possibly, zinc salts are appropriate. If the purpose is production of biodiesel, then efficiency and economy are important, which means that selection of the fastest and cheapest coagulant is appropriate, that is aluminum chloride.
References
Bilanovic D, Shelef G, Sukenik A (1998) Flocculation of microalgae with cationic polymers: effects of medium salinity. Biomass 17:65–76
Buelna G, Bhattarai KK, de la Noue J, Taiganides EP (1990) Evaluation of various flocculants for the recovery of algal biomass growth on pig-waste. Biol Wastes 31:211–222
Gregory J, Duan J (2001) Hydrolyzing metal salts as coagulants. Pure Appl Chem 73:2017–2026
Gsordas A, Wang J-K (2004) An integrated photobioreactor and foam fractionation unit for the growth and harvest of Chaetoceros spp. in open systems. Aquacult Eng 30:15–30
Knuckey R, Brown M, Robert R, Frampton D (2006) Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquacul Engin 35:300–313
Kotzabasis K, Hatziathanasiou A, Bengoa-Ruigomez MV, Kentouri M, Divanach P (1999) Methanol as alternative carbon source for quicker efficient production of the microalgae Chlorella minutissima: Role of the concentration and frequency of administration. J Biotechnol 70:357–362
Lebeau T, Robert JM (2003) Diatom cultivation and biotechnologically relevant products. Part I: cultivation at various scales. Appl Microbiol Biotechnol 60:612–623
Lee S, Kim S, Kim J, Kwon G, Yoon B, Oh H (1998) Effects of harvesting method and growth stage on the flocculation of the green alga Botryococcus braunii. Lett Apl Microbiol 27:14–28
Maeda K, Kuramochi H, Shinkawa T, Fukui K (2002) Solubility of two salts containing sulfate and chloride ions in water for ternary systems at 313 K. J Chem Eng Data 47:1472–1475
Makridis P, Vadstein O (1999) Food size selectivity of Artemia franciscana at three developmental stages. J Plankt Research 21:2191–2201
Millamena O, Aujero E, Borlongan I (1990) Techniques on algae harvesting and preservation for use in culture as larval food. Aquacult Eng 9:295–304
Oh H-M, Lee S, Park M-H, Kim H-S, Kim H-C, Yoon J-H, Kwon G-S, Yoon B-D (2001) Harvesting of Chlorella vulgaris using a bioflocculant from Paenibacillus sp. AM49. Biotechnol Lett 23:1229–1234
Poelman E, De Pauw N, Jeurissen B (1997) Potential of electrolytic flocculation for recovery of microalgae. Resour Conserv Recycl 19:1–10
Ponis E, Rombert R, Parisi G (2003) Nutritional value of fresh and concentrated algal diets for larval and juvenile Pacific oysters. Aquaculture 221:491–505
Price CA, Mendiola-Morgenthaler LR, Goldstein M, Breden EN, Guillard RRL (1974) Harvest of planktonic marine algae by centrifugation into gradients of silica in the CF-6 continuous-flow zonal rotor. Biol Bull 147:136–145
Pushparaj B, Pelosi E, Torzillo G, Materassi R (1993) Microbial biomass recover using a synthetic cationic polymer. Biores Tech 43:59–62
Rossingol N, Vandajon L, Jaouen L, Quemeneur F (1999) Membrane technology for the continuous separation microalgae/culture medium: compared performances of cross-flow microfiltration and unfiltration. Aquaculture 20:191–208
Strasser B, Strasser R (1995) Measuring fast fluorescence transients to address environmental questions: The JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. Kluwer, Dordrecht, pp 977–980
Widholm JM (1972) The use fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:89–94
Yu J, Tse S-W (1997) Flocculation of Pseudomonas with aluminum sulfate for enhanced biodegradation of synthetic dyes. Biotechnol Tech 11:479–482
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papazi, A., Makridis, P. & Divanach, P. Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol 22, 349–355 (2010). https://doi.org/10.1007/s10811-009-9465-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-009-9465-2