Abstract
Synthetic or natural cationic polymers, such as polyacrylamide and chitosan, are exploited as coagulants due to their characteristics, such as a high positive surface charge. Due to variations in culture conditions, such as species and cell concentration, different interactions of coagulants with microalgae occur, affecting the efficiency of biomass recovery. Thus, in this study, the separation efficiency of biomass of the microalgae Scenedesmus obliquus was evaluated by coagulation-flocculation with the synthetic polymer polyacrylamide and natural polymer chitosan to optimize the dose and pH by means of a rotational central composite design. Biomass removal efficiencies greater than 90% and greater than 70% were observed for the synthetic polymer and chitosan, respectively. Notably, the reduced optical density and biological nature of microalgae make the use of response surface methodology challenging. Optimal regions with doses of 12.8 mg of synthetic polymer and 5.0 mg of chitosan were obtained per liter of microalgae sample. An optimal pH of 6.5, falling within the natural range of culture conditions was indicated for both coagulants, minimizing reagent costs. The interaction with microalgae and the potential application of chitosan and other natural coagulants that are efficient and economically viable should be explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coagulation-flocculation is a simple and reliable technique with low energy consumption that is applicable to the removal of turbidity, color, organic compounds, pathogens, oils, fats and microalgae [1,2,3]. This process consists of the addition of products that, through specific mechanisms, destabilize surface charges with the subsequent formation of flocs, which can be separated by density differences [4, 5]. Classical coagulation-flocculation and its variations, such as electrocoagulation, have been effectively used to remove a diverse range of contaminants from water [2, 6, 7].
Due to the simplicity of coagulation-flocculation, this technique is also used for recovery or harvesting of microalgae biomass [8,9,10,11]. The commercial interest in microalgae is due to the accumulation of lipids and carbohydrates, which can be used for the production of biofuel, animal feed, food, cosmetics and pharmaceuticals [12, 13]. Microalgal cells are colloidal particles on the order of 3 to 50 μm that are negatively charged and, due to the repulsion of their charges, remain stable in media [10, 14, 15]. Thus, coagulation-flocculation can be performed through the addition of certain products, such as cationic polymers, that minimize or neutralize the surface charges of these cells, inducing aggregation and the consequent formation of flocs, which can be physically separated [14].
Synthetic or natural cationic polymers are the coagulants commonly used in these processes due to their efficiency [16]. However, the application of synthetic polymers is made challenging by environmental and biomass contamination due to the nonbiodegradable intermediate byproducts that are generated, in addition to the danger they pose to human health, such as neurotoxic and carcinogenic risks [17, 18].
Chitin is a natural biopolymer whose abundance in nature is surpassed only by that of cellulose and that is found in the shells of crustaceans, such as shrimp and crab [1, 8, 19]. According to this author, chitosan, obtained from the deacetylation of chitin, has particular properties, such as the presence of primary amine groups, which are responsible for the coagulant properties of this material. Despite the environmentally favorable characteristics of this product, the acquisition cost is an obstacle in the application of chitosan in coagulation-flocculation [8, 20, 21]. Therefore, optimization studies of these coagulants are conducted to study the conditions that promote greater recovery of microalgae biomass with a lower dose and overall cost.
Since the effectiveness of coagulation-flocculation depends on the interaction of the characteristics of each microalgae species, pH of the medium and coagulant, there is not enough information on the effects of coagulants on the recovery of microalgae biomass, as different doses and pH values are reported in the literature for coagulation-flocculation of microalgae [22]. Musa et al. [23] reported an optimal dose of synthetic polyacrylamide polymer of 10 mg L−1 at pH 6.5 for the recovery of Chlorella sp. However, Nayak et al. [24] suggested an optimal dose of 10 mg L−1 chitosan and pH 8.0 for the recovery of this same species. Conversely, Gupta et al. [25] described efficiencies above 90% for the recovery of Scenedesmus spp. using 100 mg L−1 chitosan at pH 7.0 and 10.0 mg L−1 synthetic cationic polymer at pH 10.0.
Thus, the variation in the reported optimal conditions reinforces that the culture conditions such as species, cell concentration, ionic strength, pH of the medium, surface charge, growth phase, composition and concentration of the culture medium influence the interaction between the coagulant and microalgae and consequently interfere with the biomass recovery efficiency [26]. In addition, most coagulation-flocculation studies of microalgae have been conducted with the species Chlorella sp., making it necessary to optimize conditions for other species, such as Scenedesmus spp.
One tool used to optimize coagulation-flocculation conditions is statistical designs that describe the behavior of a response variable under the influence of different variables [27]. Thus, based on their chemical properties, application potential and different results reported in the literature, the synthetic polymer polyacrylamide and the natural polymer chitosan were chosen to evaluate the separation efficiency of the biomass of Scenedesmus obliquus BR003 cultivated in raceway, by means of coagulation-flocculation at different doses and medium pH values; the response was assessed according to the model generated by a central composite rotational design (CCRD). Despite of that, studies of biomass separation with Scenedesmus obliquus using different coagulants are still scarce. Hence, the challenge and novelty of this study were to evaluate the recovery efficiency of microalgae cultivated on a pilot scale, in real weather conditions, which is close to industrial scale and evaluate the response using response surface method.
2 Materials and methods
2.1 Cultivation of microalgae
The strain Scenedesmus obliquus BR003 was obtained from the Microalgae Collection of the Department of Agricultural Engineering of the Federal University of Viçosa (Minas Gerais state, Brazil). The microalgae was cultivated in an open raceway system of 4000-L capacity, which simply consists of a shallow pond that uses sunlight for biomass production [28]. Cultivation was performed according to Rocha et al. [29] in L4-m culture medium with agricultural fertilizer ratios as shown in Table 1. The pH of culture was maintained between 6.5 and 7.5. The average daily radiation intensity was 752.1 µmols m−2 s−1 from direct sunlight, with a temperature variation between 22 and 30 ºC. The microalgae were harvested until use for coagulation-flocculation tests on the 10th day of cultivation during the stationary growth phase.
2.2 Obtaining and preparing coagulants
In the coagulation-flocculation tests, a synthetic polymer based on polyacrylamide and chitosan powder extracted from shrimp shells (Sigma-Aldrich) were used. The standard solutions of both coagulants were prepared at high concentrations to avoid dilution of the microalgae suspension [21].
To prepare the synthetic polymer solution, the powder was first dried in an oven at 105 °C for 24 h, after which 5.0 g of the polymer was dissolved at 60 °C in 300 mL of distilled water under constant stirring. After total dissolution, the volume was brought to 500 mL to obtain a stock solution of 10.0 g L−1. As recommended by Bleeke et al. [30], the solution was stored at 4 °C for a maximum of 15 days. Finally, from this solution, dilutions corresponding to doses between 3.0 and 18.0 mg L−1 were used.
The standard chitosan solution was prepared on the day of the tests to avoid degradation. A total of 100.0 mg of chitosan was dissolved in 10 mL of HCl solution (0.1 M) under constant stirring for 10 min. Then, the solution was diluted in 100 mL of deionized water to obtain a chitosan stock solution of 1.0 g L−1 [11]. From this solution, different dilutions were prepared, corresponding to the desired concentrations between 5.0 and 20.0 mg L−1. The change in the final volume of the samples after the addition of the coagulants was considered insignificant, as also described by Gerchman et al. [31].
2.3 Determination of the optical density
The microalgae S. obliquus was cultivated until the period corresponding to the end of the logarithmic phase and the beginning of the stationary phase. The occurrence of this phase was determined by growth analysis for approximately 10 days [32, 33]. The optical density (OD), which is the absorbance of the microalgae suspension, was determined at a wavelength of 750 nm to obtain the cell growth curve and coagulant efficiency. OD readings were performed with a spectrophotometer (Thermo Scientific-Multiskan Go) before and after the coagulation-flocculation assays [30, 34, 35]. Thus, the efficiency of biomass removal for each coagulant under study was identified and compared. Efficiency was determined according to the recovery efficiency equation [16].
At the end of the growth curve, the concentration of dry biomass in the form of suspended solids was determined using the gravimetric method [5, 36].
2.4 Determination of coagulation-flocculation efficiency
A jar test (AT700, Alpha Technochemical), was used to optimize the coagulation-flocculation process. A schematic of the test is shown in Fig. 1.
The beakers were filled with 500 mL of microalgae suspension that had been previously filtered to remove old cells and dirt, resulting in a mean initial absorbance of 0.055. Coagulant doses of 3.0–18.0 mg L−1 synthetic polymer and 5.0–20.0 mg L−1 chitosan, in addition to pH values between 4.0 and 9.0, were studied. The ranges of values were chosen after preliminary tests and based on the study of Ahmad et al. [37]. The pH was adjusted before each test using HCl (2 M) and NaOH (2 M) solutions.
After pH correction, the jar test process was next. In the rapid mixing phase, the coagulants were added, and the microalgae suspension was stirred at 160 rpm for 1 min. Then, in the slow mixing phase the suspension was stirred at 30 rpm for 10 min according to the method adapted from Lananan et al. [10] and Oliveira et al. [5]. This procedure was followed by sedimentation for 20 min, which was also chosen on the basis of previous tests. At the end of the tests, samples were collected 2 cm from the surface and subjected to absorbance readings.
2.5 Analysis of zeta potential
The zeta potential values of the studied coagulants and the microalgae suspension were evaluated to understand the mechanism of action of the coagulants. The analyses were performed in the Packaging Laboratory of the Department of Food Engineering of the Federal University of Viçosa using a Zetasizer Nano-ZS (Malvern) device.
2.6 Experimental design
A central composite rotational design (CCRD) was used to optimize the coagulant dosage and pH in the recovery of microalgae biomass through coagulation-flocculation under the effect of different coagulant doses and pH values. The variables were selected after performing preliminary tests, with the aim of establishing the smallest possible number of tests so that all runs could be performed on the same day, reducing the interference of time.
The CCRD consisted of a 22 factorial scheme with four additional axial points and six central points, totaling 14 runs, which were performed randomly. The high and low levels were chosen from the results obtained in preliminary tests.
The coagulant dosages and pH levels studied are shown in Table 2.
The effects of the coagulant dose and pH were evaluated by analysis of variance (ANOVA). The prediction validity of the generated models was also evaluated. Contour plots showing the behavior of the response variable as a function of the variation in the levels of coagulant dose and pH were generated [38].
3 Results and discussion
3.1 Efficiency of coagulants
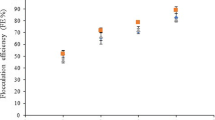
Studies with synthetic polyacrylamide-based polymers and natural chitosan-based polymers have demonstrated the potential of these coagulants in the biomass harvesting of different microalgae species [11, 16, 23, 24]. In the present study, removals above 90% and 75% (Table 3) were achieved using polyacrylamide and chitosan, respectively. The indicator variable was the optical density (OD) of the supernatant, which was analyzed in a spectrophotometer at a wavelength of 750 nm (Table 3).
Polyacrylamide was effective at doses above 5.19 mg L−1. Furthermore, the pH did not influence the performance of this coagulant, since there were good removals of biomass in the entire pH range studied. The natural pH of microalgae suspensions can vary from 6.0 to 9.0 depending on the culture conditions, such as the development phase, alkalinity and carbon dioxide dissolved in the medium [23, 39]. Thus, pH adjustment is not necessary when polyacrylamide is used to harvest S. obliquus biomass, as also reported by Musa et al. [23] and Nguyen et al. [39], since the natural pH of this target suspension was approximately 6.5, which is within the range of action of the coagulant.
Different commercial polyacrylamide products were tested by Bleeke et al. [30] for separation of microalgae Chlorella sp., Scenedesmus aculminatus and Chlamydomonas reinhardtii. Concentrations from 1.5 to 4.0 mg L−1, depending on the microalgae species, resulted in cell removal efficiency greater than 90%. The high molecular weight, due to the long chains, and cationic charge of this type of polymer resulted in desired efficiencies for cultivation conditions and different microalgae species [23, 30, 39]. Despite the characteristics of polyacrylamide, Chen et al. [32] obtained harvesting efficiencies of Scenedesmus sp. of approximately 20% using a dose of 0.05 g L−1 in the pH range of the medium between 7.0 and 11.0. These authors report that polymers properties and their interaction with the microalgae solution can influence the performance of this coagulant.
In this study, a minimum chitosan dose of 5.0 mg L−1 was sufficient to achieve greater than 70% OD removal at pH 6.5 (Table 3). The highest dose studied (20.0 mg L−1) resulted in an efficiency of 48%. This behavior may be due to the restabilization of charges in the system, considering the conditions of this run (pH 6.5).
In addition, chitosan is more efficient in the pH range between 6.0 and 8.0 because it loses its charge and ability to coagulate in a more alkaline medium [31]. Gupta et al. [21] found greater than 70% recovery of Scenedesmus spp. at pH 7.0 and a dose of 40.0 mg L−1. The best dose reported by the authors was higher than that found in the present study, but it is noteworthy that different characteristics of the culture medium and biomass concentrations may result in different optimal doses even at similar pH values.
Due to the acid character of the standard chitosan solution (pH 2.6) and the amount of solution added to the jars, there was a reduction in pH at the end of some tests, as shown in Fig. 2. This same behavior was reported by Chen et al. [32], who reported a pH reduction of 10.0 to 7.0 and biomass harvesting efficiency of 95% when using a dose of 80.0 mg L−1 chitosan. Yunos et al. [11] also reported pH variations with doses from 5 to 50.0 mg L−1. Unlike the present study, these authors reported that despite the acidity of the chitosan solution, there was an insignificant reduction in the pH of the medium, which still fell within the studied range, from 7.0 to 8.0.
Although chitosan is cited in the literature as an efficient natural coagulant for the recovery of microalgae, it should be noted that the efficiency and doses of chitosan-microalgae mixtures depend on the species used, contamination in the culture and the characteristics of the chitosan solution, such as the degree of deacetylation, molar mass and purity [1, 11, 20]. This is observed in the variation of optimal dose results, between 10 and 120 mg L−1, in addition to media with pH between 6.0 and 8.0 discussed in the literature [11, 21, 24, 35, 40]. Besides, results of efficiency lower than 20% using chitosan to recover consortium of microalgae of different species can also be found [20].
Since the coagulation-flocculation conditions using chitosan cannot be generalized, the search for more homogeneous cultivation conditions, the understanding of the interaction of microalgae with coagulants must be deepened. Thus, when using chitosan for microalgae biomass recovery, optimization is still necessary to establish the best dose and pH that promote the highest biomass harvesting efficiency of the target species [40].
3.2 Zeta potential and coagulation mechanisms
Since the zeta potential measures the electrical potential of particles in a liquid, it is an important indicator of colloidal dispersion stability [41, 42]. The coagulation mechanisms that occur in the microalgae recovery process depend on the surface characteristics of the microalgae cells and the coagulants used [13]. Microalgae have a negative surface charge, mainly related to the presence of carboxyl (–COOH), hydroxyl (–OH) and phosphate (PO43−) functional groups [15, 24, 25]. Thus, the greater the positive electrical charge of the coagulant is, the greater the tendency of coagulation to occur by charge neutralization and patch mechanisms, whereas negative charges indicate that a bridging mechanism can occur [43, 44].
The species used in this study has a charge of approximately –4 to − 12 mV, which caused variations in pH (Table 4). In addition, the most negative charge was observed at pH values close to neutrality.
Polyacrylamide and chitosan are positively charged at pH 3.5 and 2.6, respectively (Table 4). Since microalgae have a higher negative charge at a pH close to neutrality, the efficiency of cationic polymers is better at this pH value due to charge destabilization because the electrostatic interaction between the microalgae cells and the coagulant improves [32]. In addition, acidic media increase the protonation of the amine groups in chitosan, making chitosan more cationic and, therefore, more attracted by anions [37].
Studies of Dong et al. [43] using chitosan and polyacrylamide in medium with pH 5.0, 9.0 and 12.0 demonstrated that these coagulants had better efficiency in medium with pH 5.0. It was observed that in the zeta potential profile of the chitosan solution, the compounds are more protonated at more acidic pH. From pH 8.0, the chitosan zeta potential charge reaches 0 mV; thus, the polymer has its coagulation-flocculation capacity reduced, since the positive and negative charges on its surface cancel each other out.
The difference in charge between the studied cationic coagulants and microalgae (Table 4) indicates that the charge of the microalgae may be neutralized because positively charged ions are adsorbed to the cell surface and reduce electrostatic repulsion between particles [37, 45]. However, the coagulation-flocculation mechanisms of microalgae in the presence of cationic polymers are still not well understood, and the relevant processes can occur separately or together [14, 15]. Thus, due to the cationic characteristics of chitosan and polyacrylamide, it is assumed that the coagulation of the microalgae-polymer system involves neutralization and a patch mechanism, and the formation of bridges may also occur [23, 32, 35, 39, 43, 44].
Ahmad et al. [37] explained that the charges in a microalgae suspension are destabilized when a positively charged material, such as chitosan, is added because the positive regions are adsorbed to the negative regions of the cells; thus, during agitation, these aggregated particles collide. The authors also highlighted that by adding a concentration of positive charges higher than that necessary to destabilize the negative charges, charge restabilization may occur, and the efficiency of coagulation-flocculation will reduce the formation of flocs.
Due to bridge formation between particles and polymers with the same charge, a high cationic charge can first neutralize the charges of the microalgae suspension, and the balance of positive charges in the nuclei acts as a chelating agent [21, 24, 30]. According to these authors, bridge formation requires a lower dose than neutralization, which explains the variation in doses used in the literature and the consequent variations in microalgae-polymer interactions in different conditions. Since these mechanisms act together and polymers and microalgae have different surface characteristics, such as different active groups, it is not possible to say with certainty which mechanism dominated in the coagulation-flocculation process.
Besides, other parameters are important to understand the coagulation-flocculation process, like the floc formation and functional groups of coagulants, as well as compounds that may interact inside coagulants. The process of floc formation in stable conditions will not occur; this way, the properties of the coagulant destabilize the particles and collide with each other to form flocs with a larger size that can easily precipitate [46]. These authors shows that functional groups can contribute to the coagulating/flocculating activity of natural coagulants. Once that increasing positive charged groups allows interactions with the negative charged microalgae, this way improves the binding capabilities of natural coagulants. In studies with chitosan, the chitin has amine groups that are positively charged and contribute to the efficiency of the harvesting process [1, 8, 19, 46].
3.3 Obtaining the prediction model and response surfaces
Table 3 shows that the greatest removal of cells measured by OD was observed for the synthetic polymer regardless of pH and that doses higher than 5.19 mg L−1 promoted greater removal of microalgae biomass. With chitosan addition, removal occurred in the studied pH range between 4 and 9, as shown in Table 3, and the most significant removal occurred in the pH range between 6.0 and 8.0. In addition, a dose similar to that used for the synthetic polymer yielded an efficiency greater than 70%.
Optical density (OD) measurements are appropriate for cultures at low concentrations, as is the case in this study. However, the readings performed at 750 nm resulted in a low initial absorbance (with mean values between 0.05 and 0.06). The discrepancies in the results are related to the limited determination accuracy of microalgal crops with low concentration, as also reported by Delrue et al. [47].
The significance of the variables was different for each model; thus, the less significant variables were eliminated, and the hierarchy of variables was considered to generate a simpler design that maintained good predictive ability. The removal of OD using the synthetic polymer has a significant effect on the dose. Although pH is an important variable in the coagulation-flocculation process, this factor was nonsignificant because the coagulant acted efficiently in the entire pH range studied, with no difference in OD removal (considering the initial conditions of low OD). In this case, it can be inferred that for this coagulant, coagulation-flocculation without pH adjustment promotes a reduction in reagent cost, as long as the process is performed at pH values and microalgae inputs within the range considered in this study [23].
pH plays a key role in coagulation-flocculation with chitosan because the protonation of this coagulant is pH dependent. For this reason, even if the pH did not show significance in the tests, it was retained in the models [21].
Response surface methodologies, such as CCRD, have the advantage of allowing inferences about experiments with multiple variables and fitting models with a reduced number of runs and were initially applied to methods related to chromatography. However, research with other responses can be developed by applying the relevant principles [27]. Tests with this type of methodology should be performed under very controlled conditions. In the present experiment, even with rigorous laboratory control, the values of the coefficients denoting the performance of the model were considered small.
The second-order fitted models for the responses obtained using the synthetic polymer (R2 equal to 65.2%) and chitosan (R2 equal to 68.2%) are presented in Eqs. 1 and 2, respectively. Models can be considered indicators of a trend when R2 < 0.60 and can be used for predictive purposes above this value [48]. The polyacrylamide efficiencies were satisfactory, but the low initial OD probably reduced the predictive power of the model.
The optimal doses generated by the model for microalgae removal were 12.8 mg L−1 and 5.0 mg L−1 when using synthetic polymer and chitosan, respectively. These values indicate the ideal conditions for the maximum recovery of microalgae biomass. It is important to note that although the effect of pH was not significant in both models, it is a relevant variable in this type of assay. In this study, using the coagulant at the natural pH (approximately 6.5) of the microalgae resulted in good process efficiency and a cost reduction with respect to pH change, as also reported by Musa et al. [23].
The obtained contour plots (Fig. 3) indicate the regions and conditions with the best efficiency. Figure 3a shows that the pH did not influence the recovery efficiency of microalgae and that the best doses were between 10.0 and 14.0 mg L−1. In addition, Fig. 3b shows that there are optimal regions at extreme pH and dose points; thus, from an economic point of view, one can choose the pH and dose that provide lower costs.
The validation of the model was performed by independent tests at the optimal point predicted by the model for each coagulant. The results of these tests are shown in Table 5.
As observed in Table 5, the model predicts removed absorbance values of 0.0598 and 0.0481 for the synthetic polymer and chitosan, respectively. These conditions would be equivalent to almost 100% removal in the initial mean conditions of the tests (mean initial DO between 0.05 and 0.06). The results confirmed the efficiency of the coagulants studied and the satisfactory optimization of the parameters studied for the microalgae S. obliquus. The dose of chitosan indicated for the conditions of this study was lower than that reported in the literature, which can be attributed to the concentration of biomass (on average 0.1 g L−1, as determined at the end of the stationary phase) and the type of chitosan used.
Regarding chitosan, a cost of $9.60 per kilogram of microalgae extracted was estimated for the conditions studied here, i.e. processed chitosan with a relatively high degree of purity. Although costly, chitosan can be viable; for example, the production of shrimp, whose residues are a source of chitosan, is intense in the Northeast region of Brazil, and this same region is the national center of microalgae production. Thus, the costs could be reduced with the acquisition and use of local products [49]. Chitosan has the advantages of no risks of supernatant contamination or toxic effects to the environment [50]. Although the use of chitosan involves doses higher than those required for polyacrylamide, the latter is related to environmental risks, biomass contamination and carcinogenic potential [18]. In addition to chitosan, other natural coagulants are promising, such as Moringa oleifera seeds and Guazuma ulmifolia tree bark [51].
4 Conclusions
The use of coagulants for the recovery of S. obliquus biomass is important to improve the microalgal biomass recovery process. Polyacrylamide has the advantage of being efficient for different cultivation conditions and microalgae species; however, its deleterious effects on health and the environment require replacement by other less harmful coagulants. Chitosan is efficient for the recovery of S. obliquus. However, it has relatively narrow operating conditions, especially regarding pH range, due to the protonation of the active groups responsible for coagulation. In this study, more than 90% and 70% of OD removal were obtained using polyacrylamide and chitosan, respectively. Optimal regions with doses of 12.8 mg of synthetic polymer and 5.0 mg of chitosan were obtained per liter of microalgae sample. An optimal pH of 6.5, falling within the natural range of culture conditions, was indicated for both coagulants, minimizing reagent costs. This use at full scale should be based on an evaluation of its economic viability. In addition, for future research it is important to explore other natural coagulants with efficient biomass recovery for different microalgae crops that are sustainable and economically viable to reduce the use of synthetic coagulants. Besides, using CCRD in the optimization of coagulation-flocculation of microalgae is challenging due to the biological nature of these organisms.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Oladoja NA (2015) Headway on natural polymeric coagulants in water and wastewater treatment operations. J Water Process Eng 6:174–192. https://doi.org/10.1016/j.jwpe.2015.04.004
Saleh TA, Mustaqeem M, Khaled M (2022) Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ Nanotechnol, Monitor Manag 17:100617
Kurniawan SB, Ahmad A, Ilmron MF, Abdullah SRS, Othman AR, Hasan HA (2022) Potential of microalgae cultivation using nutrient-rich wastewater and harvesting performance by biocoagulants/bioflocculants: mechanism, multi-conversion of biomass into valuable products, and future challenges. J Clean Prod 365:132806
Abomohra AE, Jin W, Sagar V, Ismail GA (2018) Optimization of chemical flocculation of Scenedesmus obliquus grown on municipal wastewater for improved biodiesel recovery. Renew Energy 115:880–886. https://doi.org/10.1016/j.renene.2017.09.019
Oliveira GA, Carissimi E, Monje-ramírez I, Velasquez-orta SB, Rodrigues RT, Ledesma MTO (2018) Bioresource technology comparison between coagulation- flocculation and ozone- flotation for Scenedesmus microalgal biomolecule recovery and nutrient removal from wastewater in a high-rate algal pond. Biores Technol 259(March):334–342. https://doi.org/10.1016/j.biortech.2018.03.072
Ahmad A, Das S, Ghangrekar MM (2020) Removal of xenobiotics from wastewater by electrocoagulation: a mini-review. J Indian Chem Soc 97:493–500
Ahmad A, Priyadarshini M, Das S, Ghangrekar MM (2022) Electrocoagulation as an efficacious technology for the treatment of wastewater containing active pharmaceutical compounds: a review. Sep Sci Technol 57(8):1234–1256
Yang Z, Hou J, Miao L (2021) Harvesting freshwater microalgae with natural polymer flocculants. Algal Res 57:102358
Hamid SHA, Lananan F, Khatoon H, Jusoh A, Endut A (2016) A study of coagulating protein of Moringa oleifera in microalgae bio- flocculation. Int Biodeterior Biodegradation 113:310–317. https://doi.org/10.1016/j.ibiod.2016.03.027
Lananan F, Mohd Yunos FH, Mohd Nasir N, Abu Bakar NS, Lam SS, Jusoh A (2016) Optimization of biomass harvesting of microalgae, Chlorella sp. utilizing autoflocculating microalgae, Ankistrodesmus sp. as bio-flocculant. Int Biodeterior Biodegradation 113:391–396. https://doi.org/10.1016/j.ibiod.2016.04.022
Yunos FHM, Nasir NM, Hartini H, Jusoh W, Khatoon H, Shiung S, Jusoh A (2017) Harvesting of microalgae ( Chlorella sp.) from aquaculture bioflocs using an environmental-friendly chitosan-based bio-coagulant. Int Biodeterior Biodegradation 124:243–249. https://doi.org/10.1016/j.ibiod.2017.07.016
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenerg 53:29–38. https://doi.org/10.1016/j.biombioe.2012.12.019
Enamala MK, Enamala S, Chavali M, Donepudi J (2018) Production of biofuels from microalgae—a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sustain Energy Rev 94(May 2017):49–68. https://doi.org/10.1016/j.rser.2018.05.012
Mubarak M, Shaija A, Suchithra TV (2019) Journal of Environmental Chemical Engineering Flocculation : An effective way to harvest microalgae for biodiesel production. J Environ Chem Eng 7(4):103221. https://doi.org/10.1016/j.jece.2019.103221
Pugazhendhi A, Shobana S, Bakonyi P, Nemestóthy N, Xia A, Rajesh Banu J, Kumar G (2019) A review on chemical mechanism of microalgae flocculation via polymers. Biotechnol Rep 20(2018):e00302. https://doi.org/10.1016/j.btre.2018.e00302
Roselet F, Vandamme D, Roselet M, Muylaert K, Abreu PC (2015) Screening of commercial natural and synthetic cationic polymers for flocculation of freshwater and marine microalgae and effects of molecular weight and charge density. Algal Res 10(1):183–188. https://doi.org/10.1016/j.algal.2015.05.008
Cai Q, Song K, Cai P, Tian C, Wang C, Xiao B (2022) Harvesting of different microalgae through 100-μm-pore-sized screen filtration assisted by cationic polyacrylamide and specific extracellular organic matter. Sep Purif Technol 280:119918
Rudén C (2004) Acrylamide and cancer risk — expert risk assessments and the public debate. Food Chem Toxicol 42:335–349. https://doi.org/10.1016/j.fct.2003.10.017
Niemi C, Gentili FG (2021) The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol Plant 173(2):536–542. https://doi.org/10.1111/ppl.13409
Granados MR, Acién FG, Gómez C, Fernández-Sevilla JM, Molina Grima E (2012) Evaluation of flocculants for the recovery of freshwater microalgae. Biores Technol 118:102–110. https://doi.org/10.1016/j.biortech.2012.05.018
Gupta SK, Kumar M, Guldhe A, Ansari FA, Rawat I, Kanney K, Bux F (2014) Design and development of polyamine polymer for harvesting microalgae for biofuels production q. Energy Convers Manage 85:537–544. https://doi.org/10.1016/j.enconman.2014.05.059
Kumar S, Kumar NM, Guldhe A, Ahmad F, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117(July 2017):62–68. https://doi.org/10.1016/j.ecoleng.2018.04.005
Musa M, Wolf J, Stephens E, Hankamer B, Brown R, Rainey TJ (2020) Separation and purification technology cationic polyacrylamide induced flocculation and turbulent dewatering of microalgae on a Britt Dynamic Drainage Jar. Sep Purif Technol 233:116004. https://doi.org/10.1016/j.seppur.2019.116004
Nayak M, Rashid N, Suh WI, Lee B, Chang YK (2019) Performance evaluation of different cationic flocculants through pH modulation for efficient harvesting of Chlorella sp HS2 and their impact on water reusability. Renew Energy 136:819–827
Gupta SK, Kumar NM, Guldhe A, Ansari FA, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68
Udom I, Zaribaf BH, Halfhide T, Gillie B, Dalrymple O, Zhang Q, Ergas SJ (2013) Bioresource technology harvesting microalgae grown on wastewater. Biores Technol 139:101–106. https://doi.org/10.1016/j.biortech.2013.04.002
Bezerra MA, Santellil RE, Oliveria EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Kumar K, Mishra SK, Shrivastav A, Park MS, Yang J (2015) Recent trends in the mass cultivation of algae in raceway ponds. Renew Sustain Energy Rev 51:875–885. https://doi.org/10.1016/j.rser.2015.06.033
Rocha DN, Martins MA, Soares J, Vaz MGMV, de Leite MO, Covell L, Mendes LBB (2019) Combination of trace elements and salt stress in different cultivation modes improves the lipid productivity of Scenedesmus spp. Bioresour Technol 289(April):121644. https://doi.org/10.1016/j.biortech.2019.121644
Bleeke F, Milas M, Winckelmann D (2015) Optimization of freshwater microalgal biomass harvest using polymeric flocculants. Int Aquat Res 7:235–244. https://doi.org/10.1007/s40071-015-0108-8
Gerchman Y, Vasker B, Tavasi M, Mishael Y, Kinel-Tahan Y, Yehoshua Y (2017) Effective harvesting of microalgae: comparison of different polymeric flocculants. Biores Technol 228:141–146. https://doi.org/10.1016/j.biortech.2016.12.040
Chen L, Wang C, Wang W, Wei J (2013) Optimal conditions of different flocculation methods for harvesting Scenedesmus sp. cultivated in an open-pond system. Biores Technol 133:9–15. https://doi.org/10.1016/j.biortech.2013.01.071
Letelier-Gordo CO, Holdt SL, Francisci DD, Karakashev DB (2014) Effective harvesting of the microalgae Chlorella protothecoides via bioflocculation with cationic starch. Biores Technol 167:214–218. https://doi.org/10.1016/j.biortech.2014.06.014
Griffiths MJ, Garcin C, Hille RPV, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85:119–123. https://doi.org/10.1016/j.mimet.2011.02.005
Lam GP, Vermuë MH, Olivieri G, Van Den Broek LAM, Barbosa MJ, Eppink MHM, Kleinegris DMM (2014) Cationic polymers for successful flocculation of marine microalgae. Biores Technol 169:804–807. https://doi.org/10.1016/j.biortech.2014.07.070
APHA - American Public Health Association (2017) American Water Works Association, Water Environment Foundation. Standard methods for the examination of water and wastewater, 23 ed. APHA, Washington
Ahmad AL, Mat Yasin NH, Derek CJC, Lim JK (2011) Optimization of microalgae coagulation process using chitosan. Chem Eng J 173(3):879–882. https://doi.org/10.1016/j.cej.2011.07.070
Hauwa A, Mohamed RMS, Al-Gheethi A, Wurochekke AA, Hashim MKA (2017) Harvesting of Botryococcus sp. biomass from greywater by natural coagulants. Waste Biomass Valor 9(10):1841. https://doi.org/10.1007/s12649-017-9958-1
Nguyen LN, Labeeuw L, Commault AS, Emmerton B, Ralph PJ, Hasan A, Guo W, Ngo H, Nghiem LD (2019) Environmental technology & innovation validation of a cationic polyacrylamide flocculant for the harvesting fresh and seawater microalgal biomass. Environ Technol Innov 16:100466. https://doi.org/10.1016/j.eti.2019.100466
Xu Y, Purton S, Baganz F (2013) Chitosan flocculation to aid the harvesting of the microalgae Chlorella sorokiniana. Biores Technol 129:296–301
Hamid SHA, Lananan F, Din WNS, Lam SS, Khatoon H, Endut A, Jusoh A (2014) Harvesting microalgae, Chlorella sp. by bio-flocculation of Moringa oleifera seed derivatives from aquaculture wastewater phytoremediation. Int Biodeterior Biodegradation 95(PA):270–275. https://doi.org/10.1016/j.ibiod.2014.06.021
Zhang W, Song R, Cao B, Yang X, Wang D, Fu X, Song Y (2018) Variations of floc morphology and extracellular organic matters (EOM) in relation to floc filterability under algae flocculation harvesting using polymeric titanium coagulants (PTCs). Bioresour Technol 256(December 2017):350–357. https://doi.org/10.1016/j.biortech.2018.02.011
Dong C, Chen W, Liu C (2014) Flocculation of algal cells by amphoteric chitosan-based flocculant. Biores Technol 170:239–247. https://doi.org/10.1016/j.biortech.2014.07.108
Bratby J (2016) Coagulation and floculation in water and wastewater treatment, 3a edn. IWA Publishing, Londres
Loganathan K, Saththasivam J, Sarp S (2018) Removal of microalgae from seawater using chitosan-alum/ferric chloride dual coagulations. Desalination 433(November 2017):25–32. https://doi.org/10.1016/j.desal.2018.01.012
Kurniawan SB, Imron MF, Chik CENCE, Owodunni AA, Ahmad A, Alnawajha MM, Hasan HA (2022) What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci Total Environ 806:150902
Delrue F, Imbert Y, Fleury G, Peltier G, Sassi JF (2015) Using coagulation flocculation to harvest Chlamydomonas reinhardtii: coagulant and flocculant efficiencies, and reuse of the liquid phase as growth medium. Algal Res 9:283–290. https://doi.org/10.1016/j.algal.2015.04.004
Barros Neto B, Scarminio IS, Bruns RE (2007) Como fazer experimentos. 3° ed. Editora Unicamp, Campinas
Prado AGS, Torres JD, Faria EA, Dias SCL (2004) Comparative adsorption studies of indigo carmine dye on chitin and chitosan. J Colloid Interface Sci 277(1):43–47. https://doi.org/10.1016/j.jcis.2004.04.056
Wu Z, Zhu Y, Huang W, Zhang C, Li T, Zhang Y, Li A (2012) Evaluation of flocculation induced by pH increase for harvesting microalgae and reuse of flocculated medium. Biores Technol 110:496–502. https://doi.org/10.1016/j.biortech.2012.01.101
Dias A, Borges AC, Rosa AP, Martins MA (2021) Green coagulants recovering Scenedesmus obliquus: an optimization study. Chemosphere 262:127881
Funding
This work was partially funded by Coordination for the Improvement of Higher Education Personnel (CAPES Finance Code 001).
Author information
Authors and Affiliations
Contributions
AD: conceptualization, methodology, investigation, writing—original draft; ACB: writing—review and editing, conceptualization, supervision, project administration, funding acquisition, resources; DNR: methodology, writing—review and editing; MAM: supervision, writing—review and editing; APR: supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, A., Borges, A.C., Rocha, D.N. et al. Scenedesmus obliquus recovery using polyacrylamide and chitosan: an optimization study. Biomass Conv. Bioref. 14, 16535–16544 (2024). https://doi.org/10.1007/s13399-023-03972-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03972-w