Abstract
In this paper, an electrochemical approach was described for preparing graphene with different microstructures, starting from aqueous solutions of graphene oxide (GO), processed under direct voltage. The process relied on the electrochemical reduction of GO sheets deposited on the surface of Cu electrode. The same electrode is first used as the anode for the electrophoretic deposition of GO film, and then as the cathode, allowing for the effective reduction of GO. Paper-like graphene was observed on the electrode after the reduction, while crumpled graphene existed in the solution, which could be further transformed to graphene scroll by sonication. The samples were characterized by scanning electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis. The results showed that the oxygen functional groups are significantly removed after the reduction. The mechanism for this method was also proposed in this paper. Moreover, a scale-up device was designed to make this method more applicable for commercialization. This method has the potential for large-scale production of graphene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene, a two-dimensional (2D) honeycomb lattice of sp 2-bonded carbon atoms, holds great promise for potential applications in many fields for its unique electronic, optical, and thermal properties [1–3]. The industrial application of graphene will require large-scale and cost-effective production methods, while providing a balance between ease of application and final material quality. Currently, several approaches have been proposed for the synthesis of graphene production, among which the reduction of graphene oxide (GO) obtained from ultrasonic exfoliation of oxidized graphite is the most convenient way to yield large quantities of graphene sheets [4, 5]. The reduction of GO is normally achieved by a thermal or chemical process. However, they typically require inert atmospheres, high temperatures, or the use of hazardous solvents [6, 7]. Therefore, developing a safe and environmentally friendly way of producing graphene is becoming more and more necessary.
The electrochemical reduction of GO is an effective, controllable, and environmentally friendly way to produce graphene whereby it discards the use of toxic chemicals and high temperature in the reduction process. Recently, several research works have been reported [8–15]. Typically, the electrochemical reduction of GO can be carried out via two different approaches: one-step electrochemical reduction approach and two-step electrochemical reduction approach [16].
In the one-step electrochemical approach, the GO sheets are directly electrochemically reduced from an aqueous colloidal suspension in the presence of buffer electrolyte to produce electrochemically reduced graphene oxide (ERGO) film on an electrode surface [16–21]. Typically, buffer electrolyte is used with the GO colloidal suspension to form a medium and the selection of the appropriate pH for the medium is also essential to ensure good deposition of the ERGO onto the electrode substrate. In the two-step electrochemical approach, GO is first deposited onto the surface of an electrode by dip-coating [10], drop-casting [22, 23], layer-by-layer [11], or spray-coating method [24], which acts as a substrate and is subsequently dried out to form a GO-coated electrode. The GO-coated electrode is then subjected to electrochemical reduction using a standard three-electrode electrochemical system in the presence of a buffer or supporting electrolyte to produce ERGO films on the electrode [25–29]. However, the quantity of ERGO film obtained on an electrode with a limited area was confined to a few tens of micrograms, leading to low-yield production.
The electrophoretic deposition (EPD) method is a well-developed and economical method that has been successfully applied for the deposition of GO due to its high deposition rate, good thickness controllability, and simplicity of scale up [30]. Herein, different from the existing methods mentioned above, we show a versatile approach for controlling the adsorption of GO at surfaces and electrochemically reducing the GO in situ, which is processed entirely in GO solution without preparing GO-coated electrode and buffer electrolyte. In particular, by choosing the right deposition and electro-reduction conditions, we demonstrate that GO can be well reduced to paper-like graphene and crumpled graphene. Crumpled graphene can be further transformed to graphene scroll by sonication. The products are characterized by morphology characterization and spectroscopic characterization. The results show that the oxygen functional groups are significantly removed after the reduction. Furthermore, a scale-up device is designed to make this method more applicable for commercialization. This approach has the potential for large-scale production of graphene.
2 Experimental section
2.1 Preparation of GO suspension
Graphite oxide was purchased from the Sixth Element Ltd. Graphite oxide (15 g) was dispersed in water and sonicated for 120 min at room temperature. Then a well-dispersed and stable suspension in water containing 1 mg ml−1 of graphene oxide (GO) platelets was obtained.
2.2 Synthesis of ERGO
A small reaction unit (250 mm × 250 mm × 10 mm) was used to study and choose the reaction condition. Two copper electrodes (250 mm × 250 mm) acted as working electrodes with 10 mm spacing (SFig. 1 in ESM, according to the results of the research, the time of complete deposition of GO was increased and the reduction degree of ERGO was decreased as the space was increased. 10 mm is the optimum space). The main process involved controlling the adsorption of GO at surfaces and electrochemically reducing the GO in situ. In order to choose the optimum condition of deposition process, the influence of the concentration of GO solution (0.1, 0.5 and 1 mg ml−1) and the voltage potential (10, 30 V) on the EPD process of GO was carried out. The current–time curve was recorded to examine the time requirement for the EPD. To investigate the influence of the voltage potential and the reduction time, the opposite wires connected to the power supply was exchanged, the GO-deposited electrode was changed from positive to negative. Electrochemical reduction of GO was carried out on the cathode at 60 V for different time points.
2.3 Scale-up synthesis of ERGO
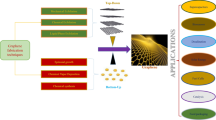
As shown in Fig. 1, the scale-up electrochemical device consists of a DC power supply, a junction box, copper electrodes, and reaction tank. The reaction tank (15 l) is embedded with 24 copper electrodes (250 mm × 250 mm) that act as working electrodes. The spacing of two electrodes is about 10 mm. Electric power to the electrodes is supplied by connecting the adjacent electrodes to an opposite pole of the DC power supply. The adjacent electrodes act alternatively as anodes and cathodes. In order to electrochemically reduce the GO in situ, a switch selector is installed in the junction box to exchange the opposite wires connected to the power supply.
The scale-up synthesis of ERGO was carried out in the electrochemical device. Under an applied potential of 30 V for 30 min, the GO in solution was deposited onto the anode completely to fabricate GO films. Then the opposite wires connected to the power supply was exchanged, namely, the GO-deposited electrode was changed from positive to negative. Electrochemical reduction was carried out on the cathode at 60 V for 180 min. After the reduction, ERGO1 film was observed on the electrode, while ERGO2 was found to exist in the solution. After the sonication of ERGO2 for 1 h, ERGO3 was obtained. The ERGO1 was air-dried at room temperature for 24 h. The ERGO2 and ERGO3 were thoroughly washed with deionized water and dried at 50 °C.
2.4 Characterization
The morphology of the samples was observed with a field emission scanning electron microscopy (Quanta 200FEG, FEI). The X-ray diffractions (XRD) of the samples were measured using an X-ray diffractometer (D/max2550VB3+/PC, Rigaku, Japan) with reference target: Cu Ka radiation. Fourier transform infrared (FT-IR) spectra were obtained through a BRUKER EQUINOXSS spectrometer that use the attenuated total reflectance. X-ray photoelectron spectroscopy (XPS) analysis was performed on a RBD upgraded PHI-5000C ESCA system (Perkin Elmer) with Mg Kα radiation (hν = 1253.6 eV). Thermogravimetric analysis (TGA) was done in a STA 449 C Jupiter simultaneous thermal analyzer. The weight loss of the samples was monitored from room temperature to 600 °C at a heating rate of 10 °C min−1 in nitrogen atmosphere.
3 Results and discussion
Typically, the current–time curve is recorded to examine the time requirement for the electrochemical deposition and reduction of GO in the constant potential reduction technique. To investigate the influence of the concentration of GO solution and the voltage potential on the EPD process of GO, the current–time curves for the EPD process with different concentrations of GO in the small reaction unit are recorded. Figure 2a, b shows the current–time curves for the EPD process with different concentrations of GO at 10 and 30 V. A common feature in the curves is a decrease of currents at first, followed by a stable current. GO sheets are negatively charged due to the deprotonation of the –COOH groups in water. GO sheets are electrophoretically drawn to the anode, which causes a large current in the initial stage. When the majority of charged GO in solution is deposited onto the copper, the current tends to be stable. It is found that the curves shift up with the increase in the concentration of GO, which indicates that the current increases with the increase in the concentration of GO. When the GO is deposited completely, the GO solution will be transparent and an adsorbed layer (micrometer) can be observed. The time when the phenomenon happens is defined as complete deposition time. It takes longer to completely deposit GO at 1 mg ml−1 (3000 s) than at 0.1 mg ml−1 (900 s) under the potential 10 V. However, the complete deposition time decreased with the increased potential, as shown in Fig. 2c. It takes shorter time to completely deposit 1 mg ml−1 GO at 30 V (1260 s) than 10 V (3000 s), which is similar to the deposition time of 0.1 mg ml−1 GO at 10 V (900 s). Therefore, the EPD process of 1 mg ml−1 GO under 30 V for 30 min is more effective, which is chosen as the optimum condition for the EPD process.
a The current–time curves of the electrophoretic deposition (EPD) process of different concentrations of GO at 10 V, b the current–time curves of the EPD process of different concentrations of GO at 30 V c the current–time curves of the EPD process of 1 mg ml−1 GO at 30 V and d FT-IR spectra of GO at 30 V for different deposition time
Then deposition products were compared by FT-IR characterization. Figure 2d shows the FT-IR transmittance spectra of GO, GO1 (deposited on the anode at 30 V for 30 min), and GO2 (deposited on the anode at 30 V for 90 min). The spectrum of GO illustrates C–O stretching vibrations at 1045 cm−1, C–O–C stretching vibrations at 1226 cm−1, C = O in carboxylic acid, and carbonyl moieties at 1722 cm−1; C = C at 1620 cm−1 assigns to skeletal vibrations of unoxidized graphitic domains. The band at 3397 and 1396 cm−1 can be ascribed to the O–H stretching mode and deformation vibration of intercalated water, respectively [31]. The intensity of carboxylates and carbonyl groups decrease dramatically with the deposition time, demonstrating that graphene oxide is reduced to some extent. This is consistent with the results of An et al. [19]. However, the reduction level of anodic reduction is limited, which is different from cathodic reduction. To compare the reduction efficiency of the anodic reduction with cathodic reduction, after the EPD of GO (deposited on the anode at 30 V for 30 min), the voltage was reversed, namely, the GO-deposited electrode was changed from positive to negative. GO3 was reduced on the cathode at 30 V for 60 min. As shown in Fig. 2d, the intensity of oxide functional groups in GO3 decreases dramatically. GO3 is better reduced than GO2 which is reduced for the same time. Therefore, the cathodic reduction is a more effective reduction of GO after the EPD process.
To reduce GO more effectively, the influence of the voltage on the reduction was investigated. Figure 3a shows the FT-IR transmittance spectra of GO and ERGO on the electrode obtained at different potentials (30, 45, 60 V) for 60 min, the intensities of the peaks corresponding to the oxygen functionalities all decreased dramatically with the voltage increased to 60 V. These data reveal that the GO is reduced more effectively with the voltage increased. Figure 3b shows XRD spectra of GO and ERGO obtained at different potentials (30, 45, 60 V) for 60 min. GO exhibits a very sharp diffraction peak at 2θ = 10.8° with d-spacing of 0.818 nm. A very sharp diffraction peak at 2θ = 10.9° and a weak peak at 23.9° are found when the reductive voltage was 30 V. The intensities of peak become weak at 2θ = 10.9° with the reductive voltage increased to 60 V. Meanwhile, a broad peak of ERGO at 2θ = 23.9° is observed. Those give evidence that the reduction degree of GO increased with the voltage increased. Therefore, 60 V is used in the electrochemical reduction process.
The recorded current–time curve for the reduction at 60 V exhibits three stages (Fig. 4a): a fast initial increase to a maximum over the first 200 s followed by a decrease to 2000 s, then a gradual decline to a stable level. This was because the electrochemical reduction started on GO sheets adjacent to the electrode and then the reduced graphene that was conductive transferred the charge to reduce the GO sheets further. As the GO was consumed, the current decreased, approaching zero when the reduction was complete. The curve exhibits a common feature in the electrochemical reduction of GO [28, 32]. Figure 4b shows the FT-IR spectra of GO and ERGO on the electrode at 60 V for different reduction times (60, 120, 180 min). The intensities of the peaks corresponding to the oxygen functionalities all decrease dramatically or even disappear after the reduction. These results reveal that the GO has been successfully reduced with the prolonged reduction time. When the reductive time reaches 180 min, the reduction degree is the highest. XRD spectra of GO and ERGO on the electrode at 60 V for different time points are shown in Fig. 4c. GO exhibited a very sharp diffraction peak at 2θ = 10.9°. After 60 min of reduction, the intensities of peak become weak at 2θ = 10.9°. Meanwhile, a new and weak peak of ERGO at 2θ = 23.9° is observed. The diffraction peaks at 2θ = 10.9° disappeared when the reductive time reached 240 min. Therefore, the optimum condition of reduction process is under the applied potential of 60 V for 180 min.
The electrochemical reduction of GO suspension on large scale was carried out in the scale-up electrochemical setup. The current–time curves are shown in Fig. 5. The feature in the curve is consistent with the curve of the small setup.
SEM was used to characterize the morphology of ERGO. The SEM image of the cross section of the ERGO1 (Fig. 6a, b) shows the layered microstructure. The packing morphology of the ERGO1 is similar to GO paper-like materials formed by filtration, and the thickness of ERGO1 is uniform. The morphology of ERGO2 resembles crumpled paper (Fig. 6c, d). Like crumpled paper balls, crumpled graphene loosely pack into a three-dimensional (3D) structure. The ERGO3 shows scrolled structure which is curled up and stakes orderly after sonication (Fig. 6e, f). It was reported that short (1 h) and high-energy (500 W) sonication was necessary for efficient graphene scroll formation [33]. Further research will be done on the scrolled graphene.
Figure 7 shows the XRD patterns of GO, ERGO1, and ERGO2. GO exhibits a very sharp diffraction peak at 2θ = 10.8° with d-spacing of 0.818 nm. The interlayer distance of ERGO1 and ERGO2 drastically decreases to 0.361 nm (2θ = 24.6°) and 0.386 nm (2θ = 23°), respectively which indicates that the oxygen-containing group of GO had been efficiently removed. While the XRD patterns of ERGO both display amorphous structures. However, the interlayer space of ERGO1 is more similar to pristine graphite, demonstrating that ERGO1 is deoxygenated effectively.
As shown in Fig. 8, the C1s spectrum of GO consists of four different peaks: C = C in aromatic rings (284.3 eV); C–O (286.3 eV); C = O (287.6 eV); COOH (289.0 eV) [34]. In the C1s XPS spectrum of ERGO, the intensities of all C1s peaks of the carbons binding to oxygen decreased obviously, revealing that considerable oxygen-containing functional groups are removed. However, the C1s XPS spectrum of ERGO1 exhibits their peak intensities smaller than those in ERGO2. The changes of C/O ratio of ERGO indicate the different reduction degree. After reduction, the C/O ratio is improved to approximately 5.8:1 and 4.6:1, respectively. Figure 8d shows the FT-IR transmittance spectra of GO, ERGO1, and ERGO2. The intensity of the peaks related to oxygen functional groups of ERGO all decrease dramatically, demonstrating that graphene oxide is well reduced. There were small peaks at 1045 cm−1 (C–O) and 1722 cm−1(C = O) remaining in ERGO2. This shows, along with the chemical analysis by XPS, that the oxygen functional groups were significantly reduced, although the reduction degree of ERGO1 was a little higher.
Figure 9 shows the TGA graphs of GO, ERGO1, and ERGO2. As for GO, there is a weight loss of ~20 wt% around 150 °C, likely due to evaporation of water molecules. As the temperature increases from 150 to 300 °C, GO exhibits a significant weight loss of 29.8 wt%, indicating the removal of oxygen functional groups which are extruded as carbon dioxide [35]. As for ERGO1 and ERGO2, a small amount (~4, 8 wt%, respectively) of weight loss up to 150 °C is due to the fact that much of the water of ERGO is removed. As the temperature increases, the ERGO2 loses weight more rapidly than that of ERGO1, suggesting that more amount of residual oxygen groups exists in ERGO2. As the temperature increases from 600 °C, the weight loss of ERGO1 and ERGO2 is much less than that of GO, indicating that GO is reduced effectively.
The mechanism of the electrochemical reduction and the formation of different nanostructures can be explained as shown in Fig. 10: GO sheets in solution are completely deposited on the electrode by EPD to fabricate GO film. After the voltage in the electrodes is reversed, the GO film adjacent to the electrode is firstly reduced in suit during the electrochemical reduction (ECR). The reduced graphene adjacent to the electrode that is conductive will transfer the charge to the GO sheets outside which are reduced further. However, these partially reduced GO sheets with negatively charged oxygen functional groups fall off from the cathode by the electrostatic force and are crumpled loosely by the different force direction. Crumpled graphene can be further transformed to graphene scroll by sonication. This also can explain the fact that the reduction degree of ERGO1 is higher than that of ERGO2. ERGO2 is not in touch with the electrode during the reduction due to the falling from the cathode, which influences the reduction degree.
Schematic illustration of the reduction mechanism and the formation of different nanostructures: a GO film is deposited on the electrode by EPD, b in suit ECR of GO is carried out. The GO film adjacent to the electrode is firstly reduced, c the partially reduced GO sheets with residual oxygen functional groups fall off from the electrode by the electrostatic force and are crumpled loosely by the different force direction and d the graphene scroll which is curled up and staked orderly is formed after sonication
The possible reaction on the cathode is as follows:
This reaction mechanism indicates the essential role of hydrogen ions in the reduction process [24]. As shown in Fig. 2c, the initial current increase is observed. This is because GO sheets are negatively charged due to the deprotonation of the –COOH groups in water. And an initial current increase is observed in Fig. 4a, which can be accounted for the charge transfer reactions. Therefore, along with the characterization, the GO sheets on the cathode are well reduced.
4 Conclusions
In summary, we described a highly versatile method that relies on a voltage applied to trigger the electrochemical reduction of GO sheets deposited on the surface of Cu electrode. A scale-up device is designed to make this method more applicable for the large-scale production of graphene. By choosing the right deposition and electro-reduction conditions, we demonstrate that GO can be well reduced to paper-like graphene and crumpled graphene. Crumpled graphene can be further scrolled into graphene scroll by sonication. The characterization results show that the oxygen functional groups are significantly decreased after the reduction, although the reduction degree of ERGO1 is a little higher. The mechanism is also proposed to explain the formation of diverse graphene. Further research about the properties and application of the production will be carried out. Our approach is simple and effective, making it possible to produce graphene with different microstructures. We anticipate that it can be applied in commercialization.
References
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Katsnelson MI (2007) Graphene: carbon in two dimensions. Mater Today 10:20–27
Rogers JA (2008) Electronic materials: making graphene for macroelectronics. Nat Nanotechnol 3:254–255
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Mao S, Pu H, Chen J (2012) Graphene oxide and its reduction: modeling and experimental progress. RSC Adv 2:2643–2662
Luo D, Zhang G, Liu J (2011) Evaluation criteria for reduced graphene oxide. J Phys Chem C 115:11327–11335
Pei SF, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Liu N, Luo F, Wu HX, Liu YH, Zhang C, Chen J (2008) One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater 18:1518–1525
Liu S, Wang J, Zeng J (2010) “Green” electrochemical synthesis of Pt/graphene sheet nanocomposite film and its electrocatalytic property. J Power Sour 195:4628–4633
Wang Z, Zhou X, Zhang J (2009) Direct electrochemical reduction of single-layer graphene oxide and subsequent functionalization with glucose oxidase. J Phys Chem C 113:14071–14075
Ramesha GK, Sampath S (2009) Electrochemical reduction of oriented graphene oxide films: an in situ Raman spectroelectrochemical study. J Phys Chem C 113:7985–7989
Shao Y, Wang J, Engelhard M, Wang C, Lin Y (2010) Facile and controllable electrochemical reduction of graphene oxide and its applications. J Mater Chem 20:743–748
Raj MA, John SA (2013) Fabrication of electrochemically reduced graphene oxide films on glassy carbon electrode by self-assembly method and their electrocatalytic application. J Phys Chem C 117:4326–4335
Peng XY, Liu XX, Diamond D (2011) Synthesis of electrochemically-reduced graphene oxide film with controllable size and thickness and its use in supercapacitor. Carbon 49:3488–3496
Hasan SA, Rigueur JL, Harl RR, Krejci AJ, Gonzalo-Juan I, Rogers BR, Dickerson JH (2010) Transferable graphene oxide films with tunable microstructures. ACS Nano 4:7367–7372
Toh SY, Loh KS, Kamarudin SK, Daud WRW (2014) Graphene production via electrochemical reduction of graphene oxide: synthesis and characterization. Chem Eur J 251:422–434
Chen L, Tang Y, Wang K, Luo S (2011) Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem Commun 13:133–137
Liu C, Wang K, Luo S, Tang Y, Chen L (2011) Direct electrodeposition of graphene enabling the one-step synthesis of graphene–metal nanocomposite films. Small 7:1203
An SJ, ZhuY Lee SH (2010) Thin film fabrication and simultaneous anodic reduction of deposited graphene oxide platelets by electrophoretic deposition. J Phys Chem Lett 1:1259–1263
Tong H, Zhu J, Chen J, Han Y, Yang S, Ding B, Zhang X (2013) Electrochemical reduction of graphene oxide and its electrochemical capacitive performance. J Solid State Electrochem 17:2857–2863
Guo HL, Wang XF, Qian QY, Wang FB, Xia XH (2009) A green approach to the synthesis of graphene nanosheets. ACS Nano 9:2653–2659
Li W, Liu J, Yan C (2013) Reduced graphene oxide with tunable C/O ratio and its activity towards vanadium redox pairs for an all vanadium redox flow battery. Carbon 55:313–320
Zhang X, Zhang D, Chen Y, Sun X, Ma Y (2012) Electrochemical reduction of graphene oxide films: preparation, characterization and their electrochemical properties. Chin Sci Bull 57:3045–3050
Zhou Y, Chen J, Wang F, Sheng Z, Xia X (2010) A facile approach to the synthesis of highly electroactive Pt nanoparticles on graphene as an anode catalyst for direct methanol fuel cells. Chem Commun 46:5951–5953
Liu S, Ou J, Wang J, Liu X, Yang S (2011) A simple two-step electrochemical synthesis of graphene sheets film on the ITO electrode as supercapacitors. J Appl Electrochem 41:881–884
Eda G, Fanchini G, Chhowalla M (2008) Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat Nanotechnol 3:270–274
Dogan HO, Ekinci D, Demir U (2013) Atomic scale imaging and spectroscopic characterization of electrochemically reduced graphene oxide. Surf Sci 611:54–59
Zhou M, Wang YL, Zhai YM, Zhai JF, Ren W, Wang F, Dong S (2009) Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem Eur J 15:6116–6120
Kauppila J, Kunnas P, Damlin P (2013) Electrochemical reduction of graphene oxide films in aqueous and organic solutions. Electrochim Acta 89:84–89
Hong S, Jung S, Kang S, Kim Y, Chen X, Stankovich S, Ruoff SR, Baik S (2008) Dielectrophoretic deposition of graphite oxide soot particles. J Nanosci Nanotechnol 8:424–427
Acik M, Lee G, Mattevi C, Pirkle A, Wallace RM, Chhowalla M, Chabal Y (2011) The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J Phys Chem C 115:19761–19781
Harima Y, Setodoi S, Imae I, Komaguchi K, Ooyama Y, Ohshita J (2011) Electrochemical reduction of graphene oxide in organic solvents. Electrochim Acta 56:5363–5368
Viculis LM, Mack JJ, Kaner RB (2003) A chemical route to carbon nanoscrolls. Science 299:1361
Mattevi C, Eda G, Agnoli S, Miller S, Mkhoyan KA, Celik O, Chhowalla M (2009) Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv Funct Mater 19:2577–2583
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia YY, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Acknowledgments
This research was supported by National High Technology Research and Development Program of China (No. 2012AA030303) and Basic Research Key Program of Shanghai (No. 12JC1408600).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shang, Y., Zhang, D., Liu, Y. et al. Simultaneous synthesis of diverse graphene via electrochemical reduction of graphene oxide. J Appl Electrochem 45, 453–462 (2015). https://doi.org/10.1007/s10800-015-0818-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0818-z