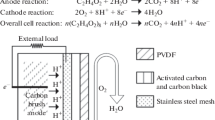
Abstract
We present a complete testing environment for the parallel performance characterization of biofuel cells. Besides rapid-assembly electrode fixtures and an aseptic electrochemical reactor, it comprises a 24-channel electrical testing system that bridges the gap between simple load resistors and costly multi-channel potentiostats. The computer-controlled testing system features active current control to enable the forced operation of half-cell electrodes, whereas galvanic isolation between individual channels ensures interference-free operation of multiple fuel cells immersed in a common testing solution. Implemented into the control software is an automated procedure for the step-wise recording of polarization curves. This way, performance overestimation due to a too fast increase in load current can be circumvented. As an applicational example, three abiotically catalyzed glucose fuel cells are characterized simultaneously in a common testing solution. Complete disclosure of the electrical system (incl. printed circuit board layout, control software, and circuit diagrams) in the online supplementary material accompanying this paper allows researchers to replicate our setup in their lab and can serve as inspiration for the design of similar systems adapted to specific requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Low-power biofuel cells, that directly generate electricity from the electro-oxidation of organic fuels, are currently being considered as micro-power generators to replace conventional batteries in distributed devices [1–9]. Besides the investigation of electrode processes and reaction mechanisms, the accurate characterization of biofuel cell power output in terms of polarization curves (current density-potential plots) thus comes increasingly into focus.
1.1 Requirements for time-efficient biofuel cell characterization
For time-efficient biofuel cell development it is essential to characterize the performance of multiple fuel cells and half-cell electrodes in a parallel fashion. One reason is that due to the slow load change response of biofuel cells (up to several hours) time-consuming experiments are required to assess their sustainable performance. Although this behavior is well documented in published experimental results [2, 3, 10–12], most researchers do not consider that under such conditions a too fast sweep rate during the recording of polarization curves leads to an overestimation of performance. Furthermore, the characterization of long-term stability, often over periods of several weeks or months, becomes mandatory when the application of biofuel cells as reliable power supply is intended [1, 8]. In some cases it is also advantageous to characterize multiple biofuel cells exposed to the same testing solution, either due to the relatively elaborate control of solution parameters or to ensure a high degree of comparability in the experiment. Prerequisite for this technique is the complete galvanic isolation between the individual fuel cells to prevent cross-channel interference.
1.2 Limitations of established biofuel cell characterization approaches
A commonly employed technique to record biofuel cell polarization curves is the use of variable load resistors, often combined with the measurement of individual electrode potentials against a reference electrode [1, 2, 13, 14]. While easily parallelizable at low cost, this technique comes with two main limitations. Firstly, the passive approach mandates that always a complete fuel cell with anode and cathode is operated, and the forced operation of half-cell electrodes against an arbitrary counter electrode is therefore not possible. Secondly, the load current cannot be actively controlled at a constant value, for instance to maintain a defined reactant consumption rate at the electrodes and thus investigate mass-transfer related effects. These limitations can be overcome by using potentiostat/galvanostat systems for the precise control of either cell potential (potentiostatic mode) or load current (galvanostatic mode) to record polarization curves, both techniques leading to comparable results [15]. Unfortunately, even relatively expensive multi-channel potentiostats/galvanostats do not always provide complete galvanic isolation between individual channels, impeding the characterization of multiple fuel cells immersed in a common reactant solution. Furthermore, potentiostats provide more functionality than required for the automated recording of polarization curves, namely fast voltage sweep and data acquisition rates to perform for example cyclic voltammetry experiments. Consequently, they are only available at comparably high cost. Most research budgets thus do not allow for the procurement of a large number of these devices to establish a parallel characterization environment. This is also reflected in current literature, where mostly only single experiments or a small number of repetitions are reported (see [1, 7] and references therein).
1.3 A dedicated testing environment for biofuel cell characterization
The aim of the present work is to provide researchers with a dedicated testing environment for parallel performance characterization of low-power biofuel cells. Its main component is an electrical testing system that bridges the gap between the limited controllability of simple load resistors and the application of oversized (and thus costly) potentiostat/galvanostat systems. Thereto its functionality has been focused on the following features relevant for highly parallel biofuel cell performance characterization:
-
Individual operation of multiple fuel cells under galvanostatic load, also when immersed in a common testing solution.
-
Separate recording of multiple anode and cathode potentials against a common reference electrode.
-
Operation of individual half-cell electrodes against arbitrary counter electrodes.
-
Optional use of two separate reference electrodes for anode and cathode of the same fuel cell.
-
Fully automated recording of polarization curves by a stepwise change in load current.
-
Operation time or voltage drift as criterion for automatic load change.
In this paper, we report upon the design and validation of a complete testing environment that besides the electrical testing system also includes rapid-assembly electrode fixtures and an aseptic electrochemical reactor. Furthermore, we demonstrate its application for the automated parallel performance characterization of abiotically catalyzed glucose fuel cells [16], immersed in a common reactant solution.
2 Electrical testing system
In Fig. 1a, the overall concept of the electrical testing system is shown exemplary for a single channel. It comprises an electronic load to apply a defined load current to the fuel cell, and a data acquisition unit to individually record the fuel cell’s electrode potentials against reference electrodes. Both systems are connected to the fuel cell via an interface box. A standard personal computer is used for control, graphical data representation, and data storage. The individual components of the system as well as their interconnection are described in the following; a more detailed description is available in the on-line supplementary material section.
2.1 Electronic load
As an electronic load, we selected a stimulus generator (STG 2008, Multichannel Systems, Reutlingen, Germany), originally developed for electrical stimulation in electrophysiology research [17, 18]. A single device comprises eight galvanically isolated channels, which can be individually controlled as current sink or source (galvanostat). The alternative operation mode, where a constant voltage can be supplied at the output terminals, has not been implemented in the presented measurement setup since the STG 2008 does not feature auxiliary sensing connections at the cell terminal. Precise control of the actual voltage present at the electrodes can thus not be achieved.
While the standard STG 2008 model delivers a maximum current of ±800 μA per channel, the manufacturer also offers custom versions with a maximum current of up to ±3.2 mA. For our application, we had the maximum current limited to ±200 μA by the manufacturer, the dynamic range spanning 1–200 μA. Also, the compliance voltage of the current channels was limited to 11 V, so that under no circumstances (e.g., broken connection leads) can a hazardous DC voltage build up at the terminals.
In the presented measurement setup, we integrated three STG 2008 units, corresponding to a total of 24 independent channels for fuel cell characterization. The individual stimulus generators are connected to a single personal computer via the USB 1.1 port.
2.2 Data acquisition
For data acquisition, we chose an integrated multimeter/data acquisition system (Keithley 2700, Keithley, Germering, Germany). Equipped with two, 40-channel (two-wire configuration) differential multiplexer modules (Keithley 7702, Keithley, Germering, Germany), its measurement capacity is sufficient to record the data of the 24 fuel cell channels realized in our system. A desirable feature of multiplexed data acquisition is the sequential measurement loop, during which only one voltage channel at a time is connected to the multimeter. The galvanic isolation of the individual channels is thus always maintained. Computer control of the data acquisition unit is established via the GPIB bus by using an interface card (KPCI-488, Keithley, Germering, Germany) for the computer’s PCI slot.
2.3 Interface box
Through a custom-made interface box, the two wires connecting the fuel cell electrodes to the load circuit are routed to the stimulus generator, whereas the separate wire pairs to measure the potential of anode and cathode against individual reference electrodes are routed to the data acquisition system (Fig. 1a). A 1-kΩ shunt resistor is integrated into each load circuit to monitor the actual load current. During open-circuit measurements, a normally open switching relay in each load circuit completely disconnects the fuel cell from the electronic load. The relays are actuated with a microprocessor-controlled relay multiplexer (μC SA07011G3, IMTEK—Electronic Service Center, Freiburg, Germany), addressed by the personal computer via the RS 232 link. Both the circuit layout and the construction of the interface box are described in the supplemental online material.
Each electronic load is connected to the interface box via a 25-pole d-sub connector fitted with a matching octopus cable. Similarly, a total of four 50-pole centronics-style connectors interlink the interface box and the data acquisition unit. An orderly and reliable connection of the individual fuel cells to the interface box is established via standard eight-wire Cat. 5 patch cables with RJ-45 (8P8C) plugs (Fig. 1b).
2.4 Fuel cell connection schematics
To illustrate the versatility of the presented measurement setup, the three applicational scenarios for two-chamber fuel cells, single chamber fuel cells, and generic electrical generators are described in the following. In these examples, the patch cable from the interface box is connected to a Cat. 5E RJ-45 insert, the type typically used in computer network installations. Fuel cell connection leads are attached to the printed circuit gold pads at the insert’s back.
A conventional two-chamber fuel cell, where cathode and anode are immersed in separate beakers connected by an ion bridge, is shown in Fig. 2a. In this setup, the voltage drop across the ion-bridge is eliminated from the measurement by using two separate reference electrodes for cathode and anode, each placed in close vicinity of the electrode.
The setup for characterization of a single-chamber fuel cell, where anode and cathode are placed at close distance in the same beaker, is shown in Fig. 2b. Here, it is preferable to use only one reference electrode, provided the voltage drop across the electrolyte is negligible. To this end, the reference electrode connections for anode and cathode can be short-circuit and connected to a single reference electrode in the testing solution.
In some cases, it may not be practical to include a reference electrode for the measurement of electrode potentials, e.g., with batteries or non-electrochemical micro generators. In this case, the reference electrode connections for anode and cathode are short-circuit and preferably connected to the anode or the negative terminal of the device. The recorded anode potential thus will always be zero, whereas the measured cathode potential corresponds to the overall voltage of the system under investigation (Fig. 2c).
3 Control software
A screenshot of the software’s user interface, realized in a Visual Basic environment (Microsoft VisualStudio.NET 2005, source code available in the online supplementary material section), is shown exemplary for a single channel in Fig. 3. The following sections describe the software functionality in detail.
3.1 Channel parameters
The parameters to be specified separately for each channel include measurement interval, file name for data storage, and the geometric area of the cell or electrode under investigation. The electrode potential of interest can be selected between cathode vs. reference, anode vs. reference, and cathode vs. anode (complete fuel cell). For the potential of interest, the actual potential drift is calculated from the difference between two subsequently recorded potentials, divided by the time span between the measurements. The average potential drift is calculated as arithmetic mean value over a user-definable look-back time.
3.2 Experimental modes
Implemented into the software are three separate experimental modes for open-circuit voltage and continuous galvanostatic load measurements, and the stepwise recording of a galvanostatic load curve over a selectable current range.
In open-circuit voltage mode, the current is set to zero and the switching relay in the load circuit is set to the open position to completely disconnect the fuel cell from the electronic load.
When selecting the continuous galvanostatic load mode, the current through the load circuit is controlled indefinitely at a constant value. This experimental mode is for instance relevant to characterize the long-term performance stability of a fuel cell operating at defined current.
The galvanostatic load curve mode is used to record a fuel cell polarization curve under galvanostatic control. The procedure is fully automated and has been implemented as a stepwise alteration of load current over a selectable range. Here the relatively slow response of biofuel cells to current transients is taken into account, which is likely to lead to an overestimated performance when a too fast continuous current scan is applied. This can be circumvented by a stepwise recording of polarization curves, in which case the stabilization of electrode potential (potential drift) serves as a criterion for load change. Alternatively, the stepwise load change procedure is useful to measure the potential drift over a certain period of time at a given load current, and thus asses the sustainability of fuel cell performance under different load conditions. Both possibilities have been implemented into the galvanostatic load curve mode. Automatic load change is only initiated when both the average potential drift is below the threshold value and a user-definable wait time has passed. This way, a purely time-controlled recording of a stepwise polarization curve can be realized by choosing a sufficiently high potential drift threshold. In any case, a load change can be initiated manually with a skip button, which overrides the limiting criterion.
The current range over which a polarization curve is recorded is defined by the following four parameters, also shown schematically in Fig. 3. Beginning from an arbitrary start value, the current is altered step-wise via a peak value towards the final stop current. Here, the introduction of a peak value allows for the two-directional recording (increasing as well as decreasing current density) of a polarization curve in one experimental sequence to investigate hysteresis effects. Upon completion of the sequence, the software automatically switches to the continuous galvanostatic load mode, with the load current set to the after value.
3.3 Channel interdependencies
To characterize several fuel cells simultaneously in an identical fashion (e.g., when performing replicate experiments in parallel), channel interdependencies can be established in two ways. The first option is a master-slave relationship between individual channels. In this case, all assigned channels are controlled from the master channel, and subject to the same experimental procedure. For automatic load change in the galvanostatic load curve mode, only the settings of the master channel are considered, potential drift or wait time of the associated channels are ignored. Alternatively, several channels can be combined in switching groups during the recording of polarization curves. Also in this case, the load current on all channels of the switching group is changed simultaneously. In contrast to the master-slave concept, however, a load change is only initiated when all channels combined in a switching group meet the load change criterion, either specified as potential drift threshold or wait time.
4 Electrode fixtures and aseptic electrochemical reactor
4.1 Fixtures for complete fuel cells and single electrodes
Often fuel cell electrodes, especially of the flat-plane type as used in abiotically catalyzed glucose fuel cells [10, 19, 20], mandate a stable frame for mechanical support. To this end, we conceived rapid-assembly fixtures, into which electrodes and membranes can be stacked between 2-mm silicon rubber gaskets and 5-mm-thick polycarbonate frame and backing. The whole assembly is clamped together with M5 nylon screws. In Fig. 4, two versions of the realized electrode fixture are illustrated. In version (a), a geometric electrode area of 2.25 cm2 is exposed to the testing medium. This version is preferably used to characterize both complete fuel cells and larger half-cell electrodes. Version (b) is designed for the simultaneous characterization of four smaller half-cell electrodes in a single holder assembly. Of each electrode only a geometric area of 0.25 cm2 is exposed to the solution.
Rapid-assembly electrode fixtures for complete fuel cells and single electrodes. a Fixture for a single fuel cell or electrode. b Fixture for four single electrodes. Components: 1 polycarbonate frame, 2 silicone rubber gasket, 3 cathode, 4 separator membrane, 5 anode, 6 single electrode, 7 silicone rubber gasket, 8 polycarbonate backing. For each electrode separate wire connections for voltage and load current are implemented
4.2 Aseptic electrochemical reactor
The reactant solution in biofuel cell experiments is often an ideal culture medium for the growth of microorganisms. For performance characterization, it is therefore essential that an environment is created where either sterile conditions or the exclusive presence of a certain microbial population can be sustained. To this end, we modified a plastic desiccator (# 1008.1, Carl Roth, Karlsruhe, Germany) into an aseptic electrochemical reactor. The vessel is large enough to accommodate up to six of the presented electrode fixtures for parallel performance characterization of fuel cells or half-cell electrodes in a common reactant solution. The modifications to the plastic desiccator are shown in Fig. 5 and are described in the following.
To minimize geometric effects upon gas-purging of the solution or due to electrolyte resistance, the fuel cells (or e.g., platinum mesh counter electrodes in case of half cell investigations) as well as reference electrode tip and aeration frit are placed symmetrically inside the vessel. Openings in the polycarbonate lid are either sealed with silicone elastomer or equipped with Luer-style connectors and fittings. Microorganism contamination is avoided by equipping both the aeration glass tube and the medium exchange port with sterile filters. To prevent pressure build-up, a 10-cm-long silicon rubber tube is used at the gas outlet instead of a sterile filter. The complete assembly is autoclavable with the fuel cells in place, and can be installed in a temperature-controlled incubator to maintain the testing solution at a desired temperature. However, heat-sensitive reference electrodes have to be separately introduced, e.g., under a sterile work bench after disinfection with ethanol.
5 Validation and application
5.1 Validation of accuracy and cross-channel interference
The measurement accuracy of the presented electrical testing setup was evaluated by using a freshly calibrated research grade potentiostat (PCI4/300 Model, Gamry Systems, Pennsylvania) as reference system. Thereto the potentiostat (operating in zero resistance ammeter mode) was connected in series with a 2.7-kΩ resistor, and integrated into the load circuit in place of a fuel cell. For load currents between 1 and 200 μA, the values recorded by the presented measurement setup differ by less than 0.25% from the current measured simultaneously with the reference potentiostat system.
Similarly, the accuracy of the voltage measurement was validated. To this end, a 2.7-kΩ resistor was connected to the measurement setup in place of a fuel cell, according to the connection schematic shown in Fig. 2c. The resistor was loaded with currents between 2 and 200 μA, corresponding to voltages between 5 and 540 mV, respectively. In parallel to the resistor, the potentiostat was connected and set to open-circuit potential measurement mode. In the voltage range between 30 and 540 mV, the values recorded with the presented measurement system differ by less than 0.1% from the potentiostat. Only at the lower voltage of 5 mV was a larger deviation of 0.6% observed, which is still acceptable and can be attributed to the ±1 mV accuracy of the reference potentiostat system.
To characterize cross-channel interference between multiple fuel cell electrodes in the same solution, six 2.25-cm2 platinum cathodes were operated against platinum mesh counter electrodes in the aseptic operating chamber as described earlier. While one cathode was kept at open circuit, the remaining five cathodes were each loaded with a current of 50 μA, and also 200 μA. The resulting shift in open-circuit potential of the unloaded cathode, caused by the uncompensated ohmic resistance of the electrolyte, amounted to 0.1 mV and 0.6 mV, respectively. In comparison to the typical biofuel cell voltages, ranging between 100 and 700 mV, this deviation is negligible.
5.2 Exemplary fuel cell performance characterization
As exemplary application of the presented testing environment the performance of three abiotically catalyzed glucose fuel cells, intended as tissue implantable power supply for medical implants, was characterized simultaneously. The construction details of these fuel cells are reported elsewhere [21]. In short, they consist of a self-supporting Raney-type platinum anode, and a thin-layer platinum cathode deposited on a slitted silicon chip. Both electrodes have a geometrical area of 2.25 cm2 and are separated by a porous filter membrane. Also included into the assembly is a filter membrane in front of the cathode, accounting for the diffusion resistance expected from tissue capsule formation around medical implants.
The fuel cell electrodes and membranes were mounted in the rapid-assembly fixtures and placed symmetrically in the aseptic electrochemical reactor as described earlier. The performance was characterized under sterile conditions in phosphate buffered saline containing 3 × 10−3 mol L−1 glucose at 37°C. The solution was continuously purged with a mixture of humidified nitrogen and air (total volume flow approx. 2 L min−1), the oxygen partial pressure corresponding to 33 mbar (approx. 3.5% oxygen saturation, estimated lower physiological range [10]). For performance characterization, the load current density was increased in steps of 4.4 μA cm−2 from 0.0 μA cm−2 to a maximum value of 17.8 μA cm−2 every 12 h. Polarization curves were constructed from the electrode potentials recorded versus the saturated calomel electrode (SCE; KE11, Sensortechnik, Meinsberg, Germany) after 12 h of operation at a given load current density.
In Fig. 6a, the evolution of load current and electrode potentials during the automatic recording of a polarization curve are shown exemplary for one fuel cell. Due to the slow load change response of the fuel cell electrodes, it takes up to approx. 5 h until the potentials stabilize. While under these circumstances a too fast increase in load current would lead to the overestimation of fuel cell performance, the stepwise recording of the polarization curve allows identifying the time period after which the fuel cell electrode potentials stabilize under load. Furthermore, the performance sustainability can be quantified by calculating the voltage drift (degradation) over a given time period.
Example of data recorded with the presented testing environment. a Evolution of load current and electrode potentials during the automated recording of a polarization curve with stepwise increased load current density. b Corresponding polarization curve of the complete fuel cell, constructed from the electrode potentials recorded after 12 h of operation at a given load current density. c Separate polarization curves of anode and cathode. The cathode polarization curve recorded at the higher oxygen partial pressure is shown for comparison, as indicated in the figure. Given in b and c is the average of three simultaneous experiments, bars represent maximum and minimum values
In Fig. 6b, the dependency of cell voltage and power density of the complete fuel cell on current density is shown. The maximum power density of 2.2 μW cm−2 (sample standard deviation: ±0.1 μW cm−2) is recorded at a current density of 4.4 μA cm−2. At this point, the parallel characterization of three identical fuel cells exposed to the same testing solution allows for a high degree of confidence in the obtained experimental results.
Relevant for the optimization of fuel cell performance is the identification of the limiting electrode reaction. By assessing the polarization plots of the individual electrodes (Fig. 6c), the major share of fuel cell polarization can be attributed to the cathode. Noticeable is the increase in cathode polarization at current densities above 4.4 μA cm−2, suggesting the onset of an oxygen mass transfer limitation. The results from repeating the experiment under the higher oxygen partial pressure of 65 mbar (approx. 7.0% oxygen saturation) validate this hypothesis: at identical current densities (and thus oxygen consumption rates) the increased oxygen availability results in more positive cathode potentials (Fig. 6c).
While the presented testing setup allows for an exact repetition of load-current steps independent of cell potential, this would be very difficult to achieve with passive-load resistors where the current (density) is always dependent on the overall cell potential. Similarly, Fig. 6c illustrates a further advantage over passive-load resistors. The active current control allows for a further increase in load current density, although the overall cell potential becomes negative. Despite the poor cathode performance, the anode can thus be independently further characterized as half-cell electrode at higher current densities (13.2 μA cm−2 and 17.8 μA cm−2 in Fig. 6).
6 Conclusions
In this work, we presented the design and validation of a dedicated testing environment for the automated biofuel cell performance characterization. Its application was demonstrated by simultaneously recording the polarization curves of three fuel cells. Thereto a galvanostatic technique with step-wise increase in load current was employed, which helps to prevent performance overestimation by a too fast current sweep rate.
Besides rapid-assembly electrode fixtures and an aseptic electrochemical reactor, our setup comprises a computer-controlled electrical testing system with 24 individual load channels. Specifically designed for the recording of polarization curves under galvanostatic control, the electrical testing system bridges the gap between simple load resistors and expensive potentiostat systems commonly used for biofuel cell performance characterization. The main advantages over simple load resistors are the possibility to operate individual half-cells against arbitrary counter electrodes as well as the fully automated recording of polarization curves. Here, the complete galvanic isolation between the individual channels ensures the interference-free operation of multiple fuel cells immersed in a common reactant solution.
With its functional range focused on the recording of polarization curves, the cost of our system is approximately only one-third the commercial multi-channel potentiostat/galvanostat system with the same number of channels. A highly parallel testing environment to characterize biofuel cell performance can thus be established at a significantly lower expense.
Of modular design, the presented setup can be easily adapted to meet different requirements. Individual components can be interchanged, for instance to realize higher load currents or higher data acquisition rates for the characterization of transient effects. Though motivated by biofuel cell research, the concept may thus also find an application to characterize the performance of other micro-power sources in a highly parallel fashion, including conventional micro fuel cells [22], mechanical [23], as well as thermoelectric [24] micro-generators, and miniature batteries [25].
A more detailed description of the electrical system (including printed circuit board layout, control software, and circuit diagrams) is available in the online supplementary material accompanying this paper. This allows researchers to replicate our setup in their lab, and can also serve as an inspiration for the design of similar systems adapted to specific requirements.
References
Bullen RA, Arnot TC, Lakeman JB, Walsh FC (2006) Biosens Bioelectron 21:2015
Logan BE, Hamelers B, Rozendal R, Schroeder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Environ Sci Technol 40:5181
Walker AL, Walker CW (2006) J Power Sources 160:123
Apblett C, Ingersoll D, Roberts G, Minteer S, Atanassov P (2006) In: Proceedings of PowerMEMS 2006—the sixth international workshop on micro and nanotechnology for power generation and energy conversion applications, Berkeley, California, 29 Nov–1 Dec 2006, pp 271–274
Heller A (2004) Phys Chem Chem Phys 6:209
Katz E, Shipway AN, Willner I (2003) In: Vielstich W, Gasteiger HA, Lamm A (eds) Handbook of fuel cells—fundamentals, technology and applications. Wiley, New York
Shukla AK, Suresh P, Berchmans S, Rajendran A (2004) Curr Sci 87:455
Barton SC, Gallaway J, Atanassov P (2004) Chem Rev 104:4867
Davis F, Higson SPJ (2007) Biosens Bioelectron 22:1224
Kerzenmacher S, Ducrée J, Zengerle R, von Stetten F (2008) J Power Sources 182:66
Rhoads A, Beyenal H, Lewandowski Z (2005) Environ Sci Technol 39:4666
Fischback MB, Youn JK, Zhao XY, Wang P, Park HG, Chang HN, Kim J, Ha S (2006) Electroanalysis 18:2016
Ringeisen BR, Henderson E, Wu PK, Pietron J, Ray R, Little B, Biffinger JC, Jones-Meehan JM (2006) Environ Sci Technol 40:2629
Haoyu E, Cheng S, Scott K, Logan B (2007) J Power Sources 171:275
Hamann CH, Vielstich W (1998) Elektrochemie. Wiley-VCH, Weinheim
Kerzenmacher S, Ducrée J, Zengerle R, von Stetten F (2008) J Power Sources 182:1
Ross JD, Ross JD, Reddy NE, Bakkum DJ, Potter SM, DeWeerth SP (2007) In: Proceedings of the 29th IEEE engineering in medicine and biology society annual international conference, IEEE, Lyon, France, 23–26 Aug 2007, pp 4759–4762
Xiao MY, Wasling P, Hanse E, Gustafsson B (2004) Nat Neurosci 7:236
Rao JR, Richter GJ, von Sturm F, Weidlich E (1976) Bioelectrochem Bioenerg 3:139
Drake RF, Kusserow BK, Messinger S, Matsuda S (1970) Trans Am Soc Artif Intern Organs 16:199
Kerzenmacher S, Kräling U, Ducrée J, Zengerle R, von Stetten F (2008) In: Proceedings of the 4th European congress for medical and biological engineering, Springer, Antwerp, Belgium, Nov 23–27 2008, pp 2379–2383
Nguyen NT, Chan SH (2006) J Micromech Microeng 16:R1–R12
Miao P, Mitcheson PD, Holmes AS, Yeatman EM, Green TC, Stark BH (2006) Microsyst Technol 12:1079
Venkatasubramanian R, Watkins C, Caylor C, Bulman G (2006) In: Proceedings of PowerMEMS 2006—the sixth international workshop on micro and nanotechnology for power generation and energy conversion applications, Berkeley, California, 29 Nov– 1 Dec 2006, pp 1–4
Heller A (2006) Anal Bioanal Chem 385:469
Acknowledgements
We gratefully acknowledge the financial support from the European Union (Contract No. 001837 Healthy Aims). Also, we would like to thank Karl-Heinz Boven, Hans Löffler, and Michael Hesse of Multichannel Systems Germany for their support during integration of the STG 2008 stimulus generator into our setup and software.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kerzenmacher, S., Mutschler, K., Kräling, U. et al. A complete testing environment for the automated parallel performance characterization of biofuel cells: design, validation, and application. J Appl Electrochem 39, 1477–1485 (2009). https://doi.org/10.1007/s10800-009-9827-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9827-0