Abstract
The inhibiting properties and adsorption behaviour of thioacetamide (TAA), thiobenzamide (TBA) and thiocinnamamide (TCA) on mild steel in sulphuric acid solution were studied by gravimetric, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) measurements. TBA and TCA were found to be mixed type inhibitors providing good corrosion inhibition. Different mechanisms of adsorption and corrosion inhibition were observed for the tested thioamides. In the cases of TBA and TCA, the adsorption of the compounds is chemical and the values of the activation energy (Ea) and the pre-exponential factor (A) are lower than the corresponding values observed in inhibitor free solution. The adsorption of TAA is physical and the values of Ea and А are higher than the corresponding values in sulphuric acid solution. The adsorption process of TBA and TCA on the mild steel/sulphuric acid solution interface is described by Langmuir’s isotherm. A correlation between the adsorption capability and the inhibiting efficiency of the molecules and their donor–acceptor properties (E HOMO and E LUMO) has been established. It is ascertained that the protection effect of TAA depends on the amount of hydrolysis products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The inhibition of corrosion by organic compounds is usually related to their adsorption on the metal surface [1–18]. The adsorption process depends on different factors such as the nature and the charge of the metal surface, the temperature and pH of the corrosion media and the molecular structure of the organic compounds (type of functional groups, steric factor and the electron density distribution).
For numerous series of organic compounds molecular orbital calculations have been used to correlate structural and electronic parameters of the inhibitor molecules with their adsorption behaviour and therefore with the inhibition efficiency [10–29].
The aim of the present work is to study the adsorption behaviour and inhibiting properties of thioacetamide (TAA), thiobenzamide (TBA) and thiocinnamamide (TCA) on mild steel in sulphuric acid solutions, as well as to obtain the correlation between inhibitor performance, respectively adsorption capability of TAA, TBA and TCA and their chemical structure. TAA and TBA have been studied as inhibitors of mild steel corrosion in hydrochloric [26–28, 30] and in sulphuric acid solutions [29], but the correlation between the inhibition efficiency and molecular structure of the compounds is discussed in the cited works without taking into account the protonation of thioamides in acid solutions [31]. However, it was ascertained [12, 13] that the protonation of organic molecules in acidic solutions generally produces a considerable change in the partial charges distributed at the adsorption centres of the molecules as well as in the values of the energy of the highest occupied molecular orbital (E HOMO) and the energy of the lowest unoccupied molecular orbital (E LUMO), which generally relate to the donor–acceptor properties of organic molecules. Another neglected problem in studying inhibition properties of TAA is the hydrolysis of compound in acidic aqueous solutions [2, 32]. The compound is relatively stable at 20 °C, but at higher temperature the rate of TAA hydrolysis significantly increases [32].
The inhibiting properties of the selected thioamides have been estimated by the use of gravimetric measurements, potentiodynamic polarization technique and electrochemical impedance spectroscopy.
2 Experimental details
Quantum-chemical calculations were carried out using of the AM1 method [33–35]. The partial charges of the atoms in the molecules, the energies: E HOMO and E LUMO, as well as the length of valence bonds and the dihedral torsion angles were calculated.
The experiments were conducted in 0.1 M H2SO4 solutions, containing different concentrations of the organic substances: thioacetamide (TAA), thiobenzamide (TBA) and thiocinnamamide (TCA). The compounds were added to the corrosive medium as ethanol solutions with the aim of increasing their solubility. The concentration of ethanol was 2 vol.% and was maintained constant for all solutions. The solutions were prepared with doubly distilled water and A.R. grade chemicals.
Mild steel samples (C-0.039, Mn-0.38, Cu-1.112, Ni-0.07, Si-0.003, S-0.02, P-0.22%) of 4.0 × 5.0 × 0.25 cm were used for weight loss experiments. The samples were polished mechanically with abrasive paper (N:240 to N:900) degreased with acetone and rinsed with ethanol. The electrochemical experiments were performed in a conventional three-electrode glass cell with working volume of 100 mL. А working electrode was a cylinder embedded in a Teflon holder. The surface area exposed to the electrolyte was 0.5 cm2. Before experiments the electrode surface was polished mechanically and treated in the same way as described above. A Pt counter electrode and a saturated calomel electrode as reference electrode were used. All potential values are referred to the normal hydrogen electrode.
The measurements were carried out by a Solartron 1286/1250 system. The potentiodynamic polarisation curves were recorded at a sweep rate of 1 mV s−1 after the steady-state potential had been established in cathodic or anodic direction.
The impedance measurements were performed using an ac signal with amplitude 5 mV in the frequency range from 65 kHz to 0.01 Hz. The spectra were recorded under open-circuit conditions after 1 h exposure in the test solution.
3 Results and discussion
3.1 Quantum chemical calculations
Molecular orbital calculations were performed for the molecules of thioacetamide (TAA), thiobenzamide (TBA) and thiocinnamamide (TCA). In addition, calculations of the protonated forms of the molecules were carried out because in acid medium the thioamides are protonated [31] due to the presence of the free electron pairs on sulphur and nitrogen atoms. The hydrogen cation from the solution can be bonded to the S atom as well as to the N atom in the molecules of TAA, TBA and TCA. The data obtained by quantum chemical calculations show that the protonation is realized at the S atom of the compounds. The values of the distribution of partial charges in the molecules and their protonated forms are presented in Fig. 1a and b respectively. It is ascertained that the protonation produces a noticeable change of the partial charges distributed at the adsorption centers: S atom, amino and methyl groups and phenyl radicals. In Table 1, the values of electron density at S and N atoms of the protonated forms as well as of E HOMO and E LUMO for molecules in non-protonated and protonated form are summarized. The comparison of quantum chemical calculation results shows that protonation produces a considerable change of E HOMO and E LUMO values. The non-protonated molecules of thioamides have similar values for energy of the highest occupied molecular orbital (E HOMO) while for protonated forms the E HOMO values differ significantly and increase in the order TAA<TBA<TCA. The essential difference in E LUMO values was established for the molecules of tested thioamides and their protonated forms. The E LUMO values for protonated forms increase in inverse order than these for non-protonated forms. These results demonstrate that in acid medium, the values of E HOMO and E LUMO for protonated molecules have to be used for correlation of the inhibition efficiency and the chemical structure of thioamides.
3.2 Weight loss measurements
The inhibition efficiency of the selected thioamides on mild steel corrosion was examined by weight loss measurements. The influence of inhibitor concentration and temperature was investigated. The inhibition efficiency, IE w (%), for each concentration of the tested thioamides was determined from weight loss data by the following equation:
where r o and r are the average corrosion rates (g m−2 h−1) of mild steel in sulphuric acid solution in the absence and presence of inhibitors, respectively.
The dependence of the inhibition efficiency on the concentration of the compounds in 0.1 M H2SO4 at 20 °C is presented in Fig. 2. It is evident that the inhibition efficiency increases with the increase of thioamides concentration. Maximum inhibition for TCA is reached at relatively low concentration of 0.1 mM, which corresponds to the maximum solubility of the substance at these conditions. For TAA and TBA the maximum protective effect was obtained at a concentration of 1 mM.
The comparison of the values of IE w (%) at a concentration of 0.1 mM shows that the inhibition efficiency increases in the order TAA< TBA< TCA. The data in Table 1 show that E HOMO and E LUMO, as well as the electron density of S and N atoms in the protonated form of the compounds increase in the same order. This clearly indicates a dependence of the inhibition efficiency on the molecular structure parameters.
The influence of temperature on the inhibition efficiency was studied at different concentrations of selected thioamides. The dependences IE w (%) on temperature at a concentration of 0.1 mM are presented in Fig. 3. The results show that the inhibition efficiency of TBA and TCA increases with increase of temperature. This indicates that the corrosion inhibition with TBA and TCA is a result of the chemical adsorption of the compounds. In contrast, in the case of TAA the inhibition efficiency decreases with increase in temperature, probably as a result of desorption, characterizing the physical adsorption.
The effect of temperature on the corrosion rate (r) at different concentrations of TAA, TBA and TCA is shown in Fig. 4 (Arrhenius plots). It is evident that the corrosion rate increases with increase in temperature, both for the blank and for the inhibited solutions. Figure. 4b and c show that at higher concentrations TBA and TCA significantly slow down the corrosion rate within the entire temperature range. The apparent activation energy (Ea) and the pre-exponential factor (A) from the Arrhenius equation (r = A e−Ea/RT) were determined from the slopes and the intercepts respectively of the straight lines in Fig. 4 and are presented in Table 2.
Summarizing the results obtained for Ea and A in sulphuric acid solutions with and without the addition of thioamides at various concentrations, it can be concluded that the mechanism of corrosion inhibition differs in the presence of selected thioamides. In sulphuric acid solution containing TBA and TCA, the Ea and A values are lower than those in the uninhibited solution. With increase in TBA and TCA concentrations the Ea and A values decrease. For TAA the values of Ea and А are higher than those for blank acid solution. In this case however, with increase of TAA concentration both values of Ea and А increase.
3.3 Adsorption isotherms
The adsorption isotherms provide specific information about the adsorption behaviour of the inhibitors i.e. the interaction between the adsorbed molecules and metal surface as well as adsorbate/adsorbate interactions in the adsorption layer. The degree of surface coverage (θ) for different concentrations of the inhibitors in sulphuric acid solution was evaluated using the inhibition efficiency data (θ = IE w/100). Different adsorption isotherms were tested for agreement with the experimental data.
The linear relations in the ln[θ/(1 − θ)] on ln c or plots for TBA and TCA at all examined temperatures (Fig. 5a and b) suggest that the adsorption of these compounds on mild steel surface follows the Langmuir adsorption isotherm:
where B is the equilibrium constant of the adsorption process. The validity of Langmuir’s isotherm of TBA and TCA adsorption on mild steel indicates that the interaction forces between the molecules in the adsorbed layer are equal to zero. Higher values of the equilibrium constant (B) were obtained for TCA for all temperatures (Table 3). This confirms that the adsorption capability of TBA and TCA depends on their donor-acceptor properties (E HOMO and E LUMO values).
The degree of surface coverage (θ) estimated at different concentrations of TAA were tested graphically as to whether they fitted the Langmuir, Frumkin, Temkin and Flory-Huggins adsorption isotherms. However, the correlation coefficient R between θ and c or for all adsorption isotherms was below 0.65. The adsorption of TAA on the mild steel/sulphuric acid solution interface can therefore be considered as a non equilibrium process due to the TAA hydrolysis [2, 32].
3.4 Electrochemical impedance measurements
The impedance spectra of mild steel in 0.1 M H2SO4 solutions without and with addition of the tested inhibitors after 1 h of exposure at 20 °C are shown as Nyquist plots in Fig. 6. The concentration of 0.1 mM was selected to compare the effect of compounds since it corresponds to the maximum solubility of TCА.
The quantitative analysis of the experimental data was performed by a nonlinear least squares minimization method [36]. The impedance spectra in the sulphuric acid solution consist of two components: a high-frequency capacitive semicircle and a low-frequency inductive loop. The equivalent circuit describing the processes on the mild steel/solution interface is shown in Fig. 6a, where R t is the charge transfer resistance and C dl is the double-layer capacitance. The resistor R and the inductance L are elements associated with the relaxation of the adsorbed intermediates. In the presence of TAA the character of the spectrum is similar to that of an inhibitor free solution, and no significant changes in the diameter of the high-frequency semicircle associated with the charge transfer resistance R t is visible. In the presence of TBA and TCА (Fig. 6b) the diameter of the high-frequency semicircle increases and the low-frequency inductive loop disappears. This result indicates different mechanism of adsorption of TBA and TCА on the mild steel surface.
The calculated values of the corresponding parameters of the equivalent circuits in the absence and presence of the investigated compounds in the corrosive medium are summarized in Table 4. The significant increase of R t and the decrease of C dl is clear evidence of the inhibitor properties of TBA and TCA at concentration of 0.1 mM.
3.5 Polarization measurements
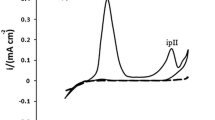
The inhibiting effect of selected thioamide compounds on the partial corrosion reactions of mild steel was investigated by use of the potentiodynamic polarisation technique. Figure 7 shows polarization curves recorded after 1 h of exposure in 0.1 M H2SO4 solution without and with addition of different concentrations of thioamides tested at 20 °C.
The inhibition efficiency was calculated using the equation:
where I corr,o and I corr are the corrosion current densities without and with inhibitor, respectively. These were determined by extrapolation of the Tafel lines and the open circuit potential of the corresponding polarization curves. The values of open circuit potential (E corr) and corrosion current densities (I corr) as well as the calculated inhibition efficiency are presented in Table 5.
The obtained polarization curves show that the TAA addition shifts the corrosion potential (E ocorr ) in negatively. This means that TAA is an inhibitor of cathodic type. However, at the highest concentration of TAA (10 mM) significant inhibition of the anodic reaction is also observed. TBA and TCA act as inhibitors of mixed type. Their addition slows down the rate of both partial corrosion reactions without shifting the corrosion potential (E ocorr ). The cathodic Tafel slopes do not change significantly in the presence of inhibitors. This fact suggests that these compounds do not change the kinetics of hydrogen evolution.
The inhibition efficiency of thioamides increases with increase in concentration (Table 5). The relatively high IE value (about 94%) in the presence of TCА indicates good protective properties of this compound toward mild steel corrosion in acid medium.
The inhibition efficiency obtained by polarization measurements for TBA and TCA show good correlation with results obtained by gravimetric measurements at 20 °C. For TAA at concentration of 0.1 mM significant difference is observed. The inhibition efficiency obtained by gravimetric measurements at this concentration of TAA is equal to 50.70%, whereas no inhibiting effect was observed from the polarization and impedance measurements.
3.6 Inhibition mechanism
The results obtained by weight loss measurements and electrochemical methods for the inhibiting properties and the adsorption behaviour of the selected thioamides clearly indicate that the mechanism of corrosion inhibition of TAA is different than this for TBA and TCA.
In general [37], the increase in inhibiting effect (IE) with temperature increase and the lower value of the activation energy (Ea) in the presence of inhibitors compared to the Ea obtained in uninhibited solution are attributed to chemical adsorption. In contrast the decrease in IE values with temperature increase and the higher value of Ea in inhibited solution are interpreted as physical adsorption. The results obtained for selected thioamides are an indication of chemical adsorption for TBA and TCA and of physical adsorption in the case of TAA. The established chemical adsorption of TBA and TCA can be consider as a result of the electron charge transfer from the adsorbed molecules of the inhibitor to the vacant d-orbital of the metal. The charge transfer could be realized by the free electron pairs at S and N atoms and by the π- electrons of benzene rings as well as by the double bonds in the TCA molecule.
The process of physical adsorption generally requires the presence of both an electrically charged metal surface and charged species in the bulk of the solution. The physical adsorption behaviour of TAA may be explained by considering the hydrolysis of TAA. In aqueous acid solutions TAA is hydrolyzed and the process is characterized by two parallel reactions [32]. These two distinct reaction paths are represented by the scheme:
The intermediate compounds for the first reaction path are acetamide (AA) and H2S. Thioacetic acid (TA) and NH3 are intermediate products of the second one. The physical adsorption of TAA can be attributed to the electrostatic interaction between the metal surface and the AA and TA molecules or between a double layer of adsorbed TAA and the above mentioned molecules, which can be considered as dipoles.
The concentrations of the hydrolysis products of TAA were calculated on the basis of data in [32] for the temperature interval between 20 and 50 °C taking into account different experimental times for weight loss and electrochemical measurements. The estimated amounts of different hydrolysis products show strong dependence on the experimental time. Under the conditions of electrochemical measurements (1 h exposure) only 0.7% of the initial concentration of TAA is hydrolyzed. Hence in this case only the TAA molecules participate in the inhibiting process and the adsorption of TAA is insignificant due to poor donor-acceptor properties of the TAA molecule. For the experimental time of the gravimetric measurements the concentration of AA and TA hydrolysis products increase (about 15% of the initial concentration of TAA is hydrolyzed). Therefore, the higher values of inhibition efficiency for TAA obtained by gravimetric measurements at a concentration of 0.1 mM can be explained by the higher amount of intermediate products.
4 Conclusion
This study of the inhibiting properties and adsorption behaviour of thioacetamide (TAA), thiobenzamide (TBA) and thiocinnamamide (TCA) on mild steel in sulphuric acid solution by gravimetric and electrochemical methods leads to the following conclusions:
-
(i)
The inhibition efficiency (IE%) of the tested thioamides depends on the molecular structure of the protonated form of the compounds. The IE% increases in the order TAA < TBA < TCA, in which order the values of E HOMO and E LUMO also increase, as well as the electron density of S and N atoms in the protonated form of the compounds.
-
(ii)
TBA and TCA were found to be mixed type inhibitors providing good corrosion inhibition.
-
(iii)
Different mechanisms of adsorption and corrosion inhibition were observed for the tested thioamides. In case of TBA and TCA, the adsorption of the compounds is chemical and the values of the activation energy (Ea) and the pre-exponential factor (A) are lower than the corresponding values in inhibitor free solution. The adsorption of TAA is physical and the values of Еа and А are higher than the corresponding values in sulphuric acid solution.
-
(iv)
The adsorption process of TBA and TCA on the mild steel /sulphuric acid solution interface is described by Langmuir’s isotherm and the adsorption capability of TBA and TCA correlates with the donor-acceptor properties of the protonated forms of the molecules. The adsorption of TAA is a non equilibrium process due to TAA hydrolysis.
-
(v)
It is ascertained that the protective effect of TAA depends on the amount of hydrolysis products.
References
Trabanelli G (1987) In: Mansfeld F (ed) Corrosion mechanisms. Marcel Dekker, New York
Reshetnikov S (1986) Inhibitory kisslotnoi korrozii metallov. Kimia, Leningrad
Lagrenee M, Mernari B, Bouanis M, Trasnel M, Bentiss F (2002) Corros Sci 44:573
Schweinsberg D, Ashworth V (1988) Corros Sci 28:539
Add El-Rehim S, Refaey S, Taha F, Saleh M, Ahmed R (2001) J Appl Electrochem 31:429
Pebere N, Duprat D, Dabosi F, Lattes A, De Savignac A (1988) J Appl Electrochem 18:225
Srhiri A, Etman M, Dabosi F (1996) Electrochim Acta 41:429
Bentiss F, Traisnel M, Lagrenee M (2001) J Appl Electrochem 31:41
Azhar M, Mernari B, Traisnal M, Bentiss F, Lagrenee M (2001) Corros Sci 43:2229
Kutej P, Vosta J, Pancir J, Macak J, Hackerman N (1995) J Electrochem Soc 142:829
Bentiss F, Traisnel M, Vezin V, Lagrenee M (2003) Corros Sci 45:371
Lazarova E, Yankova T, Neykov G (1996) J Appl Electrochem 26:757
Lazarova E, Petkova G, Raicheff R, Neykov G (2002) J Appl Electrochem 32:1355
Popova A, Christov M, Raicheva S, Sokolova Е (2004) Corros Sci 46:1333
Kutey P, Vosta J, Pancir J, Macak J, Hackerman N (1995) J Electrochem Soc 142:829
Nazmutdinov R, Shapnik M (1996) Electrochim Acta 41:2253
Quaraishi M, Sardar R (2003) J Appl Electrochem 33:1163
Martinez S, Metikos-Hukovic M (2003) J Appl Electrochem 33:1137
Kutey P, Vosta J, Pancir J, Hackerman N (1995) J Electrochem Soc 142:1847
Khaled K, Babic-Samaradziya K, Hackerman N (2004) J Appl Electrochem 34:697
Macak J, Vosta J, Hackerman N (1995) Proceeding of the 8th European Symposium on Corrosion Inhibitors, Ferrara, Italy, Sez V, p 179
Growcock F, Frenier W, Andreozzi P (1989) Corrosion 45:1007
Lukovits F, Bako I, Shaban A, Kalman E (1998) Electrochim Acta 43:131
Stoyanova A, Petkova G, Peyerimhoff S (2002) Chemical Phys 279:1
Khalil N (2003) Electrochem Acta 48:2635
Ebenso E, Ekpe U, Ita B, Offiong O, Ibok U (1999) Materias Chem Phys 60:79
Fang J, Li J (2002) J Mol Struct 593:179
Stoyanova A, Peyerimhoff S (2002) Electrochim Acta 47:1365
Ozcan M, Dehri I (2004) Prog Org Coat 51:181
Stoyanova A, Sokolova E, Raicheva S (1997) Corros Sci 39:1595
Smith M, March M (2001) March’s advanced organic chemistry: reactions mechanisms and structure. J Wiley & Sons, New York
Peeters O, Ranter C (1974) J Chem Soc Perkin II 1832
Dewar M, Thiel W (1977) J Am Chem Soc 99:4899
Dewar M, Gleicher G (1965) J Am Chem Soc 87:692
Dewar M, Zoebisch E, Healy E, Stewart J (1985) J Am Chem Soc 107:3902
Boukamp B (1986) Solid State Ionic 20:31
Szauer T, Brandt A (1981) Electrochim Acta 26:1253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazarova, E., Petkova, G., Iankova, T. et al. Inhibiting properties and adsorption of some thioamides on mild steel in sulphuric acid solutions. J Appl Electrochem 38, 1391–1399 (2008). https://doi.org/10.1007/s10800-008-9577-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9577-4