Abstract
Uniform conducting polymer films of poly(N-(1-Naphthyl) ethylene-diamine dihydrochloride), PNED, were prepared conveniently and reproducibly by the anodic oxidation of the monomer, N-(1-Naphthyl) ethylene-diamine dihydrochloride, NED, in an acidic aqueous solution using the conventional potentiodynamic technique. The different parameters influencing the preparation conditions like monomer concentration, solvent constitution, scan range, scan rate, scan repetition, rotation speed of the working electrode and the type of the substrate were investigated and the optimum preparation conditions are specified. The stability of the prepared films was tested in both aqueous and non-aqueous media. The characteristics of the polymer films and their electrochemical activity towards catalyzing some technologically promising redox reactions were also examined. The films were found to be very stable in aqueous solutions and in some organic solvents like acetone, acetonitrile, and chloroform and dimethyl sulfoxide. The film stability was found to depend on the solution pH. The polymer films were capable of catalyzing the redox processes of several natural products and amino acids e.g. vitamin C and glycine. The polymer film possesses electrochromic properties and the color of the film changes from purple to violet to dark blue and then to brown according to the preparation and/or polarization conditions. The electrochromic properties are related to polaron formation, which subsequently oxidizes to diimine species followed by the oxidation of the aromatic ring. The mechanism of the polymerization process was investigated and discussed. The process involves deprotonation reactions and a head-to-tail coupling of the oxidized monomer with cation radicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrogenerated polymer films are novel conducting and semi-conducting materials with great promise because of their wide range of potential technological applications that have attracted intense multidisciplinary research worldwide [1–4]. The excellent redox cyclability and reversible proton dopability beside other attractive properties displayed by the resulting polymer films represent attractive fields that have received a great deal of attention [5, 6]. These include applications in lightweight (rechargeable) batteries [7, 8], electro-chromic display devices [9–11], biosensors [12] and corrosion protection [13]. Some polymer films have shown good stability in aqueous media under normal atmospheric conditions [14]. Among the conducting polymers, poly(aniline), (PANI), has been studied extensively due to the commercial availability of the monomer, its relatively non-complicated synthesis, well-behaved electrochemistry, good environmental stability, high conductivity and multiple redox and protonation states [15–17]. Some ring and N-substituted PANI derivatives like 1-naphthylamine (NPA) or 1-aminoanthracene have been successfully synthesized by chemical and electrochemical methods and their electrochemical characteristics or electrochromic properties have been well-investigated [18–21]. The mechanism of electrochemical polymerization of NPA was reported to be similar to that of PANI formation. PNPA possesses many properties different from PANI derivatives, such as solubility, electrochemical and electrochromic properties [22, 23].
PNPA derivatives have been used as pH indicator materials, protective films for mild steel and electroactive electrodes in different redox processes [24–26]. Films of PNPA modified by transition metal ions were employed for quantitative detection of organic compounds [27, 28]. One of the attempts to improve the electrochemical and electrochromic properties of PNPA is the copolymerization of NPA with aniline and O-toluidine [23]. The potential used in these electrochemical copolymerization processes is as high as 1.0 V, which resulted in the formation of undesirable degradation by-products [29]. The addition of a small amount of aniline derivative to the monomer enhances the rate of polymer film deposition, significantly [30, 31]. It was also reported that, the preparation of water-soluble poly(aniline) derivatives involving orthanilic acid units has been made feasible by introducing small amounts of aniline in the feed solutions for electropolymerization [32–34]. While the electropolymerization of aniline derivatives has received a great deal of attention, the electropolymerization of PNPA, and its derivatives has had much less attention. The mechanism proposed for the electropolymerization processes involves E(CE)n reactions. This mechanism is initiated by the electrooxidation of the monomer to the corresponding cation radical, followed by polymerization and deposition of a polymer film onto the electrode surface at the appropriate oxidation potential [35–38]. Although N-(1-Naphthyl) ethylene-diamine dihydrochloride, (NED) represent a promising polymer worthy of investigation, there are no reports on the electropolymerization and characterization of this material.
In this work, we are aiming at the electropolymerization of NED from aqueous solutions to produce PNED films of beneficial applications. The optimization of the preparation conditions and the investigation of the electrochemical properties of the films besides the understanding of the mechanism of the polymerization process represent the main tasks. The electrocatalytic activity of PNED modified electrodes towards the redox processes occurring in some natural products and amino acids of industrial relevance were investigated. Detailed mechanistic investigation and discussion of the electropolymerization process in aqueous acidic solutions and the utilization of prepared films were also presented.
2 Experimental details
N-(1-Naphthyl) ethylene-diamine dihydrochloride (referred to as NED herein), BDH was used without further purification. HPLC grade ethanol (Merck) was used as received. The aqueous solutions were prepared using triply distilled water and analytical grade reagents.
A standard all glass three-electrode cell was used for the electropolymerization and the investigation of the electrochemical response of the polymer films. The working electrode was a glassy carbon (GC), gold (Au), or platinum (Pt) disk of geometric area of 0.071 cm2. A silver/silver chloride (Ag/AgCl/ 3.0 M KCl) was used as reference electrode. The counter electrode was a large area platinum spiral. Before each experiment, the working electrode was polished mechanically, with alumina powder (1.0 μm diameter), washed thoroughly with triply distilled water, and then rubbed with a smooth cloth. All electrochemical measurements were carried out using the electrochemical work station, IM6 Zhaner elektrik (Kronach, Germany). All experiments were carried out at room temperature (25 ± 0.1 °C) and the potentials were measured against and referred to the Ag/AgCl reference electrode (E o = 0.222 V).
The electropolymerization process was carried out in solutions containing 25.0 mM NED dissolved in an aqueous solution of 1.0 M HClO4 acid containing ethanol with 9:1 acid/alcohol ratio. The presence of ethanol is essential for both the complete dissolution of the monomer and the stabilization of the formed polymer film on the working electrode surface. For reproducibility, each experiment was carried out at least twice. Details of all experimental procedures were as described elsewhere [35–37].
3 Results and discussion
3.1 Optimization of the electropolymerization process
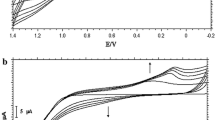
Thin films of poly(N-(1-Naphthyl) ethylene-diamine dihydrochloride), PNED, were prepared by the potentiodynamic electropolymerization technique, using 25.0 mM NED dissolved in 1.0 M HClO4/EtOH mixed solvent of a 9:1 volume ratio. The potentiodynamic technique was found to be better than the galvanostatic or potentiostatic technique. Films grown potentiodynamically were very smooth and adherent to the substrate surface whereas those formed galvanostatically or potentiostatically were not stable [36]. The working electrode was a GC disk and the potential was swept continuously at a rate of 100 mV s−1 between −1.0 and +1.2 V. The cyclic voltammograms of the first 30 cycles of the electropolymerization process are presented in Fig. 1. During the first positive scan an irreversible current peak (I) at ∼+0.56 V and an irreversible wave (II) at ∼+0.83 V were recorded. The peak (I) and wave (II) are due to the oxidation of the amino group of the monomer molecule to a radical cation then to a dication, respectively [39]. In the reverse scan a complementary peak (III) for the anodic peak (I) is recorded at ∼+0.38 V, which is attributed to the reduction of the dication radical (bipolaron). Another cathodic wave was recorded at ∼+0.04 V, that can be attributed to the reduction of the oxidized species, which are formed from the monomer. The cation radicals produced from the aromatic amine derivatives couple rapidly to give dimers, which are more easily oxidized than the parent monomer [38]. In the first cycle, the electrooxidation process is accompanied by the release of protons that are then involved in the protonation of the monomer molecules [16].
During the second forward scan, a thin film is formed on the electrode surface and an anodic wave (V) appears at ∼+0.37 V, which corresponds to the cathodic wave (IV). This wave (V) can be attributed to the oxidation of the cation radicals to oligomers. These oligomer species are brown in color and are free floating at the electrode surface. Above a certain chain length the oligomer transforms into a polymer film, which mediates the oxidation of the reactive species and an adherent purple surface film appears [40, 41]. For short polymerization times (≤5 cycles), both the anodic peak (I) and anodic wave (II) potentials shift to more negative values and a decrease of the anodic current occurs. The decrease in anodic current is attributed to the insulating nature of the initially formed film. By continuous cycling (6–15 cycles) the anodic peak (I) shifts to ca. +0.41 V and its current starts to increase, whereas the anodic wave (II) diminishes and appears as a small shoulder. The cathodic peak (III) shifts to ca. +0.38 V and its current starts to increase, and at the same time, the cathodic wave (IV) shifts to −0.05 V and the corresponding current starts to increase. Such behavior represents the characteristic behavior of a deposited electroactive substance, and is a direct indication of a conductive polymer growth [19, 37, 41]. In general, the potential of oxidation decreases as the molar mass of the species increases, reaching a nearly constant value after few couplings [42]. Film formation during potential cycling is readily recognized by the color change of the electrode surface, which can be noticed easily by the naked eye.
The deposition of thick films of PNED on the GC electrodes occurred rapidly, indicating an efficient film formation process. The direct proportionality of the amount of polymer to the anodic peak current (I) is fundamentally important for studies of the electropolymerization processes [7, 41]. The anodic peak currents of the different electropolymerization cycles were measured and plotted as a function of the cycle number, which corresponds to the reaction time, and are presented in Fig. 2. The amount of polymer formation, which is directly proportional to the film thickness, increases linearly with increase in cycle number until the 20th cycle. Between the cycle numbers, 20 and 30 stabilization of the peak current was recorded. The slope at any point on the linear relation gives the rate of polymer formation at the corresponding reaction time and implies that the polymerization process is obeying first order kinetics in the monomer concentration up to the 20th cycle [43]. Under such conditions, where the rate of electropolymerization is directly proportional to the monomer concentration, two possibilities have to be considered. The first is that the rate of polymerization is dependant on the diffusion of the monomer to the electrode surface to participate in the polymerization process and the reaction is then under diffusion control. The second is that the reaction rate is directly proportional to the charge transfer on the monomer molecules and the process is charge transfer controlled [35]. Increasing the number of cycles above 30 cycles is accompanied by a remarkable decrease in the anodic peak current. The decrease in peak current can be attributed to increased insulating properties, where a relatively compact and thick film is formed. The color of the film in these cases becomes dark brown.
Relation between the cycle number and the anodic peak currents values for the cycles between 1 and 50 during PNED formation, under the conditions of Fig. 1
The effect of initial monomer concentration on the electropolymerization process was investigated. In Fig. 3a, the cyclic voltammograms of the 30th cycle of the NED polymerization at different monomer concentrations are presented. The anodic peak current is presented as a function of the monomer concentration in Fig. 3b. It is clear from Fig. 3b that the amount of the formed polymer increases linearly with the monomer concentration in the range between 0.1 and 30.0 mM. The slope of this relation, which represents the rate of increase of polymer amount with the concentration of the monomer, is about unity, which is in excellent agreement with the theoretical predictions for adsorbed species on thin film modified electrodes [7]. At higher concentrations, a very slow increase in the peak current was recorded. Analysis of the solution after the electropolymerization process has shown that the excess monomer remained unchanged and was not involved in the electropolymerization process.
The electropolymerization process was found to be affected by the scan rate and the scan range. The best polymer films were formed when the scan range was between −1.0 and +1.2 V vs. Ag/AgCl electrode. If the scan range was shortened to more positive than −1.0 or less than +1.2 V, a decrease in the rate of film formation occurs. Extension of the scan range to more negative than −1.0 V or more positive than +1.2 V, leads to a decrease in the rate of film formation. In both cases, the films do not adhere to the working electrode surface.
The effect of scan rate on the polymer film formation is presented in Fig. 4. Figure 4a presents the cyclic voltammograms of the 30th cycle of film formation as a function of scan rate, v. The variation of the amount of polymer formed at the electrode surface, as presented by the anodic peak current, I paI with v is presented in Fig. 4b. It is clear that the peak current increases as the scan rate increases till 200 mV s−1 and then a stabilization of the peak current is attained. The increase in the wave recorded in Fig. 4a can be attributed to an increased rate of apparent diffusion; including physical diffusion and electron self-exchange [44].
The kinetics of the electropolymerization process can be understood by analyzing the cyclic voltammograms presented in Fig. 1. The oxidation peak recorded at +0.56 V is dependant on the scan rate, v. The dependence of the peak current, I paI, on the scan rate shows a linear relation between 10 and 200 mV s−1 and then stabilization in the peak current values occurs, which is strongly dependant on the monomer concentration (cf. Fig. 5a). In this relation, the anodic peak (I paI) increases as the scan rate increases up to 200 mV s−1. This can be attributed to the presence of an irreversible adsorbed electroactive substance [39]. The experimental current function (I paI/v 1/2Co) decreases with v 1/2 in a way, which is dependant on the monomer concentration (cf. Fig. 5b). This leads to the conclusion that the follow-up chemical reaction of the formed cation radical obeys higher than first-order kinetics [35–38].
(a) Relation between I paI of the 30th cycle during PNED film formation for two NED concentrations, 25.0 and 35.0 mM, at different scan rates ranging between 50 mV s−1 and 1.0 V s−1. (b) Relation between I paI/v 1/2C of the 30th cycle during PNED film formation for two NED concentrations 25.0 and 35.0 mM at different scan rates ranging between 50 mV s−1 and 1.0 V s−1
The type of substrate was found to affect the electropolymerization process. Glassy carbon disks are more appropriate than both Au and Pt disks. Figure 6 presents the cyclic voltammograms of the 30th cycle of film formation on Pt, Au, and GC. It is clear that the peak current in the case of the GC substrate is higher than that of both Pt and Au. This observation can be attributed to the amorphous nature of GC [35, 36].
The rotation of the working electrode affects the polymerization process. Rotation of the working electrode during potential cycling increases the anodic peak (I) current and the cathodic peak (II) is diminished. The increase in anodic current was found to be a linear function of the rotation speed as presented in Fig. 7a.
Although, the increase in rotation speed increases the formation peak current value, which indicates increased film thickness, the films at high rotation showed a remarkable loss in the electrochemical activity. Such a loss of electrochemical response may be explained by the formation of a compact film with lower charge transfer properties. The electrochemical response of the films at different rotation speeds for the 30th cycle was measured as a function of rotation speed. These results are presented in Fig. 7b. The best electrochemical activity is obtained whenever the working electrode is stagnant. This behavior can be explained on the basis that during electropolymerization, rotation enhances the accumulation of reaction products on the surface and hence better charge transfer conditions for electropolymerization occur. This is reflected in the increase in polymerization peak current with the increase of working electrode rotation speed (Fig. 7a).
After film formation, the electroactive response in amine free solutions shows the highest values for films formed under stagnant conditions. In these cases, the film is capable of exchanging ions with the solution, especially protons and so the electroactivity is notable. Films formed at higher speeds of rotation are adherent to the substrate surface and represent a highly compact structure that cannot exchange ions with the solutions; hence both the rate of charge transfer and the electrochemical activity are decreased.
3.2 The electrochromic properties of the PNED films
The prepared PNED films show electrochromic properties similar to those reported for electroconductive polymers such as polyaniline, which changes its color as a result of the redox reactions of the film itself [10, 18, 22, 23, 37]. The polymers films change color according to the polymerization time under the optimized conditions of scan range, scan rate and monomer concentration. For short electrochemical polymerization time corresponding to three to four cycles (<3 min) a purple film was formed. For longer polymerization times ∼5 min (10 cycles) a violet colored film was obtained. Longer polymerization times (6–22 min) produced dark blue films, which transformed into dark brown. The produced films showed multiple color changes according to the redox reactions occurring at different potentials as was observed for polyaniline films in the potential range between −0.2 and +1.2 V vs. NHE [10]. The electrochromic properties of these electroactive films find a great deal of applications in filter preparation, optics and electrochromic display devices [9 –11, 18, 22, 23].
3.3 Electroactive characteristics of the PNED film
The PNED modified electrodes obtained by the electropolymerization of the NED on GC under the optimized conditions described above were tested in amine free perchloric acid to investigate their electrochemical response. Figure 8 shows the cyclic voltammograms of the electrochemical response of the modified electrodes in 1.0 M HClO4 solution (pH = 0.01) at a scan rate of 100 mV s−1 between +0.2 and +0.7 V vs. the Ag/AgCl reference electrode. The figure represents the redox system of the polymer backbone, which exhibits only a single redox couple at ∼+0.52 V. The reversible redox wave corresponds to the oxidation of the amine nitrogen i.e. polaron state formation like the polyaniline structure [43]. The figure shows the first, third, fifth and tenth cycles, and shows that the PNED modified electrodes show stepwise decrease in the electrochemical activity. It was found that the polymer film activity decreased about ∼9% in the 3rd cycle, ∼14% in the 5th cycle, and ∼22% in the 10th cycle and then remained unchanged, whatever the number of cycles. In contrast to ordinary aniline, the peak potentials appearing in both the positive and negative branches did not change on repetition of the cycling, as if only the solution species are involved in the reaction.
The electrochemical response of the modified electrodes was found to depend on scan rate. Figure 9 presents the effect of v on the cyclic voltammograms of the modified electrodes in amine free 1.0 M HClO4 solution. It is clear from Fig. 9a that the peak current of the first cycle increases as the scan rate increases, which can be attributed to an increased contribution of capacitive charging and/or the diffusion and electron self exchange [44]. The capacitive charging ratio of the cathodic to anodic peak current (I pc/I pa) was found to increase as the scan rate increases. This means that the apparent electrochemical reversibility for the PNED film is better at relatively high scan rates, which can be attributed to the ease of movement of the counter ion (ClO −4 ) into and out of the polymer film. In Fig. 9b, the anodic peak current is plotted against the square root of v. The linear relation presented in this figure indicates that the redox reaction occurring at the polymer film/electrolyte interface is a diffusion-controlled reaction, as expected for redox reactions involving surface attached species [5, 6].
It should be pointed out that the peak current and also the wave shape of the electrochemical response of the polymer films are affected by the acid concentration. Typical cyclic voltammograms of the electrochemical response of PNED at different concentrations of HClO4 have been obtained, and similar results were obtained with other acids e.g. HCl, HNO3, and H2SO4, and even with organic acids like oxalic acid. In general, the peak current decreases the increase in the acid concentration. The value of the peak current depends on the strength of the acid and the order of increase follows HNO3 > HClO4 > HCl > H2SO4 > Oxalic (cf. Fig. 10a). This behavior can be explained on the basis of the anion effect on the peak currents and the facility of the anion to reach the interface. Although the effect of anions and acid concentration is clear as presented in Fig. 10a, the peak current is much affected by the solution pH [37]. Below pH = 1.0 and at constant ionic strength a linear relation between log I paI and pH is recorded. By successive increase of solution pH, stability in the anodic peak current value was attained. In the linear part, the relation between log I paI and the pH can be formulated mathematically as
The linear relation is presented in Fig. 10b and values of the constants a and b were calculated and found to be a = 2.41 which is equivalent to 260 μA for the peak current at pH = 0, and b = 0.0015, which represents the rate of increase of the peak current with the pH. It should be pointed out that the same holds for the cathodic peak currents which show an exponential relation to the hydrogen ion concentration. This behavior was recorded for different acids without regard to the nature of the acid, and the general trend is the increase in electroactive response as the pH of the electrolyte increases up to pH = 1.0. In neutral solutions, the polymer film loses its electrochemical activity, which means that the presence of protons in the solution is essential for the redox reaction to occur. It should be mentioned that the polymer film remains unchanged without any loss in electrochemical activity after the 10th cycle, whatever the number of potential cycles (cf. Fig. 8). The stability of the polymer film was found to depend on the strength and concentration of the acid. The presence of a strong acid retards the decomposition of the fully oxidized polymer film by shifting the equilibrium of water protonation towards the hydroxonium (H3O+) ion [37, 45].
3.4 Solubility and molar mass determination
The film is very stable since it does not lose its response even after months of exposure to air or after repetitive cycling on solvent-supporting electrolyte solution at sweep rates between 10 mV s−1 and 1.0 V s−1. In addition, immersion in common organic solvents such as ethanol, acetone, chloroform, ethyl acetate, acetonitrile and dimethyl sulfoxide do not affect the film. These facts, which indicate a stiff structure and probably a high molar mass, prevent any study of molar mass or spectroscopy in solution [39]. Similar to many other conducting polymers, the PNED films are insoluble in common solvents, therefore their exact chemical structure has never been determined up to now.
4 IR spectroscopy of NED and PNED
The IR absorption spectrum of a KBr pellet of the monomer, NED, and the polymer, PNED, film were recorded and are presented in Fig. 11. The spectral data are summarized in Table 1. A broad band between 3,200 and 3,700 cm−1 is due to N–H stretching of the amine or imino groups of the PNED film, whereas two absorption peaks corresponding to the N–H stretching vibrations of the amino groups of the NED were recorded at ∼3,390 and 3,456 cm−1 [46]. This fact suggests that the –NH2 groups are involved in the electropolymerization process. The peaks observed at ∼1,635 and 1,624 cm−1 for both the polymer and the monomer, respectively, are attributed to the bending vibration of the N–H bond. The C–N stretching bands, which are characteristic of an aromatic amine, were observed between ∼1,323 and 1,225 cm−1. The absorption peak recorded at about ∼1,450 cm−1 can be ascribed to the C=C stretching vibration [47]. The observed band in the region of ∼1,083 cm−1 is due to the ClO −4 vibrational band.
The absorption peak corresponding to the stretching vibrations of the C=N bonds was recorded at ∼1,605 cm−1. Analysis of the absorption peak pattern between ∼991 and 628 cm−1, which arises from the C–H out-of-plane bending modes, enables us to assign the substitution pattern of the aromatic ring [40, 47, 48].
5 Mechanism of the electropolymerization process
The mechanism of polymerization of NED is based on the consideration that the oxidation of a conducting polymer is easier than the monomer itself, and the oxidation occurs at the secondary amine group of the naphthylamine molecule as the chain end [49]. In the actual reaction, chain growth and electron transfer may not be distinguished separately. From the electrochemical data, NED molecules oxidize at ∼+0.56 V, and during the anodic polymerization the species being oxidized must not be the end of the polymer but the solution species, i.e. the monomer molecule or its oligomers. The polymerization has to take place in the solution phase between two oxidized NEDs by, for instance, successive dimerization:
A molecular mass of the polymer can be achieved if successive dimerization e.g. four times occurs, and the resultant product will precipitate on the electrode surface. If this mechanism is actually operating, the anion doping should be quite restricted and the electrical conductivity will remain very low, in agreement with the experiments described above. Accordingly, the radical cation, NED•+, formed in the first charge transfer steps presented in Fig. 12 follows the pathways indicated in the figure. In such a mechanism the aromatic amine cation radicals, NED•+, may dimerize by either C–N coupling (III) or C–C coupling (VI). The most probable coupling mechanism is the C–N coupling, because of the steric hindrance of the C–C coupling [19, 37, 38], and hence dimer IV will represent the major product.
These dimers oxidize to the corresponding dications at lower potentials than the monomer itself. The polymer film obtained is, most likely, a linear chain structure similar to poly (aniline) and poly (naphthylamine) [19, 37, 49, 50]. The electropolymerization mechanism proceeds via successive head-to-tail coupling of oxidized monomers with its oligomeric radical cations. As shown in Fig. 12, the oxidation of the monomer (I) produces the radical cation (II) which dimerize to the dimer cation (III) that gives the structure (IV) by mesomeric effect. The NED radical cation can adopt a coplanar conformation that facilitates a strong electronic interaction between the two-phenyl rings, to stabilize the radical [51]. The radical cations (II) and (III) are quite reactive and undergo head-to-tail coupling to give the dimer (IV), which is subsequently oxidized and react with other monomer radical cations to produce a polymer with a structure (V). The final structure of the polymer film is, most likely, similar to that of poly (aniline) or poly (p-phenylene). The mechanism proposed in Fig. 12 is consistent with the E(CE)n reaction revealed from the results of the cyclic voltammetry and confirmed by the IR spectra [37, 52].
6 Applications of the PNED modified electrode
Electrode modification has been widely employed to optimize the performance of many electrochemical processes [53, 54]. Chemically modified electrodes in particular have been used to facilitate electron transfer reactions [55] and the preferential accumulation of analytes at electrode surfaces [56].
PNED modified electrodes are capable of decreasing the overpotential and/or improving the reversibility of the redox processes of several natural products like vitamin C (ascorbic acid), benzoic acid, boric acid, citric acid, oxalic acid, sodium benzoate, sodium citrate, sodium oxalate, tartaric acid, and also for several amino acids such as l-glycine, β-alanine, l-lysine, l-cystine, l-lucien and glutamic acid. The decrease of the overpotential of the oxidation processes means that there is a decrease in the background currents and limited interference from other dissolved species, which is reflected in higher selectivity and better performance. The improvement in electrochemical reversibility is reflected in sharper current response peaks and prevention of electrode fouling from oxidation products i.e. increased stability. Both of these factors are important in obtaining lower detection limits in electroanalytical applications.
The optimized PNED modified electrodes were used as working electrodes for testing different redox reactions of technological relevance. Diagnostic cyclic voltammetry was then carried out using solutions of 10.0 mM of each of the tested compounds in 1.0 M HClO4 at a scan rate 100 mV s−1, and in the potential range between +0.2 and +0.7 V vs. Ag/AgCl at these modified surfaces. A comparison of the behavior of PNED modified electrodes with that obtained by bare glassy carbon electrodes was made and the oxidation potentials and the anodic peak current values are presented in Table 2.
Figure 13 presents typical cyclic voltammograms of the electrochemical oxidation of 10.0 mM citric acid on both the bare GC and the modified GC-PNED electrode under the above described conditions. It is clear that the modified electrode has a very clear response to the oxidation of citric acid compared to the bare GC electrode, and a definite anodic peak current value of 143.0 μA was recorded. Beside the remarkable decrease in the overpotential of the oxidation process, which is reflected in the lower value of the peak potential, E pa, there is a large increase in the peak current corresponding to the oxidation reaction.
The PNED modified electrode, was found to be sensitive for any variation of the concentration of the species to be oxidized. A typical example of the sensitivity of the modified electrode towards variation of the ascorbic acid concentration compared to bare GC electrode is presented in Table 3. The anodic peak currents were measured in solutions of concentrations from 0.0 mM to 10.0 mM of ascorbic acid in a background solution of 0.1 M sodium citrate/0.1 M NaH2PO4 at a scan rate 100 mV s−1 in the potential range between +0.2 and +0.7 V. These are presented in Fig. 14. The linear relation of this figure can be considered as a calibration curve for the determination of unknown ascorbic acid concentration in the specified range.
7 Conclusions
-
Stable, electroactive conducting PNED films are produced on GC electrodes from a solution of 25.0 mM NED dissolved in 1.0 M HClO4/EtOH (9:1 volume ratio) mixed solvent by potentiodynamic polymerization at a scan rate of 100 mV s−1 in a scan range between −1.0 and +1.2 V.
-
The prepared modified electrodes are very stable in aqueous acidic solution and show electroactive response in the potential range between +0.2 and +0.7 V.
-
PNED films electrogenerated under stagnant conditions give the best electrochemical response.
-
The PNED films show clear electrochromic properties for promising optical applications.
-
The polymerization process obeys an E(CE)n mechanism in which C–N coupling of the cation radicals is the main process.
-
PNED is capable of decreasing the overpotential and/or improving the reversibility of the redox processes for several natural products and several amino acids, e.g. ascorbic acid.
References
Liu L, Zhao C, Zhao Y, Jia N, Zhou Q, Yan M, Jiang Z (2005) Eur Polym J 41:2117
Geetha S, Trivedi DC (2005) Synth Met 155:232
E
Barrios EM, Mujica GA, Velasquez CL, Martinez Y (2006) J Electroanal Chem 586:128
Diaz A, Logan JA (1980) J Electroanal Chem 111:111
Kitani A, Yano J, Kunai A, Saski K (1987) J Electroanal Chem 221:69
Heinze J (1990) In: Topics in current chemistry, vol 152. Springer-Verlag, Berlin
Lee MH, Luo YC, Do JS (2005) J Power Sources 146:340
Garnier F, Tourillon G, Gazaud M, Dubois JE (1983) J Electroanal Chem 148:299
Kobayashi T, Yoneyama H, Tamura H (1984) J Electroanal Chem 161:419
Byker HJ (2001) Electrochim Acta 46:2015
Gerard M, Chaubey A, Malhotra BD (2002) Biosens Bioelect 17:345
Bereket G, Hur E, Sahin Y (2005) Appl Surf Sci 252:1233
Huang WS, Humphery BD, MacDiarmid AG (1986) J Chem Soc, Faraday Trans 1 82:2385
Yano J, Kitani A, Vasquez R, Sasaki K (1985) Nippon Kagaku Kaishi 1124
Pekmez N, Pekmez K, Yildiz A (1993) J Electroanal Chem 348:389
Chan HSO, Ng SC, Leong LS, Tan KL (1995) Synth Met 68:199
Skotheim TA (ed) (1998) Handbook of conducting polymers, vols 1–2. Marcel Dekker, New York
Arevalo AH, Fernandez H, Silber JJ, Sereno L (1990) Electrochim Acta 35:741
Moon DK, Osakada K, Maruyana T, Kubota K, Yamamoto T (1993) Macromolecules 26:6992
Ohsaka T, Ohba M, Sata M, Oyama N (1991) J Electroanal Chem 300:51
Schmitz BK, Euler WB (1995) J Electroanal Chem 399:47
Chung CY, Gopalan A, Wen TC (2001) Mater Chem Phys 71:148
Xu YJ, Xie QJ, Hu MQ, Nie LH, Yao SZ (1995) J Electroanal Chem 389:85
Meneguzzi A, Pham MC, Ferreira CA, Lacroiz JC, Aeiyach S, Lacaze PC (1999) Synth Met 102:1390
Meneguzzi A, Ferreira CA, Pham MC, Delamar M, Lacaze PC (1999) Electrochim Acta 44:2149
Huang SS, Lin HG, Yu RQ (1992) Anal Chim Acta 262:331
D
Stilwell DE, Park SM (1988) J Electrochem Soc 135:2497
Wei Y, Jang GW, Chan CC, Hsueh KF, Hariharan R, Patel SA, Whitecar CK (1990) J Phys Chem 94:7716
Wei Y, Hariharan R, Patel SA (1990) Macromolecules 23:785
Lee JY, Cui CQ (1996) J Electroanal Chem 403:109
Kilmartin PA, Wright GA (1997) Synth Met 88:153
Roy BC, Gupta MD, Bhowmik L, Ray JK (1999) Synth Met 100:233
Ismail KM, Khalifa ZM, Abdel-Azzem M, Badawy WA (2002) Electrochem Acta 47:1867
Badawy WA, Ismail KM, Medany SS (2006) Electrochim Acta 51:6353
Badawy WA, Ismail KM, Khalifa ZM Electrochim Acta (submitted)
Vettorazzi N, Silber JJ, Sereno L (1981) J Electroanal Chem 125:459
Barbero C, Silber JJ, Sereno L (1989) J Electroanal Chem 263:333
Oyama N, Ohsaka T, Shimizu T (1985) Anal Chem 57:1526
Wei Y, Sun Y, Tang X (1989) J Phys Chem 93:4878
Berdas JL (1986) In: Skotheim TA (ed) Handbook of conducting polymers. Marcel Dekker, New York, p 859
Genies EM, Lapkowski M (1988) Synth Met 24:61
Komura T, Yokono Y, Yamaguchi T, Takahashi K (1999) J Electroanal Chem 478:9
Nguyen MT, Dao LH (1990) J Electroanal Chem 289:37
Chiba K, Ohsaka T, Ohnuki Y, Oyama N (1987) J Electroanal Chem 219:117
Dolphin D, Wick A (1977) Tabulation of infrared spectral data. Wiley, New York
Ohnuki Y, Matsuda H, Oyama N (1984) Nippon Kagaku Kaishi 1801
Diaz AF, Crowley J, Bargon J, Gardini GP, Torrance JB (1981) J Electroanal Chem 121:355
Hand RL, Nelson RF (1974) J Am Chem Soc 96:850
Genies EM, Tsintavis C (1985) J Electroanal Chem 195:109
Hertl P, Rieker A, Speiser B (1986) J Electroanal Chem 2000:147
Baldwin RP, Thomsen KN (1991) Talanta 38:1
Wang J (1993) Anal Chem 65:450R
Wantanabe M, Nagasaka H, Ogata N (1995) J Phys Chem 32:12294
Khoo SB, Cai QT (1996) Electroanalysis 6:549
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10800-007-9340-2
Rights and permissions
About this article
Cite this article
Badawy, W.A., Ismail, K.M. & Khalifa, Z.M. Conducting poly(N-(1-Naphthyl) ethylene-diamine dihydrochloride) electropolymerization, characterization and electroanalytical applications. J Appl Electrochem 37, 593–604 (2007). https://doi.org/10.1007/s10800-007-9290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9290-8