Abstract
Purpose
Intravenous glucocorticoids (GCs) are the mainstay of treatment for severe forms of Graves’ orbitopathy (GO). Our aim was to assess the effectiveness and safety of a modified monthly regimen (mMR) and to compare them with those of the established weekly regimen (WR).
Methods
This was a prospective non-randomized single-center study involving 62 patients, divided into two therapeutic groups depending on their referral time. Thirty-one subjects, admitted in the period 2017–2018, were treated with mMR, total dose—5.5 g, with intake of oral GCs after completion of intravenous infusions. Thirty subjects, who were referred in the period 2019–2020, were treated with WR, total dose—4.5 g One patient refused to be part of the WR group and was treated with mMR. Eye status and therapeutic response were evaluated on the 1st, 3rd and 6th months, quality of life—at 3rd and 6th month.
Results
At 1st month and 3rd month, there was no significant difference in the therapeutic response between the two groups. At 3rd month, the proportion of patients with improvement in soft tissue manifestations and subjective complaints was significantly higher in mMR group (65.6% vs. 40% and 81.3% vs. 46.7%, respectively) and the same manifestations were of significantly milder degree.
At 3rd month, significant improvement in quality of life was found without significant difference between the two groups.
At 6th month, worsening of GO occurred in 3 patients from WR group, while in 5 patients from mMR group further improvement was found.
Conclusions
The two GC regimens have comparable efficacy with small differences in the time of onset of the effect and its duration, as well as in the effectiveness on some ocular manifestations.
Trial registration number NCT05793359/29.03.2023, retrospectively registered..
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intravenous glucocorticoids (GCs) have been used for decades in the treatment of the severe forms of Graves’ orbitopathy (GO). Geest et al. carried out the first prospective randomized double-blinded placebo-controlled study to evaluate the effectiveness of pulse therapy with methylprednisolone (MP) [1]. Despite the small number of patients (15 patients), the results clearly showed high effectiveness of intravenous GCs for the treatment of active moderate-to-severe GO in the absence of serious side effects. Macchia et al.[2] were the first research team to directly compare the effectiveness of intravenous and oral GCs. They observed a significantly better effect of intravenous GCs (84% vs. 57%, p < 0.01), as well as a lower incidence of adverse reactions compared to oral GCs. Subsequent studies confirmed the better efficacy and safety of the intravenous administration compared to oral [3,4,5,6].
Nowadays, intravenous GCs remain the mainstay of treatment for active moderate-to-severe GO [7]. The consensus of the European Thyroid Association (ETA) and European Group on Graves’ Orbitopathy (EUGOGO) from 2016 recommends that GC treatment in moderate-to-severe form of GO should be administered as follows: 500 mg MP as intravenous infusion per week for 6 weeks, followed by 250 mg MP per week for 6 weeks with a total cumulative dose 4.5 g with a proven good benefit/risk ratio (weekly regimen, WR) [8]. The effectiveness of this regimen, assessed in various studies, is between 35 and 80%, and regarding CAS reduction 64–83% [3]. The current consensus of ETA and EUGOGO recommends usage of weekly infusions of MP with 50% reduction of the dose in the second half of the treatment course and total cumulative dose of 4.5 g or 7.5 g combined or not with Mycophenolate [7]. However, in some contries different GC schemes are still used in accord with National Health System requirements and standards.
There are few comparative studies evaluating the effectiveness of different intravenous GC regimens for the treatment of GO [9,10,11,12,13,14]. A large multicenter randomized clinical trial involving 159 patients with active moderate-to-severe GO examined the efficacy and tolerability of three different cumulative doses—7.47, 4.98 and 2.25 g MP with the same duration of the GC course [9]. It was found that the short-term effectiveness of treatment is positively correlated with the applied cumulative dose (52%, 35% and 28% for the high-, moderate- and low-dose regimens, respectively). The long-term efficacy of moderate-dose and high-dose regimens was equivalent, but with fewer side effects for moderate doses. Zhu et al. conducted a prospective randomized study comparing the effect of two GC regimens—WR and daily regimen (DR) with total cumulative dose of 4.5 g MP [12]. At 1st month, the authors found no difference between the two regimens in terms of efficacy, but at 3rd month, WR proved to be significantly more effective and with a better safety profile. It should be underlined that in this study, despite the randomization, there was a selection bias—patients on the WR had longer duration of GO and lower levels of TRAb compared to patients on the DR, which might have affected the therapeutic effectiveness of the applied GC regimens. In contrast to the previous study, Mu et al. observed similar efficacy of DR and WR, but with better tolerability for WR. In this study, the duration of DR and its total cumulative dose were similar to that of WR and the baseline characteristics of the patients from the two regimens groups were comparable. Roy et al. carried out a study comparing the effectiveness and safety of a monthly regimen (MR) with total cumulative dose of 6 g and a low-dose peroral prednisolone treatment [14]. MR was superior to peroral GCs in terms of the overall response at 6th month (80.6% vs. 38.7%, p < 0.01) and 12th month (87.1% vs. 54.8%, p < 0.01), but with comparable safety profile. One limitation of this study is the selection bias—at baseline patients from the MR group had significantly more severe diplopia and lower TSH. In addition, judging by the average baseline values of TSH, the majority of the enrolled patients had uncontrolled hyperthyroidism, which definitely influenced the results. In a recent retrospective observational study of 140 patients with GO, Serbian authors compared the efficacy and safety of WR and combined (intravenous and peroral) GC regimen [10]. They found that the efficiency of WR was 51% and that of the combined regimen—65%, p = 0.07. However, in the group on WR, the frequency of side effects was significantly lower (38% vs. 74%, p < 0.001), and exacerbations of ocular manifestations were significantly more frequent (26% vs. 0%, p < 0.001). Some of the weaknesses of this study are its retrospective designs, the lack of randomization and the substantially higher cumulative dose of the combined GC regimen compared to WR (10.2 g vs. 4.5 g). A recent meta-analysis by Jia et al. [15] including 10 studies and 593 patients, concluded that MR and WR are a better therapeutic option regarding the overall response, proptosis, and adverse events compared to oral GCs and DR.
Cases of severe cardiovascular and hepatic impairment following intravenous GC treatment of GO have been reported in the literature [16,17,18,19,20,21,22,23]. A meta-analysis of 14 studies (over 1000 patients with GO) found that morbidity and mortality from GC treatment were 6.5% and 0.6%, respectively [24]. In order to minimize the risk of adverse reactions during the GC course, it is accepted that the single daily dose should not exceed 750 mg MP, the cumulative dose MP should be less than 8 g, and the GC infusions should not be done on consecutive days [7]. Otherwise, the risk is doubled, including for severe cardiovascular, cerebrovascular and hepatic disorders [24].
The reported suboptimal efficacy of the WR and the recent changes in the recommended total cumulative dose demonstrate that the search for an optimal glucocorticoid regimen for treatment of severe GO is still ongoing and more studies on this topic are needed. On the other hand, in some countries, including Bulgaria, weekly hospital admissions or intravenous administration of high doses glucocorticoids in outpatient settings are not possible, due to the National Health System standards. That is why in the Endocrinology facilities in Bulgaria a modified MR (mMR) has been applied for the last decades as a standard of care. The aim of the present study was to evaluate the effectiveness and safety of the mMR and to compare them with those of the established WR for treatment of moderate-to-severe GO.
Experimental
Patient selection
During the period 2017–2020 in our tertiary care inpatient clinical center 221 patients with GO with different severity and activity were admitted. The diagnosis of GO was made on the basis of the typical clinical manifestations (soft tissue involvement, proptosis, motility deficits, diplopia and subjective complaints) in the setting of Graves’ disease (GD), autoimunne hypothyroidism or without overt thyroid dysfunction (euthyroid GO). Additionally, TRAb were measured in all patients by radioreceptor assay (Thermofisher, Brahms Diagnostic, Germany) together with measurement of TSH, fT4, thyroid-peroxidase antibodies, anti-thyreoglobulin antibodies and routine biochemical tests. In selected cases (hypothyroidism, euthyroidism, unilateral form, asymmetrical bilateral form) a computed tomography scan was performed to confirm the diagnosis of GO. All GO patients underwent comprehensive ophthalmological examination. Proptosis was evaluated using Hertels’ exophthalmometer and presented in mm, visual acuity was assessed by Snellen chart and presented in decimals, as well as in LogMAR units for statistical purposes, lid fissure was measured by ruler in mm. Soft tissue involvement was assessed using the color atlas proposed by EUGOGO and defined as gr. 0–1 no or mild and gr. 2–3 moderate and severe. Subjective symptoms were reported by the patients themselves using the same grading system as that of soft tissue involvement. Diplopia was evaluated separately using Gorman classification system: gr. 0—absent, gr. 1—intermittent, gr. 2—inconstant, gr. 3—constant. Intraocular pressure was measured by Goldmann applanation tonometer. Corneal staining was assessed with biomicroscope. In addition, color vision assessment, pupillary test, perimetry, fundus examination, visual evoked potentials and computed tomography imaging were performed in case of suspicion of dysthyroid optic neuropathy. Quality of life (QoL) was evaluated by a disease-specific questionnaire recommended by EUGOGO (GO-QoL), which consists of two scales assessing the changes in visual functioning and in appearance. The results were expressed as percentages, where 0% was the worst possible QoL and 100%—unimpaired QoL. GO severity and activity were defined in accord with the recommendations of ETA and EUGOGO [7].
Finally, the following selection criteria were applied: inclusion criteria: 1. Active moderate-to-severe GO, 2. No previous systemic GC treatment or orbital radiotherapy, 3. Euthyroid status under treatment; the exclusion criteria were: 1. Mild, inactive moderate-to-severe GO or sight-threatening GO, 2. Previous GC treatment or orbital radiotherapy, 3. Overt thyroid dysfunction, 4. Contraindications for optimal dose GC therapy.
After the above selection criteria were applied, 62 patients with active moderate-to-severe GO were enrolled in the study.
Therapeutic groups
Patients were divided into two therapeutic groups depending on their referral time. In the period 2017–2018, all patients meeting the selection criteria were included in mMR group, and in the subsequent period—in WR group. If a patient refused to be part of WR group, they were included in mMR group (1 patient).
The mMR group consisted of 32 patients, who received pulse GC treatment in the following scheme: 3 pulses consisting of 3 intravenous infusions of 500 mg MP on alternate days administered in three consecutive months. Between the first and second pulses, as well as between the second and third pulses, 4 weekly intramuscular applications of 125 mg MP were applied. The total cumulative dose of MP for a 3-month course of treatment was 5.5 g Additionally, these patients were prescribed oral prednisolone 15 mg every other day after the third pulse for a period of 3 months.
WR group involved 30 patients who received the GC regimen recommended by EUGOGO and ETA in their consensus from 2016—500 mg MP as intravenous infusion per week for 6 weeks, followed by 250 mg MP per week for 6 weeks [8]. The total cumulative dose for a 3-month course of treatment was 4.5 g.
Assessment of the therapeutic response
The therapeutic reponse was defined as full, partial and lack of response using modified Bartalena’s major and minor criteria. Major criteria were: change in proptosis by 2 or more mm, in lid fissure by 2 or more mm, in CAS by 2 or more points, in visual acuity by 1/10 or more, transition of diplopia from one grade to another. The minor criteria were: changes in soft tissue involvement or in patient's subjective assessment by at least one degree. Full responders had improvement of at least 2 major and 1 minor criteria; partial responders—improvement of 1 major or 2 minor criteria; and non-responders—lack of improvement or deterioration in any of the criteria.
Follow-up
Clinical and laboratory follow-up was performed at the end of the 1st, 3rd and 6th month. The therapeutic response was evaluated at 1st, 3rd and 6th month. TRAb and QoL were reassessed at 3rd and 6th month. Due to ethical concerns patients who needed an additional therapy were promptly referred to orbital radiotherapy after the end of the intravenous GC course (at 3rd month).
Ethical approval
Informed consent was obtained from all patients. The research protocol was approved by the Ethics Committee of University Hospital of Endocrinology, Sofia (№ 21A / 25.07.2017). All diagnostic and therapeutic procedures performed in this study were in accordance with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Statistical analysis
The results were analyzed using SPSS version 19 (IBM SPSS, v.19.0. Armonk, NY: IBM Corp.). First, descriptive analysis and the Shapiro–Wilk test for normality were performed. Continuous variables were presented as means and standard deviations or as medians with minimum and maximum values according to data distribution. Categorical variables were presented as count and proportion. When comparing two continuous variables, the Student's t-test or the non-parametric Mann–Whitney test were used depending on the distribution of the data. Categorical variables were tested by χ2 test. A p-value of less than 0.05 indicated statistical significance.
Results
Comparison of the baseline characteristics of the patients
When comparing the baseline characteristics of the patients from mMR and WR groups, we did not find a significant difference in terms of gender, age, smoking, duration of GD and GO (Table 1). Although all patients had active GO with CAS ≥ 3, CAS was significantly higher in patients from mMR group (5.3 vs. 4.5, p < 0.01). We also observed a statistically significant difference in the severity of diplopia between the two groups, with gr. 1 diplopia being more prevalent in WR group where the frequency of gr. 0 was lower, while the proportions of the more severe forms of diplopia were comparable. When comparing the other ophthalmological indicators, as well as QoL, we did not observe statistically significant differences.
Comparison of the efficiency of the two GC regimens
When comparing the responder-status of the two groups, we observed a better initial therapeutic response in WR group with smaller number of non-responders at 1st month, but without reaching statistical significance (p = 0.08) (Table 2). At 3rd month, the proportion of non-responders in WR group remained the same, while in mMR group it decreased substantially, but again there was no statistically significant difference (p = 0.14).
At 1st month of follow-up, we observed a higher proportion of patients with a significant increase in visual acuity in WR group (40% vs. 3.1%, p < 0.01) (Table 3). At 3rd month, the percentage of patients with improvement in soft tissue manifestations and subjective complaints was significantly higher in mMR group (65.6% vs. 40% and 81.3% vs. 46.7%, respectively).
When monitoring the changes in the individual ocular manifestations, we found a significantly lower CAS at 1st month in WR group (4.5 vs. 4.9), but without a significant difference between the two therapeutic groups in terms of CAS relative reduction (Table 4). We observed a significant difference in the severity of subjective complaints and soft tissue involvement—in patients from mMR group these manifestations were significantly milder at 3rd month.
Significant improvement in QoL was observed at 3rd month for both subscales of the questionnaire, and no significant difference was found between the two therapeutic groups.
No significant difference was found in the dynamics of TRAb during treatment.
Follow-up
After the end of the GC course, 5 patients from mMR group and 6 from WR group were referred for orbital radiotherapy. One patient from mMR group and 3 from WR group were lost from follow-up after 3rd month.
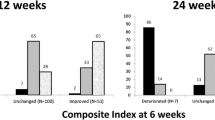
At 6th month, worsening of GO occured in 3 patients from WR group, while in 5 patients from mMR group there was an additional improvement. In the rest of the patients GO was stable.
Safety profile
During the GC treatment, a total of 30 patients experienced adverse reactions—17 from mMR group and 13 from WR group (p = 0.44). Table 5 presents the frequency of side effects in the two therapeutic groups. In both groups the most commonly observed side effects were increase in blood pressure, palpitations and sleep disturbances, mainly on the day after the intravenous infusion of MP (n.s.). Elevated intraocular pressure (p = 0.055) and increased blood sugar (p = 0.27) were more common in patients in mMR group, without reaching significance though. In one of the patients from WR group, significantly elevated liver enzymes were measured 2 weeks after the end of the GC treatment. After further tests and consultation with a gastroenterologist, the diagnosis of autoimmune hepatitis was established. Mildly elevated ALT and GGT (< threefold above the upper limit) were measured in 3 of patients in mMR group, requiring intake of a hepatoprotector. One of the patients (female, 40 years old, without risk factors for osteoporosis) from WR group suffered a tibial fracture as a result of a fall from her own height 1 week after the end of treatment. One patient in mMR group developed a gluteal abscess, most likely due to the intramuscular injections of MP administered between the intravenous infusions. Her treatment included surgical incision and drainage in combination with antibiotic therapy. Two patients from the WR group developed bacterial pneumonia within 1–2 months after discontinuation of intravenous infusions, requiring antibiotic treatment at home.
The mean increase in body weight and body mass index during the GC treatment was 1.9 kg and 0.7 kg/m2 for mMR group, and 2.9 kg and 1.3 kg/m2 for WR group (p = 0.38 and p = 0.35, respectively).
Discussion
When comparing the therapeutic response in patients treated with mMR and WR assessed at the beginning and at the end of the treatment, we found comparable results. He et al. also compared MR and WR and did not find significant difference in the overall efficacy of the two regimens (76.5% for MR and 66.7% for WR, n.s.), either [25]. However, the MR applied in their study consisted of 4 pulses of 3 infusions of 500 mg MP each, administered at 4-week intervals, without use of intramuscular or peroral GCs between and after the pulses, which makes it different from our mMR. We observed a different pattern of change of the therapeutic response, namely: in patients from WR group the effect of treatment was evident early in the GC course being better than in mMR group (p = 0.08), but did not undergo further substantial improvement. It appears that the lower doses of MP used in the second half of the treatment course may not be sufficient to elicit a response in patients who are non-responders at 1st month. On the other hand, in mMR group the response to GC therapy improved steadily during the course of the treatment, resulting in a significant reduction in the proportion of non-responders at the 3rd month compared to the 1st month. The later findings showed that in the mMR group a significant proportion of patients who lacked an early response experienced improvement of ocular manifestations at the end of the treatment course. In accord with this observation, Bartalena et al. found that patients who lack early therapeutic response still had a 30% chance of later improvement in GO, a 60% chance of GO entering an inactive phase, and more than a 30% chance to improve the QoL [26]. The recent consensus recommends that in case of deterioration or lack of response at 6th week, first-line treatment should be discontinued and appropriate second-line therapy should be considered [7]. However, our study followed the consensus of ETA and EUGOGO from 2016, according to which GC treatment is continued under strict follow-up, and should be discontinued only in case of adverse reactions outweighing the benefits of treatment [8]. We did not observe such drug reactions, and none of our patients experienced deterioration in GO during the treatment course.
When comparing the changes in the individual ocular manifestation, we found that a higher number of patients from the mMR group had improvement in soft tissue involvement and subjective complaints (65.6% vs. 40%, p = 0.04 and 81.3% vs. 46.7%, p < 0.01, respectively) and these manifestations were significantly milder in this group at the end of the treatment. The small differences in the efficacy of the two GC regimens could be due to differences in the baseline characteristics of the two groups and/or due to the GC regimens themselves. At baseline, patients in mMR group had a significantly higher CAS (5.3 vs. 4.5, p < 0.01), and although this significant difference persisted at 1st month, there was not a statistical difference in CAS relative reduction at 1st and 3rd month, moreover, at 3rd month CAS did not differ between the two groups. There were no significant differences in baseline levels and dynamics of TRAb between the two treatment groups, either. Accordingly, the different effect of the two GC regimens is more likely to be due to the GC schemes themselves. In mMR the higher total cumulative dose may be the reason for the better effect on subjective symptoms and soft tissue manifestations. The superior effect of the higher doses on the therapeutic response and improvement of eye symptoms was reported in earlier studies as well [9, 27]
At the end of the GC course, we found a significant improvement in the QoL on both scales in both groups. The same was observed by Hoppe et al. [28] among German patients with GO. Patient satisfaction with the effectiveness of the two GC regimens seemed comparable, given the lack of a significant difference in QoL-visual functioning and QoL-appearance between the two groups.
The lower total cumulative dose and the administration of a lower single dose of MP in the second half of the treatment course are possible explanations for the observed deterioration of GO in 3 of the patients from WR group during follow-up. On the other hand, the additional significant improvement in GO registered in 5 of the patients from mMR group may be due to the higher total dose of GC or the subsequent peroral GC treatment after 3rd month. According to Bartalena et al. this is one of the possible ways to reduce the risk of deterioration of GO after abrupt cessation of intravenous GCs [9]. In addition, Tsirouki et al. reported that low-dose oral GC treatment after GC intravenous infusions was associated with better overall therapeutic response, greater CAS reduction and lower rate of exacerbations of GO [29].
Although adverse reactions were observed in approximately half of the patients of the two groups, the vast majority of them were mild, occurring in the days immediately following the intravenous infusion of MP and spontaneously disappearing or easily treatable. Three of our patients had more serious side effects—hepatotoxicity, bone fracture, severe local infection. Despite the differences in the total doses and the schemes of administration of MP, we did not find significant differences in the safety profile of the two GC regimens.
Limitations
First, the relatively small number of patients included might result in inability to establish existing patterns. Second, the inclusion of patients in the therapeutic groups was based on the time period and was not randomized. However, the choice of the respective GC regimen was not influenced by the clinical manifestations of GD or GO. In addition, the inclusion of patients in this way reduced the impact that the lack of blind assessment of the therapeutic response by the endocrinologist and ophthalmologist might have had on the results. In our study, follow-up of patients after completion of the GC course was limited by the fact that, for ethical reasons, at 3rd month patients requiring additional therapy were referred for orbital radiotherapy. Therefore, we considered it appropriate at 6th month to report only the presence of improvement/deterioration/steady state of ocular manifestations.
Conclusion
The two GC regimens have similar efficiency and safety profile with small differences in the therapeutic response and its duration, and in their effect on individual ocular manifestations. This findings imply that they could be used interchangeably in routine clinical practice.
Data availability
The data that support the findings of this study are available from the corresponding author, M.S., upon reasonable request.
References
van Geest RJ, Sasim IV, Koppeschaar HPF, Kalmann R, Stravers SN, Bijlsma WR, Mourits MP (2008) Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol 158:229–237. https://doi.org/10.1530/EJE-07-0558
Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G (2001) High-dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 24:152–158. https://doi.org/10.1007/BF03343835
Kahaly GJ, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240. https://doi.org/10.1210/jc.2005-0148
Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, Barbesino G, Mazzi B, Bartolomei MP, Lepri P (2001) Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves ’ ophthalmopathy : results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 86:3562–3567. https://doi.org/10.1210/jcem.86.8.7737
Aktaran Ş, Akarsu E, Erbağci İ, Araz M, Okumuş S, Kartal M (2006) Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract 61:45–51. https://doi.org/10.1111/j.1742-1241.2006.01004.x
Akarsu E, Buyukhatipoglu H, Aktaran Ş, Kurtul N (2011) Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin Endocrinol 74:118–124. https://doi.org/10.1111/j.1365-2265.2010.03904.x
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, Marinò M, Vaidya B, Wiersinga WM (2021) The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol 185:G43-67. https://doi.org/10.1530/EJE-21-0479
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM (2016) The 2016 European thyroid association/European group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 55:9–269. https://doi.org/10.1159/000443828
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G et al (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97:4454–4463. https://doi.org/10.1210/jc.2012-2389
Nedeljkovic Beleslin B, Ciric J, Stojkovic M, Savic S, Lalic T, Stojanovic M, Miletic M, Knezevic M, Stankovic B (2020) Comparison of efficacy and safety of parenteral versus parenteral and oral glucocorticoid therapy in Graves’ orbitopathy. Int J Clin Pract 74:0–2. https://doi.org/10.1111/ijcp.13608
Sánchez-Ortiga R, Moreno-Pérez Ó, González Sánchez V, Arias Mendoza N, Mauri Dot M, Alfayate Guerra R, López Macia A, Picó Alfonso A (2009) Treatment of Graves’ ophthalmopathy with high-dose intravenous methylprednisolone: a comparison of two dosing regimens. Endocrinol Y Nutr 56(3):118–122. https://doi.org/10.1016/S1575-0922(09)70841-1
Zhu W, Ye L, Shen L, Jiao Q, Huang F, Han R, Zhang X, Wang S, Wang W, Ning G (2014) A prospective, randomized trial of intravenous glucocorticoids therapy with different protocols for patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 99:1999–2007. https://doi.org/10.1210/jc.2013-3919
Mu P-W, Tang X-X, Wang Y-N, Lin S, Wang MM, Yin QL, Shu J, Zhu BL, Li JR, Zhou L (2020) Comparison of two regimens for patients with thyroid-associated ophthalmopathy receiving intravenous methyl prednisolone: a single center prospective randomized trial. Exp Ther Med 20:1–9. https://doi.org/10.3892/etm.2020.9282
Roy A, Dutta D, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S (2015) Efficacy and safety of low dose oral prednisolone as compared to pulse intravenous methylprednisolone in managing moderate severe Graves’ orbitopathy: a randomized controlled trial. Indian J Endocrinol Metab 19:351–358. https://doi.org/10.4103/2230-8210.152770
Jia J, Dong J, Deng L (2022) Network meta-analysis of different intravenous glucocorticoid regimes for the treatment of Graves’ orbitopathy. Front Pharmacol 13:1–9. https://doi.org/10.3389/fphar.2022.785757
Lendorf ME, Rasmussen ÅK, Fledelius HC, Feldt-Rasmussen U (2009) Cardiovascular and cerebrovascular events in temporal relationship to intravenous glucocorticoid pulse therapy in patients with severe endocrine ophthalmopathy. Thyroid 19:1431–1432. https://doi.org/10.1089/thy.2009.0069
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10:521. https://doi.org/10.1089/thy.2000.10.521
Marinól M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14:403–406. https://doi.org/10.1089/105072504774193276
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S, Beck-Peccoz P (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14:631–634. https://doi.org/10.1089/1050725041692927
Marinò M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, Campani D, Vitti P, Bartalena L (2005) Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves’ ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest 28:280–284. https://doi.org/10.1007/BF03345386
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17:357–362. https://doi.org/10.1089/thy.2006.0267
Gursoy A, Cesur M, Erdogan MF, Çorapcioglu D, Kamel N (2006) New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with Graves’ ophthalmopathy. Endocrine 29:513–516. https://doi.org/10.1385/ENDO:29:3:513
Owecki M, Sowiński J (2006) Acute myocardial infarction during high-dose methylprednisolone therapy for Graves’ ophthalmopathy. Pharm World Sci 28:73–75. https://doi.org/10.1007/s11096-006-9013-y
Zang S, Ponto KA, Kahaly GJ (2011) Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96:320–332. https://doi.org/10.1210/jc.2010-1962
He Y, Mu K, Liu R, Zhang J, Xiang N (2017) Comparison of two different regimens of intravenous methylprednisolone for patients with moderate to severe and active Graves’ ophthalmopathy: a prospective, randomized controlled trial. Endocr J 64:141–149. https://doi.org/10.1507/endocrj.EJ16-0083
Bartalena L, Veronesi G, Krassas GE, Wiersinga WM, Marcocci C, Marinò M, Salvi M, Daumerie C, Bournaud C, Stahl M et al (2017) Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves’ orbitopathy? J Endocrinol Invest 40:547–553. https://doi.org/10.1007/s40618-017-0608-z
Zangi S, Ponto KA, Pitz S, Kahaly GJ (2011) Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 34:876–880. https://doi.org/10.1007/BF03346732
Hoppe E, Lee ACH, Hoppe D, Kahaly GJ (2021) Predictive factors for changes in quality of life after steroid treatment for active moderate-to-severe Graves’ orbitopathy: a prospective trial. Eur Thyroid J 9:313–320. https://doi.org/10.1159/000508071
Tsirouki T, Bargiota A, Tigas S, Vasileiou A, Kapsalaki E, Giotaki Z, Asproudis I, Tsatsoulis A, Koukoulis G, Tsironi EE (2016) Clinical and imaging evaluation of the response to intravenous steroids in patients with Graves’ orbitopathy and analysis on who requires additional therapy. Clin Opthalmol 10:2277–2289. https://doi.org/10.2147/OPTH.S118555
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation was performed by MAS, IDD, IAY whereas data collection—by all authors. The first draft of the manuscript was written by MAS and all authors commented on previous versions of the manuscript. All authors read approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study protocol was approved by the Institutional Ethical Committee (approval number 21A/25.07.2017) and was in accordance with the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All patients included in the study signed an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stoynova, M.A., Shinkov, A.D., Dimitrova, I.D. et al. Comparison of the efficacy of two different glucocorticoid regimens for treatment of active moderate-to-severe Graves’ orbitopathy. Int Ophthalmol 43, 4747–4757 (2023). https://doi.org/10.1007/s10792-023-02875-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02875-z