Abstract
Purpose
To investigate the quantitative differences in optical coherence tomography angiography (OCTA) data between type 2 diabetes patients without clinically detectable diabetic retinopathy (DR) and healthy subjects.
Methods
Thirty-nine patients with type 2 diabetes without DR and 41 age- and sex-matched healthy controls were recruited. The vessel density and foveal avascular zone (FAZ) area in the superficial capillary plexus and deep capillary plexus were measured using Nidek RS-3000 Advance® and compared between patient cohorts. Foveal vessel density (%) and FAZ (%) were also calculated.
Results
A significant decrease in vessel density has been observed in the deep capillary plexus of the patients compared to healthy individuals (5.58 ± 0.98 mm2 versus 6.15 ± 0.89 mm2, p < 0.001). However, there were no significant differences in other parameters between cohorts (p > 0.05 in all parameters). Despite the decrease of deep capillary plexus density in the macular region, there was no significant change observed in foveal vessel density (p:0.44). It has also been observed that the duration of diabetes mellitus correlates with vessel density decrease in deep capillary plexus (R:–0.52; p < 0.001). In both groups, all parameters in deep capillary plexus were significantly higher than superficial capillary plexus (p < 0.001 for all parameters).
Conclusions
OCTA can identify quantitative changes in DCP before the manifestation of clinically apparent retinopathy. DCP-VD reduction may be an earlier finding than FAZ enlargement. Despite the reduction of VD, FVD could be preserved for a certain period of time in DM patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the most important epidemic diseases of the twenty-first century which is assumed to affect more than 439 million individuals in 2025 in the world [1]. This disease is an important public health problem due to microvascular and macrovascular complications. One of the complications in the vascular structure of the retina is development of diabetic retinopathy (DR), which occurs in more than one-third of DM cases [2]. Diabetic macular edema (DME) is the most common sight-threatening complication of DR [1]. During the progression from DM to DR, disruption of retinal microcirculation and formation of microaneurysm leads to the development of clinically detectable DR.[3]Due to severe ocular complications, some experimental studies have been performed to detect predictive signs before the appearance of clinical DR findings in DM cases. However, those methods remained experimental methods that are not useful in clinical practice [4].
Optical coherence tomography angiography (OCTA), which can monitor retinal microcirculation with a noninvasive method, has made a breakthrough in this field. The basic working principle is based on the processing of decorrelations resulting from erythrocyte movement between consecutive B-Scan retinal images obtained from the same section [5]. Thus, it became possible to detect microvascular changes in superficial and deep capillary plexus (SCP and DCP) in the retina.
OCT devices provide quantitative data about the parameters of foveal avascular zone (FAZ) and vascular density (VD) of both capillary plexuses with built-in software [6]. Various OCTA systems can use different algorithms to measure retinal vascular system such as intensity signal-based (speckle and phase variance) or amplitude-decorrelation (split spectrum angiography). Algorithms that are used, segmentation differences, repeatability and reproducibility of the devices and the way of measurement (manual or automatic) can affect the results [5]. Therefore, the device that is used for the measurement in the studies is an important factor.
Nidek RS-3000 Advance® is one of the new OCTA devices that has been reported to be highly reproducible [7]. However, to the best of our knowledge, there is no study in the literature, a study comparing healthy individuals and DM cases using RS-3000. In this study, it is aimed to compare DM cases without clinical DR findings with healthy individuals using Nidek RS-3000 Advance®.
Material and methods
This observational and cross-sectional retrospective study was conducted in accordance with the Helsinki Declaration with the approval of the local ethics committee. Informed consent was obtained from all participants.
The study was included type 2 DM patients and age and sex-matched healthy individuals with the best-corrected visual acuity (BCVA) 20/20 in all cohort. DM patients who developed DR findings on detailed fundus examination were not included in the study. Besides type 2 DM in the study group, individuals in all cohorts who have any known systemic diseases such as type 1 DM and hypertension, as well as ocular disease, trauma, or surgery excluded from the study. Other exclusion criteria were as follows; presence of any known systemic drug use, IOP > 21 mmHg, absolute spherical equivalent (SE) value (spherical error + ½ cylindric error) > 3 diopters, poor cooperativity to the measurements [Scan quality index (SQI) < 9], cases without or inability to measure choroidal thickness measurements and presence of any OCTA artifacts.
Complete ophthalmologic examination including BCVA and intraocular pressure (IOP) measurements, anterior and posterior segment examinations were performed. DM duration of the cases was recorded in years based on the date of diagnosis. Participants self-reported the disease duration. Only one eye with the best image quality was selected for statistical analysis and the right eye was preferred if the image quality was equal.
OCTA images were obtained by scanning a 4.5 × 4.5 mm macular area centered in the fovea using the RS-3000 Advance® (Nidek, Gamagori, Japan) instrument using the Angioscan-8 software. The RS-3000 Advance® is a device with an A scanning rate of 53,000 scans/sec, using 880 nm light source with 7 and 20 µm axial and transverse resolution in tissues. Images consist of 256 B scans separated by 11 µm. The brightness level of the image, which can vary from –10 to + 10, is usually set to 0 as provided automatically by the device itself. The technique to acquire and process the OCT-A cube is proprietary but is thought to use both phase/Doppler shift and amplitude variation. [7]
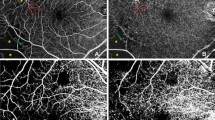
The SCP and the DCP en face images were visualized automatically segmenting 2 separate slabs defined by the arbitrary segmentation lines created by the software of the device: The en face slab for the SCP extends from 8 μm below the internal limiting membrane to the inner boundary of the inner nuclear layer (INL). The en face slab for the DCP extends from the inner boundary of the INL to 88 μm below. All images were reviewed to confirm accurate segmentation by the automated instrument software. No manual adjustment was deemed necessary.
The automated segmentation defines the en face slab for the SRL to extend from 8 μm below the internal limiting membrane to the inner boundary of the inner nuclear layer (as identified by automated segmentation). The en face slab for the DRL extends from the inner boundary of the inner nuclear.
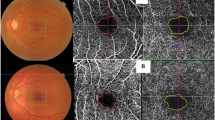
The VD measurement in SCP and DCP was figured out automatically with a device-specific software. This assessment was adjusted by contrast-thresholding on the en-face images and it was calculated by proportioning the number of pixels of the areas defined as vascular tissue to the number of pixels in the entire image. Figure 1 shows the automatic detection of retinal capillary plexuses and assessment of VD.
FAZ measurements were made by two physicians (N.S.K. and M.A.) and the mean was recorded. As described in the literature, the borders of FAZ were drawn manually by masking the clinical status of the cases (Fig. 2).
After determining the FAZ area and contrast thresholding, the device automatically overlaps an imaginary circle of 1000-µm diameter with the en-face image, centering the fovea, and quantitatively indicates what percentage of the FAZ and VD occupy this circle (Figs. 3–4). Those parameters were called the FAZ percentage (FAZ%) and foveal vessel density (FVD%).
Central macular thickness (CMT) was measured automatically by the device as the average retinal thickness in the central 1000-µm diameter.
Subfoveal choroidal thickness (SFCT) was routinely obtained in all patients using enhanced-depth imaging (EDI) technique. Subfoveal choroidal thickness (SFCT) was defined as the vertical distance between Bruch’s membrane and the chorioscleral interface at the fovea, which was manually measured by two independent graders (M.A. and N.S.K.) blinded to study parameters using the built-in caliber. Cases with more than 15% difference were re-evaluated and a consensus was achieved by discussion between the graders.
Statistics
Statistical analyses were performed with SPSS 24.0 Windows version (SPSS, Chicago, Illinois, USA). Qualitative variables are presented as numbers and percentages. Quantitative data are presented as mean ± standard deviation (SD) unless otherwise stated. Distribution pattern was determined according to the Kolmogorov–Smirnov test. The categorical variables were compared with chi-squared test and numerical variables were compared according to the distribution pattern (student’s t-test in normal distribution, Mann–Whitney U test in abnormal distribution). Correlations were analyzed with Spearman test. The inter-examiner reproducibility of the manual FAZ measurements was assessed by measuring the intraclass correlation coefficient (ICC) with %95 confidence interval (CI). In order to test adjustment differences of CMT and SFCT, an analysis of covariance (ANCOVA) was performed with the group as the dependent variable, CT and CMT as covariates. p < 0.05 was considered significant.
Power analysis was used to assess the sample (power = 0.80, α = 0.05, two-sided test, G power 3.0, Dusseldorf, Germany). A sample size calculation determined that 40 eyes of each group were required to detect a 0.5 mm2 VD difference with standard deviation 0.8 mm2.
Results
Thirty-nine eyes of 39 patients (24 females, 15 males) with Type 2 DM and 41 eyes of 41 healthy subjects (19 females, 22 males) were included in this study. The mean age of the patients was 60.74 ± 5.87 years in the DM group and 59.54 ± 6.19 years in the control group. The gender distribution and mean age did not differ between the groups (p = 0.18 and p = 0.35 respectively). The mean DM duration was 12.48 ± 6.94 years (min–max: 1–30 years). No significant difference was observed in the groups in terms of other covariant factors that may affect the OCTA metrics. Additionally, SQI of the scannings was ≥ 0.9 in all cohorts. Median (min/max) SE values were -0.5 (-2.25/2.25) in the control group and -0.25 (-1.25/2.75) in the study group (p = 0.056).
Based on the Kolmogorov–Smirnov test results, FAZ area, VD in SCP, and FVD in SCP/DCP showed normal distribution (p > 0.05 for all comparisons). The ICC between the 2 observers for the SCP FAZ area and FAZ(%) was 0.926 (95% CI 0.884–0.952) and 0.928 (95% CI 0.887–0.954) respectively. ICC for DCP FAZ area and FAZ(%) were 0.897 (95% CI 0.839–0.935) and 0.874 (95% CI 0.821–0.930) respectively.
There was no significant difference in FAZ area, FAZ%, and FVD% in the SCP and DCP between diabetic patients and healthy individuals (Table 1). While VD showed no significant difference between the two groups in SCP (p = 0.73), it decreased in diabetic patients in DCP (p < 0.001). The duration of DM correlated with VD decrease in DCP (r:-0.52; p < 0.001). In both groups, all parameters in DCP were significantly higher than that .in the SCP (p < 0.001 for all parameters; Table 1).
There was no difference between the groups regarding possible covariants CMT and SFCT. CMT (µm) was 288.49 ± 13.33 in the diabetic group and 287.12 ± 10.98 in the control group (p = 0.61). SFCT (µm) was 319.82 ± 64.49 in the diabetic group and 331.82 ± 79.12 in the control group (p = 0.46). There was no correlation between structural OCT parameters including CMT, SFCT, and OCTA metrics (p > 0.05 for all values). In ANCOVA, when the groups were compared with SFCT and CMT as the covariants, significant differences in DCP-VD persisted (p < 0.001).
Discussion
In this study, the quantitative retinal vascular analysis of DM patients was performed with OCTA.t was found that in patients whose clinical retinopathy did not develop yet, DCP-VD decreased, FVD (%) was preserved and the FAZ area did not change compared to healthy individuals. A significant negative correlation was observed between DCP-VD and DM duration. When the groups were compared using CMT and SFCT values as covariants, the difference in significance of DCP-VD persisted.
DM is a chronic disease with acute exacerbations and remissions, causing systemic and ocular comorbidities. Despite advanced diagnostic methods, it is still not easy to predict acute exacerbations and ocular comorbidities that may cause permanent complications [8]. It was reported that in patients with DM, after clinically detectable DR findings occur, progression risk of the DR increases. So early detection of DM and screening for DR have been reported to reduce the severity and prevalence of the retinopathy [9]. Hence, screening algorithms are evolving from invasive methods to in-vivo, non-invasive methods to detect subclinical findings with predictive value in order to develop preventive strategies.
Accordingly, new technologies such as OCTA, which enable non-invasive, highly reproducible measurements, have provided significant convenience in capturing these tips. In the retina which is a combination of vascular and neuronal tissues, OCTA studies showed that vascular changes may occur before neurodegenerative changes [10]. A recent OCTA study also confirmed this finding that VD was reduced in peripapillary and macular capillary plexus without loss of retinal ganglion cells in DM cases [11].
However, the different segmentation and measurement algorithms of each device caused controversial and non-interchangeable results [6]. In this study, DM cases were compared with healthy individuals using a commercial OCTA device that has not been studied in the literature before.
According to our study, FAZ area didn’t change between diabetic and healthy individuals. On the other hand, there is no consensus in the literature aligned with our findings. Although Cao et al. reported correlating findings with our study [12], contradictory results have also been published reporting that superficial or both superficial and deep FAZ increased in patients with DM without DR [13,14,15]. In those studies, FAZ enlargement was attributed to the increase of capillary non-perfusion.
Some hypotheses were reported behind the conflicting results about the FAZ area. First of all, it was speculated that the FAZ area is not a sensitive parameter due to its high variability[12] Indeed, in our cohort, the FAZ area varied between 0.18 and 0.83 mm2.
On the other hand, there is a contradiction between considering FAZ area separately in the superficial and DCP or as a unique common area to avoid segmentation errors [16]. In early studies, the FAZ area was reported to be higher in DCP in OCTA devices in accordance with our study [17,18,19]. Additionally, some studies evaluated FAZ area separately and reported that FAZ area increases only in DCP in the early stages of the DM disease [20]. But Spaide attributed the higher DCP values in healthy individuals to the segmentation error in a study in which histological images were analyzed in correlation with OCTA images[16] and proposed analyzing a single FAZ area instead of two different FAZs.
Moreover, in the version of the device used, although there is a strong correlation (ICC:0.92 and 0.89 for superficial and deep FAZ areas respectively) between manual FAZ border measurements. Based on these findings, the device may be upgraded to a version where it calculates the FAZ area as a unique area using an automated method.
Also when the groups were compared in terms of VD, it was observed that DCP-VD decreased in diabetic patients. The amount of VD reduction exceeded the repeatability coefficients reported in the literature [7]. Al-Sheikh et al.[7] reported the mean difference in VD between two sessions was 0.1 (95% CI 2.6 to − 2.5) and the coefficient of variation was 0.02 for the DCP in the 3 × 3 mm area. According to our study, the mean DCP-VD difference was 1.05 (95% CI 0.73 to 1.37).
OCTA data were analyzed together with structural OCT parameters in our study. Our analysis revealed that CMT and SFCT were not correlated with OCTA metrics. Conflicting results have been reported on the relationship between SFCT, VD, and FAZ area. Although Fernández-Vigo et al.[21] reported that parafoveal VD increased with CT, other publication reported no correlation between CT and VD [22]. As there is no difference between our groups in terms of CMT and SFCT, we concluded that our results are reliable in this respect. On the other hand, when CMT and SFCT were analyzed as covariants, the results did not change statistically.
OCTA results performed by AngioVue or Triton also showed that DM findings progressed from deep vascular layers to superficial layers which is consistent with our study [23, 24]. Some studies claimed both plexuses were affected simultaneously [12, 13]. The decrease in VD that has been observed in DM patients was attributed to impairment of neurovascular autoregulation and endothelial cell damage secondary to chronic hyperglycemia [25].
DCP is a source of retinal venules originating from vortex-like vessels [26]. From this perspective, it can be suggested that the venous component of the retinal vascular system is more prominent in the pathogenesis of DR according to OCTA studies [26]. In this context, venous bleeding, increased resistance, and pressure in the retinal venous system in DR cases also support OCTA findings [27]. On the other hand, considering the studies showing FAZ enlargement in DCP in the cases of retinal vein occlusion, it is possible to speculate that DCP may be more susceptible to ischemia [28]. Contradictingly, early histopathological studies reported that the capillary closure observed in DR is more prominent in the arterial part of the retinal vasculature [29].
Some hypotheses have been proposed to support the onset of pathological effects in DCP in DM cases. Namely, it was shown that in mild nonproliferative DR (NPDR) cases, VD decreased primarily in the DCP, before the SCP [30, 31]. VD decreased from 25.23% to 20.16% and afterward to 11.16% according to the NPDR stages [31]. Additionally, some studies reported that the number of microaneurysms in NPDR cases is higher in DCP and visual acuity correlates with DCP-VD. [32, 33]
A new noteworthy finding in our study is that despite the decreasing of VD in the macular region, FVD% which represents the VD percentage in the foveal area did not change. In this regard, two hypotheses can be proposed; hyperglycemia may cause oxygen deficiency and this may cause retinal capillary expansion or early local damage may cause compensatory dilatations of the intact vessels [34]. So it can be speculated that despite the reduction of VD, FVD could be preserved for a certain period in DM patients.
According to our study, a negative correlation was found between DM duration and DCP-VD values. As DM duration increases, VD decreases in DCP. In the early period, the severity of hyperglycemia and hypoxia may cause microvasculopathy more than the duration of the disease. However, there is no consensus on this issue. [12, 20, 35] Multifactorial etiopathogenesis of DR, differences in demographic characteristics of the cases included in the studies and based on the duration of DM according to the self-reports of the patients may cause those contradictory results.
In this study, we evaluated the cases quantitatively, but there were other studies evaluating them qualitatively, such as non-perfused areas or microaneurysms [12, 36, 37]. Cao et al.[12] mentioned microaneurysms that were not detected in biomicroscopic examination detected by OCTA, and they called it preclinical DR. However, no microaneurysms were detected in our cohort. These conflicting results may be due to the usage of different devices in these studies. Based on that, it is required to conduct studies comparing different OCTA devices side by side.
Our study has some limitations: axial lengths, simultaneous blood pressure, and glucose values are missing during OCTA imaging due to its retrospective nature. However our study has a homogeneous cohort, since the patients with SE > 3, HT, and the ones underwent treatment changes were not included due to irregular blood glucose values in the last 3 months in the study. Since the device used in the study only measured the FAZ area, we could not measure parameters such as the FAZ perimetry or a circularity index. Since our study was aimed at investigating the usability of the device in practical clinical conditions, we did not use advanced image analysis programs.
Additionally, RS-3000 Advance appears that it couldn’t overcome the general deficiencies of OCTA technology such as two-dimensional evaluation of the images, inability to evaluate intermediate capillary plexus as a separate plexus, inability to remove the projection artifacts from the DCP images, and evaluation of the DCP in SCP due to segmentation errors [5, 16]. Although it was shown to have high reproducibility levels [38], manual measurement of FAZ parameters is another limitation of the RS-3000.
In conclusion; our study suggested that OCTA can identify quantitative changes in DCP before the manifestation of clinically apparent retinopathy. DCP-VD reduction may be an earlier finding than FAZ enlargement. Despite the reduction of VD, FVD could be preserved for a certain period in DM patients. OCTA may give subclinical predictive findings in DM patients who have not yet developed clinical DR and may be a promising tool for screening of diabetic eyes for DR. Longitudinal randomized controlled studies that include cases are needed in the future to accurately determine the predictive value of OCTA before clinical retinopathy develops in DM patients.
Availability of data and material
Data and material are available.
References
Flaxman SR, Bourne RRA, Resnikoff S et al (2017) Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Heal 5:1221–1234. https://doi.org/10.1016/S2214-109X(17)30393-5
Williams R, Airey M, Baxter H et al (2004) Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye 18:963–983. https://doi.org/10.1038/sj.eye.6701476
Antonetti DA, Barber AJ, Bronson SK et al (2006) Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55:2401–2411. https://doi.org/10.2337/db05-1635
Lecleire-Collet A, Tessier LH, Massin P et al (2005) Advanced glycation end products can induce glial reaction and neuronal degeneration in retinal explants. Br J Ophthalmol 89:1631–1633. https://doi.org/10.1136/bjo.2005.079491
Spaide R, Fujimoto J, Waheed N et al (2017) Optical coherence tomography angiography. Prog Retin Eye Res 101:985–988. https://doi.org/10.1136/bjophthalmol-2016-309200
Corvi F, Pellegrini M, Erba S et al (2018) Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol 186:25–31. https://doi.org/10.1016/j.ajo.2017.11.011
Al-Sheikh M, Tepelus TC, Nazikyan T, Sadda SR (2017) Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br J Ophthalmol 101:449–452. https://doi.org/10.1136/bjophthalmol-2016-308764
Battista M, Borrelli E, Sacconi R et al (2020) Optical coherence tomography angiography in diabetes: a review. Eur J Ophthalmol 30:411–416. https://doi.org/10.1177/1120672119899901
Olafsdottir E, Andersson DKG, Dedorsson I et al (2016) Early detection of type 2 diabetes mellitus and screening for retinopathy are associated with reduced prevalence and severity of retinopathy. Acta Ophthalmol 94:232–239. https://doi.org/10.1111/aos.12954
Li Z, Wen X, Zeng P et al (2019) Do microvascular changes occur preceding neural impairment in early-stage diabetic retinopathy? Evidence based on the optic nerve head using optical coherence tomography angiography. Acta Diabetol 56:531–539. https://doi.org/10.1007/s00592-019-01288-8
Zeng Y, Cao D, Yu H et al (2019) Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol 103:1747–1752. https://doi.org/10.1136/bjophthalmol-2018-313582
Cao D, Yang D, Huang Z et al (2018) Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol 55:469–477. https://doi.org/10.1007/s00592-018-1115-1
Dimitrova G, Chihara E, Takahashi H et al (2017) Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci 58:190–196. https://doi.org/10.1167/iovs.16-20531
Vujosevic S, Muraca A, Alkabes M et al (2019) Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina 39:435–445. https://doi.org/10.1097/IAE.0000000000001990
Choi W, Waheed NK, Moult EM et al (2017) Ultrahigh speed oct angiography of retinal and choriocapillaris alterations in diabetic patients with and without retinopathy using swept source optical coherence tomography. Retina 37:11–21. https://doi.org/10.1007/s11897-014-0247-z.Pathophysiology
Spaide RF, Curcio CA (2017) Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol 135:259–262. https://doi.org/10.1001/jamaophthalmol.2016.5327
Coscas F, Sellam A, Glacet-Bernard A et al (2016) Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Investig Ophthalmol Vis Sci 57:OCT211-OCT223. Doi: https://doi.org/10.1167/iovs.15-18793
Hussain N, Hussain A (2016) Diametric measurement of foveal avascular zone in healthy young adults using optical coherence tomography angiography. Int J Retin Vitr 2:27. https://doi.org/10.1186/s40942-016-0053-8
Lupidi M, Coscas F, Cagini C et al (2016) Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am J Ophthalmol 169:9–23. https://doi.org/10.1016/j.ajo.2016.06.008
Niestrata-Ortiz M, Fichna P, Stankiewicz W, Stopa M (2019) Enlargement of the foveal avascular zone detected by optical coherence tomography angiography in diabetic children without diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 257:689–697. https://doi.org/10.1007/s00417-019-04264-8
Fernández-Vigo JI, Kudsieh B, Shi H et al (2020) Normative database and determinants of macular vessel density measured by optical coherence tomography angiography. Clin Exp Ophthalmol 48:44–52. https://doi.org/10.1111/ceo.13648
Oh J, Baik DJ, Ahn J (2020) Inter-relationship between retinal and choroidal vasculatures using optical coherence tomography angiography in normal eyes. Eur J Ophthalmol 30:48–57. https://doi.org/10.1177/1120672118816225
Scarinci F, Picconi F, Giorno P et al (2018) Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol 96:264–265. https://doi.org/10.1111/aos.13510
Carnevali A, Sacconi R, Corbelli E et al (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54:695–702. https://doi.org/10.1007/s00592-017-0996-8
Simó R, Stitt AW, Gardner TW (2018) Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 61:1902–1912. https://doi.org/10.1007/s00125-018-4692-1
Dimitrova G, Chihara E (2019) Implication of deep-vascular-layer alteration detected by optical coherence tomography angiography for the pathogenesis of diabetic retinopathy. Ophthalmologica 241:179–182. https://doi.org/10.1159/000495624
Cybulska-Heinrich AK, Baertschi M, Loesche CC et al (2015) Patients with diabetic retinopathy have high retinal venous pressure. EPMA J 6:5. https://doi.org/10.1186/s13167-015-0027-1
Suzuki N, Hirano Y, Tomiyasu T et al (2016) Retinal hemodynamics seen on optical coherence tomography angiography before and after treatment of retinal vein occlusion. Investig Opthalmology Vis Sci 57:5681. https://doi.org/10.1167/iovs-16-20648
Bek T (2000) Histopathology and pathophysiology of diabetic retinopathy. In: van Bijsterveld O (ed) Diabetic retinopathy. Martin Dunitz, London, pp 169–189
Nesper PL, Roberts PK, Onishi AC, et al (2017) Quantifying microvascular abnormalities with ıncreasing severity of diabetic retinopathy using optical coherence tomography angiography. Investig Opthalmology Vis Sci 58:BIO307. Doi: https://doi.org/10.1167/iovs.17-21787
Sambhav K, Abu-Amero KK, Chalam KV (2017) deep capillary macular perfusion indices obtained with OCT angiography correlate with degree of nonproliferative diabetic retinopathy. Eur J Ophthalmol 27:716–729. https://doi.org/10.5301/ejo.5000948
Hasegawa N, Nozaki M, Takase N et al (2016) new insights into microaneurysms in the deep capillary plexus detected by optical coherence tomography angiography in diabetic macular edema. Invest Ophthalmol Vis Sci 57:348. https://doi.org/10.1167/iovs.15-18782
Dupas B, Minvielle W, Bonnin S et al (2018) Association between vessel density and visual acuity in patients with diabetic retinopathy and poorly controlled type 1 diabetes. JAMA Ophthalmol 136:721–728. https://doi.org/10.1001/jamaophthalmol.2018.1319
Gołębiewska J, Olechowski A, Wysocka-Mincewicz M et al (2017) Optical coherence tomography angiography vessel density in children with type 1 diabetes. PLoS ONE 12:e0186479. https://doi.org/10.1371/journal.pone.0186479
Li T, Jia Y, Wang S et al (2019) Retinal microvascular abnormalities in children with type 1 diabetes mellitus without visual impairment or diabetic retinopathy. Invest Ophthalmol Vis Sci 60:990. https://doi.org/10.1167/iovs.18-25499
De Carlo TE, Chin AT, Bonini Filho MA et al (2015) Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 35:2364–2370. https://doi.org/10.1097/IAE.0000000000000882
Forte R, Haulani H, Jürgens I (2018) Quantitative and qualitative analysis of the three capillary plexuses and choriocapillaris in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina 1:333–344. https://doi.org/10.1097/iae.0000000000002376
Shiihara H, Sakamoto T, Yamashita T et al (2017) Reproducibility and differences in area of foveal avascular zone measured by three different optical coherence tomographic angiography instruments. Sci Rep 7:9853. https://doi.org/10.1038/s41598-017-09255-5
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Manisa Celal Bayar University (Date: 02.05.2018 / No:20.478.486).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Informed consents were obtained from the patients.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altinisik, M., Kahraman, N.S., Kurt, E. et al. Quantitative analysis of early retinal vascular changes in type 2 diabetic patients without clinical retinopathy by optical coherence tomography angiography. Int Ophthalmol 42, 367–375 (2022). https://doi.org/10.1007/s10792-022-02230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02230-8