Abstract
Aims
To evaluate the microvascular and neural differences of the optic nerve head (ONH) between type 2 diabetes mellitus (T2DM) subjects and controls.
Methods
This was a cross-sectional observational study. One hundred and eight eyes of 108 T2DM subjects with or without diabetic retinopathy (DR) (54 preclinical DR and 54 mild-to-moderate DR) were included. Fifty-two eyes of 52 healthy subjects were included as controls. The 4.5-mm Angio Disc scan mode and the ganglion cell complex scan mode were performed with all participants using AngioVue software 2.0 of the optical coherence tomography angiography (OCTA) device.
Results
Regarding ONH radial peripapillary capillary (RPC) density, the peripapillary region was mainly significantly reduced in the No-DR (NDR) group. Moreover, the RPC density of the peripapillary region and the inside optic disc area were significantly reduced in the non-proliferative DR (NPDR) group. When compared to the controls, significantly reduced peripapillary capillary density in six sections was observed in the NPDR group. However, reduced density was observed in only two sections in the NDR group. The NPDR group had significantly increased focal loss volume (FLV) and reduced peripapillary RNFL thickness in the inferior nasal section compared to those in the controls, but similar changes were not observed in the NDR group. A regression model identified RPCs inside the optic disc as a significant parameter in early-stage DR detection. In the NPDR group, BCVA showed a significantly negative correlation with RPCs inside the optic disc and a significantly positive correlation with FLV.
Conclusions
OCTA findings of the ONH area may provide evidence that microvascular changes occur preceding neural impairment in early-stage DR. However, further researches are still needed to support the statement. Reduced ONH perfusion inside the optic disc may be one of the crucial biomarkers in early-stage DR detection and is a possible sensitive visual acuity predictor in early-stage DR subjects. With the ONH mode, OCTA may be a more promising tool in DR screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is one of the serious complications of diabetes mellitus (DM), and it is a major cause of sight-loss worldwide [1, 2]. One hundred million patients worldwide were affected by DR in 2010, and more than 190 million are expected to be affected by 2030 [3, 4]. The pathogenesis of DR is not completely clear. Microvasculopathy in the retina has been classically regarded as the pivotal initiating step [5]. The loss of pericytes has been considered as the initially detectable histologic evidence in the retina of DM subjects [6]. However, in the past few years, emerging evidence has suggested that neurodegeneration may occur before microvascular changes in preclinical DR [7, 8]. The potential relationship between neurodegeneration and microvascular impairment has been frequently discussed [9]. Therefore, it remains controversial whether microvascular dropout or neural impairment occurs first in early-stage DR.
A real-time cross-sectional imaging machine, optical coherence tomography angiography (OCTA), has been widely applied in retinopathy diagnoses. Compared to traditional diagnostic techniques, such as fluorescein fundus angiography (FFA), OCTA is less invasive, more convenient and safer because intravenous injection of dyes is not needed in the examination [10]. The microvascular dropout of the macular area in preclinical and early-stage DR has been frequently reported in recent OCTA studies [11,12,13]. OCTA seems to be a promising tool for screening the macular area and follow-up in DR subjects. However, the optic nerve head (ONH) area is usually analysed in glaucoma and optic neuropathy, while it has rarely been studied in DR [14]. As is implied by growing evidence, DR is a neurovascular disease. It is crucial and meaningful to further study the ONH area in DR subjects. Our previous study [15] showed that ONH capillary density may be reduced in the No-DR (NDR) compared to healthy controls, but the shortcoming was that non-proliferative DR (NPDR) subjects and detailed parameters, such as different regions of the peripapillary area, were not included, which made the evidence for the presence of DR incomplete. Based on the preliminary conclusions, the overall goal of our study was to provide evidence, using OCTA, whether microvascular changes or neural impairment occurs first after the onset of DR. In addition, the importance of ONH perfusion in early-stage DR detection and the effect on visual acuity in early-stage DR were evaluated.
Methods
Subjects
This was a cross-sectional observational study. The study adhered to the tenets of the Declaration of Helsinki and was approved by the research ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. One hundred and eight eyes of 108 T2DM subjects (54 preclinical DR and 54 mild-to-moderate DR) were included. Another 52 eyes of 52 healthy subjects were included as controls. The subjects were recruited from the ophthalmology department of the DM centre between January 2018 and October 2018. Informed consent was obtained from all subjects to be included in the study. DR stages were confirmed based on the criteria released by the American Diabetes Association (ADA) in 2017 [16]. When only microaneurysms were observed in the FFA, the DR stage was defined as mild NPDR. The criteria for severe NPDR were as follows: (1) intraretinal haemorrhage ≥ 20 dots per quadrant, (2) venous beading in two or more quadrants, or (3) intraretinal microvascular abnormalities in one or more quadrants. Moderate NPDR was defined as the stage between mild NPDR and severe NPDR. The more serious eye was selected if the severity was different in both eyes, while a random eye was selected if the severity was the same. The inclusion criteria included a diagnosis of T2DM with NDR or mild-to-moderate NPDR, a T2DM duration more than 1 year and age not less than 40 years. A diagnosis of T2DM with NDR or mild-to-moderate NPDR was provided by two senior ophthalmologists through thorough ophthalmic examinations, including LogMAR best-corrected visual acuity (BCVA), intraocular pressure (IOP) measurement, dilated fundus examination, Early Treatment Diabetic Retinopathy Study 7 standard 35-mm colour fundus photographs (7F-ETDRS) (Canon, Inc., Tokyo, Japan), FFA (Microclear, Inc., Suzhou, China) and OCTA (Optovue, Inc., Fremont, CA, USA). General characteristics included age, gender, laterality, haemoglobin Alc (HbAlc) levels, DM duration, presence of hypertension, body mass index (BMI) and presence of diabetic complications (diabetic foot and diabetic renal disease) were collected. A diagnosis of hypertension (> 130/80 mmHg) and diabetic renal disease was made according to associated guidelines [17, 18] by a cardiology specialist and a nephrology specialist, respectively. The exclusion criteria were as follows: (1) moderate or high myopia (a refractive error higher than − 3 dioptres or an axial length greater than 26 mm); (2) glaucoma, optic neuropathy, and other retinal diseases; (3) dioptric media opacity that may have affected imaging; (4) a history of intraocular surgery; and (5) macular oedema in OCTA or FFA. Thorough ophthalmic examinations were also performed in the healthy controls.
OCTA image collection and analysis
OCTA images were obtained using the RTVue XR Avanti device with AngioVue 2.0 (Optovue Inc., Fremont, CA, USA). The device has a speed of 70,000 A scans per second and a light source of 840 nm in the scan. The split-spectrum amplitude-decorrelation angiography (SSADA) algorithm was used to analyse the OCTA images. The 4.5-mm Angio Disc scan mode and the ganglion cell complex (GCC) scan mode were used for all subjects. All examinations were performed by experienced ophthalmic examiners. The 4.5-mm Angio Disc scan mode was used to capture a 4.5 × 4.5 mm area centred on the optic disc. The area was divided into different sections (Fig. 1); therefore, the radial peripapillary capillary (RPC) density in these sections and peripapillary thicknesses were automatically calculated by the software AngioVue 2.0. The GCC scan mode (Fig. 2) was applied to measure the thickness of the retinal GCC including the retinal nerve fibre layer, ganglion cell layer and inner plexiform layer. Average GCC thickness, focal loss volume (FLV) and global loss volume (GLV) were automatically calculated by the software AngioVue 2.0. FLV was the sum of the fractional deviation (FD) in the area where significant focal loss (SFL) existed. SFL was defined as a pattern deviation more than 1.65 standard deviations below the normal average (5% of normal distribution). GLV was the sum of FD in the area where FD was negative. Images that had a scan quality of less than 6 were excluded.
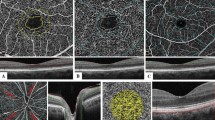
ONH section division and radial peripapillary capillary density differences in the three groups. a, b Right and left eyes. A 4.5 × 4.5 mm OCTA image (left) of the corresponding layer in the ONH (right: horizontal OCT B-scan of nerve fibre layer-internal limiting membrane; area between the red and green line). Inside optic disc: area surrounded by the inner blue circle; peripapillary region: area between two blue rings. NS nasal superior, NI nasal inferior, IN inferior nasal, IT inferior tempo, TI tempo inferior, TS tempo superior, ST superior tempo, SN superior nasal. c Right eye of a 55-year-old male control. A 4.5 × 4.5 mm OCTA image (above) of the corresponding layer in the ONH (below). d Right eye of a 51-year-old male with no diabetic retinopathy (NDR). A 4.5 × 4.5 mm OCTA image (above) of the corresponding layer in the ONH (below). e Right eye of a 52-year-old male with non-proliferative diabetic retinopathy (NPDR). A 4.5 × 4.5 mm OCTA image (above) of the corresponding layer in the ONH (below). Reduced RPC density in the NDR group (d above) was less prominent than that in the NPDR group (e above) when compared with the controls (c above). (Color figure online)
Ganglion cell complex differences in the three groups. The same eyes as in Fig. 1. Thickness map: left; deviation map: middle; normal database (NDB) reference: right. a Right eye of a 55-year-old male control. b Right eye of a 51-year-old male with no diabetic retinopathy (NDR). c Right eye of a 52-year-old male with non-proliferative diabetic retinopathy (NPDR). A gradually reduced ganglion cell complex thickness and an increased abnormality in the NDB reference map were presented from the controls to the NDR group as well as from the NDR group to the NPDR group
Statistical analysis
Statistical analyses were performed using SPSS 24.0 (SPSS Inc,. Chicago, IL, USA), a commercially available statistical software. A one-way ANOVA was applied to analyse the numerical variables among the three groups (NDR, NPDR, and healthy controls), and Bonferroni’s post hoc analysis was performed to evaluate the significant differences. Categorical variables were analysed using a chi-squared test. Statistically significant variables between NDR and NPDR in the univariate analysis were included in a multivariable binary logistic regression model that compared NDR and NPDR using backward elimination analysis. The receiver operating characteristic (ROC) curve was then generated based on the combined parameter binary logistic regression model. The area under the curve (AUC), sensitivity, and specificity were reported according to the curve. In addition, for the NPDR group, a backward multiple linear regression was performed between logMAR BCVA and the OCTA parameters (capillary density inside optic disc, peripapillary capillary density, peripapillary RNFL thickness, GCC thickness, FLV and GLV).
Results
Patient characteristics
One hundred and eight eyes of 108 T2DM subjects (54 preclinical DR and 54 mild-to-moderate DR) and 52 eyes of 52 matched healthy controls were included in this study. Subjects with NPDR had significantly longer DM duration times than those with NDR. Moreover, subjects with NPDR and NDR had a higher BMI and worse BCVA, respectively, than healthy controls. No significant differences among groups were found with regard to age, gender, eye laterality and other variables. Details are shown in Table 1.
OCTA findings
With regard to ONH RPC density, the densities of the whole image and the peripapillary region were significantly reduced in the NDR group compared to the controls (whole image: 48.49 ± 3.37% versus 50.17 ± 2.1%, p = 0.02; peripapillary region: 50.14 ± 5.12% versus 52.22 ± 5.02%, p = 0.046), while the capillary density inside the optic disc was significantly reduced in the NPDR group compared to the NDR group (46.47 ± 5.01% versus 50.14 ± 5.12%, p < 0.01). However, all the parameters in the ONH RPC density were significantly reduced in the NPDR group when compared to the controls (whole image: 47.03 ± 3.11% versus 50.17 ± 2.11%; inside optic disc: 46.47 ± 5.01% versus 52.22 ± 5.02%; peripapillary region: 49.52 ± 3.85% versus 52.50 ± 2.74%). The NPDR group had significantly increased FLV compared to the NDR group and the controls (NDR: 2.28 ± 2.83% versus 1.06 ± 1.02%, p < 0.01; control: 2.28 ± 2.83% versus 0.86 ± 0.68%, p < 0.01). Moreover, GLV was also significantly increased in the NPDR group when compared to the controls (4.47 ± 5.37% versus 2.29 ± 2.38%, p = 0.01). No significant differences among the groups were found in the peripapillary RNFL thickness. Details are shown in Tables 2, 3. Classical figures of RPC density differences and GCC differences in the three groups are presented in Figs. 1 and 2, respectively.
The peripapillary region was then divided into eight sections and statistical data between each of two groups were compared (Table 2). When compared to the controls, significantly reduced capillary density in six sections (nasal superior, nasal inferior, inferior nasal, inferior tempo, tempo inferior and tempo superior) was observed in the NPDR group, while significantly reduced capillary density in two sections (inferior nasal and tempo superior) was observed in the NDR group. The NPDR group had significantly reduced peripapillary RNFL thickness in the inferior nasal section compared to the controls.
DM duration, capillary density inside the optic disc and FLV, which are parameters that were statistically significant between the NPDR and NDR groups in a one-way ANOVA, were included for analysis in a multivariable binary logistic regression model between the NPDR and NDR groups. The dependent variable in the regression model was whether subjects have DR. The regression model identified DM duration (OR 1.12, CI [1.03, 1.22], p < 0.01) and capillary density inside the optic disc (OR 0.82, CI [0.72, 0.93], p < 0.01) as significant. Next, ROC curves were generated. The AUC for the combined parameter binary logistic regression model was 0.84 (p < 0.001) with sensitivity and specificity of 74% and 82%, respectively.
For the NPDR group, a backward multiple linear regression between LogMAR BCVA and the OCTA parameters was performed. LogMAR BCVA showed a significantly negative correlation with capillary density inside the optic disc (β = − 0.3, p = 0.03) and showed a significantly positive correlation with FLV (β = 0.37, p = 0.01) (Fig. 3).
Receiver operating characteristic curve (ROC) for the regression model. ROC for the combined parameter binary logistic regression model between non-proliferative diabetic retinopathy (NPDR) and no diabetic retinopathy (NDR): blue line; reference line: green line. AUC area under the curve, SE sensitivity, SP specificity. (Color figure online)
Discussion
Do microvascular changes occur preceding neural impairment in early-stage DR? Using OCTA, we have found evidence of the ONH area to support this statement. The macular area differences between DM objects and the controls have recently been intensively studied using OCTA. Previous studies showed that the increased foveal avascular zone was accompanied by reduced macular vessel density, which may be useful parameters in the detection of early-stage DR [11, 19, 20]. However, OCTA studies on quantifying the ONH changes in DM objects were relatively lacking and incomplete. Inconsistent with our previous study [15], Cao [21] compared ONH perfusion between an NDR group and controls using OCTA, and they found that significantly reduced ONH perfusion existed in the NDR group. However, the major drawback was that NPDR objects were not included in their study, which cannot answer the question whether microvascular changes or neural impairments occurred first at the onset of DR. In our present study, NPDR patients were included. When compared to the controls, reduced ONH capillary density was observed only in two sections of the peripapillary region in the NDR group, while the same reduction was noticed both in most sections of the peripapillary region and inside the optic disc in the NPDR group. Moreover, a gradual change in neural impairment, including increased FLV and reduced RNFL thickness in the inferior nasal section, was presented in the NPDR group when compared to the controls. It seems that microvascular dropout began in the preclinical DR stage and was followed by further microvascular and neural impairment in the mild-to-moderate DR stage. That is, microvascular changes in the ONH may occur preceding neural impairment in early-stage DR. A possible explanation is that RPCs may contribute to RNFL nourishment. Mansoori’s study [22] revealed that RPC density had a significant positive correlation with RNFL in OCTA images. Anatomically speaking, RPCs, which are parallel to the retinal ganglion cell axons, are the most superficial layers of capillaries located in the inner part of the RNFL. They may originate from the arterioles of the ganglion cell layer and lay around the ONH to supply superficial RNFL [23, 24].
Similar to our study, Van Dijk’s research also showed no significant difference in the RNFL or GCC thickness between NDR objects and the healthy controls [25]. By contrast, Carpineto’s study [7] revealed a significant reduction in RNFL or GCC thickness even in NDR subjects using Cirrus spectral domain OCT (SD-OCT) when compared with the control group. Moreover, localised retinal dysfunction was detected in quite a few type 2 NDR subjects using multifocal electroretinograms (mfERG) in a recent study [26]. Histologically, glial, microglial and other cells are involved in the neural apoptosis of the retina and the retinal ganglion cell apoptosis may be the initial losses noticed in the diabetic retina. It has been illustrated in some rodent models that apoptosis of retinal ganglion cells begins 5 or 10 weeks after the onset of hyperglycaemia [3, 27]. It is an undeniable fact that neurodegeneration occurs in early-stage DR [28]. According to a large-scale randomise clinical trial, early-stage DR subjects in the placebo group had more obvious dysfunction in mfERG than those in the neuroprotective drugs group [29].
However, recent researches have laid special emphasis on the pathophysiology of the neurovascular unit [3, 9]. In the theory, swelling of glial cells was considered as part of the neuroinflammatory process. At the beginning, inflammatory process may not decrease RNFL or GCC thickness because of neural cell swelling. As the process develops, the degeneration and death of neural cells may occur. It is possible that some of the included NDR and NPDR subjects were in “swelling stage” in different degree, thus the reduction of RNFL or GCC thickness was not so prominent and the standard deviation of FLV and GLV was high. The apoptosis of retinal neurons may be the consequence of gradually reduced perfusion and the neural impairment degree may be altered for different factors such as ethnicity, DM type, DM duration and HbAlc levels [30]. In regard to our results, a significantly increased FLV was not presented until NPDR occurred. A longer DM duration and a higher HbAlc value were more likely to contribute to the difference in neural parameters between Carpineto’s study and ours, as Araszkiewicz found that the RNFL or GCC thickness was closely correlated with DM duration and HbAlc values [30]. In addition, according to Zang’s study, a lower variation of the RPC density than that of the RNFL may also contribute to the difference [31]. To some degree, the specificity of neural parameters may be not as good as that of RPC density in DR screening. Another possible reason for the difference may be the analysis pattern. RNFL thickness was divided into four sections with an even distribution in Cirrus SD-OCT, while section division of the peripapillary RNFL thickness was closely matched to the RPC sectors in OCTA. Accordingly, Leite et al. showed a significant difference between OCTA and Cirrus SD-OCT in RNFL thickness measurement [32].
Interestingly, we also noticed that capillary density reduction developed from the peripapillary region to inside the disc. Jia’s finding [33] may provide a reason for this reduction pattern of ONH perfusion in DM development. In their study, a decreased trend in the RPC density was observed with increasing distance from the ONH. We hypothesised that the RPC network was similar to the retinal vascular network. Lower density may be responsible for lower reserve capacity; therefore, the peripapillary RPCs may initially shrink because they are more sensitive to ischaemia and the lack of oxygen induced by hyperglycaemia at the onset of DM. It is a possible protection mechanism of the retina in DR patients. Our study revealed that significantly reduced RPCs in the nasal, inferior and temporal quadrants were observed in the NPDR group compared to the controls, while no significant changes in RPCs in the superior quadrant in the same group were noticed. In agreement with our findings, some publications implied that the superior quadrant had a higher RPC density than other regions [34, 35], which may be evidence that reinforces the above hypothesis.
The combined model between NDR and NPDR showed that a lower RPC density inside the disc and longer DM duration times were observed in the NPDR group, which suggested that capillary density inside the optic disc may be a sensitive predictor in early-stage DR screening, especially in type 2 DM subjects with a long duration. Several well-designed statistical models including macular parameters have been employed in previous studies to assess the severity of DR, but none of them analysed ONH perfusion [11, 36]. To our knowledge, this was the first study to assess the ONH parameter in early-stage (mild-to-moderate) DR screening models. We suggest a combined model of macular and ONH parameters in early-stage DR screening using OCTA should be performed in further research. In addition, we found that a lower RPC density inside the disc and higher FLV may contribute to worse visual acuity. In previous publications [15, 37], lower macular vessel density was also closely related to worse visual acuity in preclinical and early-stage DR. Previous histological evidence [38, 39] illustrated that RPCs came from the superficial vascular plexuses (SVPs), which ascended to the nerve fibre layer. SVPs mainly supplied the macular area and were frequently discussed in previous studies. The pre-capillary arterial parts fed RPCs from the SVPs while the post-capillary venous parts drained RPCs into the SVPs [38,39,40]. Last year, David Huang’s research team updated the segmentation method accordingly in OCTA and, therefore, enabled observation of the RPC network images that were separated from the original superficial plexus images in the updated software [40]. A possible relationship exists between the RPC network and the macular vascular network [39, 40]. Therefore, the reduction of capillary density in the ONH may be related to other vascular plexuses in the retina, which may be a reflection of ischaemia in DR. Ischaemia causes the death of photoreceptors and other neural cells and subsequently contributes to impaired vision [3].
The limitations of our study included that it was a cross-sectional observational study. In addition, assessments of retinal neurodysfunction such as mfERG have not been performed. A longitudinal study with retinal neurodysfunction assessments should be performed to explain the relationship between microvascular and neural impairments in early-stage DR. Moreover, the sample size was relatively small. Another limitation was that automatic segmentation was applied in density calculations. The possibility of segmentation errors may exist.
In conclusion, OCTA findings of the ONH area may provide evidence that microvascular changes occur preceding neural impairment in early-stage DR. However, further researches are still needed to support the statement. Reduced ONH perfusion inside the optic disc may be one of the crucial biomarkers in early-stage DR detection, and it is a possible sensitive visual acuity predictor in early-stage DR subjects. With the ONH mode, OCTA may be a more promising tool in DR screening.
References
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R (2016) Diabetic retinopathy. Nat Rev Dis Primers 2:16012
Stitt AW, Curtis TM, Chen M et al (2016) The progress in understanding and treatment of diabetic retinopathy. Progr Retinal Eye Res 51:156–186
Lechner J, O’Leary OE, Stitt AW (2017) The pathology associated with diabetic retinopathy. Vis Res 139:7–14
Chua J, Lim CXY, Wong TY, Sabanayagam C (2018) Diabetic retinopathy in the Asia-Pacific. Asia Pac J Ophthalmol (Phila) 7(1):3–16
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48
Carpineto P, Toto L, Aloia R et al (2016) Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye (Lond) 30(5):673–679
Moran EP, Wang Z, Chen J, Sapieha P, Smith LE, Ma JX (2016) Neurovascular cross talk in diabetic retinopathy: pathophysiological roles and therapeutic implications. Am J Physiol Heart Circ Physiol 311(3):H738–H749
Simo R, Stitt AW, Gardner TW (2018) Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61(9):1902–1912
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55
Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA (2018) Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci 59(10):4292–4298
Kim K, Kim ES, Yu SY (2018) Optical coherence tomography angiography analysis of foveal microvascular changes and inner retinal layer thinning in patients with diabetes. Br J Ophthalmol 102(9):1226–1231
Kaizu Y, Nakao S, Sekiryu H et al (2018) Retinal flow density by optical coherence tomography angiography is useful for detection of nonperfused areas in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 256(12):2275–2282
Alnawaiseh M, Lahme L, Müller V, Rosentreter A, Eter N (2018) Correlation of flow density, as measured using optical coherence tomography angiography, with structural and functional parameters in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 256(3):589–597
Li Z, Alzogool M, Xiao J, Zhang S, Zeng P, Lan Y (2018) Optical coherence tomography angiography findings of neurovascular changes in type 2 diabetes mellitus patients without clinical diabetic retinopathy. Acta Diabetol 55(10):1075–1082
Solomon SD, Chew E, Duh EJ et al (2017) Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 40(3):412–418
Whelton PK, Carey RM, Aronow WS et al (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71(6):1269–1324
Tuttle KR, Bakris GL, Bilous RW et al (2014) Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 37(10):2864–2883
Carnevali A, Sacconi R, Corbelli E et al (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54(7):695–702
Simonett JM, Scarinci F, Picconi F et al (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol 95(8):e751–e755
Cao D, Yang D, Yu H et al (2018) Optic nerve head perfusion changes preceding peripapillary retinal nerve fiber layer thinning in preclinical diabetic retinopathy. Clin Exp Ophthalmol. https://doi.org/10.1111/ceo.13390
Mansoori T, Sivaswamy J, Gamalapati JS, Balakrishna N (2018) Topography and correlation of radial peripapillary capillary density network with retinal nerve fibre layer thickness. Int Ophthalmol 38(3):967–974
Chan G, Balaratnasingam C, Xu J et al (2015) In vivo optical imaging of human retinal capillary networks using speckle variance optical coherence tomography with quantitative clinico-histological correlation. Microvasc Res 100:32–39
Scoles D, Gray DC, Hunter JJ et al (2009) In-vivo imaging of retinal nerve fiber layer vasculature: imaging histology comparison. BMC Ophthalmol 9:9
van Dijk HW, Verbraak FD, Kok PH et al (2012) Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci 53(6):2715–2719
Santos AR, Ribeiro L, Bandello F et al (2017) Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes 66(9):2503–2510
Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW (2007) Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 48(11):5152–5159
Ziccardi L, Parisi V, Picconi F et al (2018) Early and localized retinal dysfunction in patients with type 1 diabetes mellitus studied by multifocal electroretinogram. Acta Diabetol 55(11):1191–1200
Simo R, Hernandez C, Porta M et al (2018) Effects of topically administered neuroprotective drugs in early stages of diabetic retinopathy. Results of the EUROCONDOR Clinical Trial. Diabetes. https://doi.org/10.2337/db18-0682
Araszkiewicz A, Zozulinska-Ziolkiewicz D et al (2012) Neurodegeneration of the retina in type 1 diabetic patients. Pol Arch Med Wewn 122(10):464–470
Zang P, Gao SS, Hwang TS et al (2017) Automated boundary detection of the optic disc and layer segmentation of the peripapillary retina in volumetric structural and angiographic optical coherence tomography. Biomed Opt Express 8(3):1306
Leite MT, Rao HL, Weinreb RN et al (2011) Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmol 151(1):85–92.e81
Jia Y, Simonett JM, Wang J et al (2017) Wide-field OCT angiography investigation of the relationship between radial peripapillary capillary plexus density and nerve fiber layer thickness. Invest Ophthalmol Vis Sci 58(12):5188–5194
Wang Q, Chan SY, Yang JY et al (2017) Density of the macular and radial peripapillary capillary network measured by optical coherence tomography angiography. Acta Ophthalmol 95(6):e511–e512
Triolo G, Rabiolo A, Shemonski ND et al (2017) Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Invest Ophthalmol Vis Sci 58(13):5713–5722
Eladawi N, Elmogy M, Khalifa F et al (2018) Early diabetic retinopathy diagnosis based on local retinal blood vessel analysis in optical coherence tomography angiography (OCTA) images. Med Phys. https://doi.org/10.1002/mp.13142
Samara WA, Shahlaee A, Adam MK et al (2017) Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 124(2):235–244
Snodderly DM, Weinhaus RS, Choi JC (1992) Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci 12(4):1169–1193
Stone J, van Driel D, Valter K, Rees S, Provis J (2008) The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res 1189:58–69
Campbell JP, Zhang M, Hwang TS et al (2017) Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep 7:42201
Acknowledgements
The research was funded by the National Natural Science Foundation of China 81570845 and the Natural Science Foundation of Guangdong Province 2015A030313019.
Funding
National Natural Science Foundation of China 81570845; Natural Science Foundation of Guangdong Province 2015A030313019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed By Antonio Secchi.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Wen, X., Zeng, P. et al. Do microvascular changes occur preceding neural impairment in early-stage diabetic retinopathy? Evidence based on the optic nerve head using optical coherence tomography angiography. Acta Diabetol 56, 531–539 (2019). https://doi.org/10.1007/s00592-019-01288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01288-8