Abstract
Purpose
To compare retinal capillary plexus vessel densities, choroidal thickness, optic disc vessel densities and foveal avascular zone measurements between normal subjects and subjects with obstructive sleep apnea syndrome (OSAS) using spectral-domain OCT angiography (OCTA).
Methods
Forty eyes of patients with overall OSAS, 20 eyes of patients with mild-moderate OSAS, 20 eyes of patients with severe OSAS on polysomnography and 21 controls were evaluated with the RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA). Superficial and deep retinal capillary plexus vessel densities, choroidal thickness and foveal avascular zone (FAZ) parameters (area, diameter, acircularity index of the FAZ, foveal density-300) were measured for all subjects.
Results
Superficial capillary plexus vessel densities of the groups were similar. Deep capillary plexus (DCP) vessel densities of all three OSAS groups decreased in the parafoveal region (< 0.001). In the perifoveal region, DCP vessel densities were decreased in the Overall OSAS group (p = 0.023) and in the Severe OSAS group (p = 0.021), whereas it was similar in the Mild-moderate OSAS group. Choroidal thickness decreased in all three OSAS groups (p < 0.001). All FAZ measurements and foveal density-300 measurements were similar compared to the control group.
Conclusion
Our findings indicate that in severe OSAS patients, deep parafoveal, perifoveal vascular densities decrease and the choroid layer becomes thinner. When evaluating a patient with OSAS, it should be kept in mind that there may be eye involvement due to the disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a disease with recurrent partial or total obstruction of the upper respiratory tract that causes oxygen desaturation, hypercapnia, and intermittent hypoxia during sleep [1]. Hypercapnia and hypoxia cause endothelial dysfunction, inflammation and oxidative stress, which contribute to the development of many systemic diseases in OSAS patients [2]. The retina is one of the tissues in the body that requires the most oxygen per unit weight. Autonomic nervous system innervation is not seen in retinal blood vessels, so recurrent hypercapnia and hypoxia in OSAS patients may adversely affect retinal blood circulation [3]. To date, many ophthalmic diseases such as glaucoma, non-arteritic anterior ischemic optic neuropathy, optic disc edema, and retinal vein occlusion have been associated with OSAS [4,5,6,7].
Many imaging devices can be used in the diagnosis and follow-up of retinal diseases. The most recent of these methods is the optical coherence tomography angiography (OCTA), which allows non-invasive visualization of the retinal vascular network in detail without using contrast media [8]. In the literature, OCTA has been used in a very few number of studies to demonstrate alterations in the retinal vascular network in OSAS patients [9, 10]. In this study, we aimed to demonstrate the effects of OSAS on the retina by evaluating previously unavailable parameters such as foveal avascular zone measurement using OCTA.
Material and methods
This is a prospective case–control study and was conducted at Ankara Numune Training and Research Hospital between June 2018–June 2019, in accordance with the principles of the Helsinki Declaration and with the approval of Ankara Numune Training and Research Hospital Institutional Ethics Committee. Informed consents were obtained from all subjects.
Standard full-night polysomnography (PSG) was performed in individuals over 18 years old who presented to the ENT outpatient clinic with OSAS symptoms (e.g. snoring, daytime sleepiness, witnessed apnea). The patients were examined for the first time by polysomnography and they had no known OSAS history. Forty patients who were diagnosed as OSAS with apnea–hypopnea indices (AHI) greater than 5 (as determined by the number of apnea and hypopnea per sleep-hour) were included in the study. Twenty-one healthy adults without OSAS symptoms were included in the control group. OSAS was clinically excluded in these control subjects for them giving less than 3 “yes” answers to the 8 questions in the STOP-Bang questionnaire. All participants were examined by OCTA (RTVue XR Avanti; Optovue, Inc., Fremont, CA) after a complete ophthalmic examination. Since OSAS systemic involvement will affect both eyes in a similar way; one eye of each patient was included in the study. One eye of each patient was identified for inclusion by simple randomization, for which random computer-generated numbers were used. Forty patients with OSAS were divided into two groups according to the severity of the disease (AHI = 5–30: mild-moderate OSAS; AHI > 30: severe OSAS). OCTA measurements of the three groups (overall OSAS, mild-moderate OSAS, and severe OSAS) were compared with those of the control group. In addition, the correlation between the OCTA parameters and the time spent with SpO2 below 90% as detected by PSG was examined.
Patients with hypertension, diabetes, hyperlipidemia, smoking, glaucoma suspect (Intraocular pressure (IOP) > 21 mmHg with applanation tonometry, glaucomatous disc appearance (generalised/focal enlargement of the cup, disc haemorrhage, usually at superior & inferior poles of neuroretinal rim thinning, asymmetry of cupping between patient's eyes, loss of nerve fibre layer, parapapillary atrophy), positive family history for glaucoma were considered as glaucoma suspect.), history of previous ophthalmic surgery, and a spherical equivalent of > 2D were excluded from the study in that these conditions may confound our results by affecting ocular perfusion. In addition, none of the patients included in the study had a known systemic, vascular, and/or autoimmune disease.
STOP–bang questionnaire and polysomnography
The STOP-Bang questionnaire is an easily applicable survey that allows OSAS to be excluded without polysomnography. There are 8 questions in the questionnaire, where one's snoring, daytime fatigue, and drowsiness, history of witnessed apnea, use of antihypertensive drugs, body mass index greater than 35, age greater than 50, neck circumference greater than 40 cm and gender is questioned. OSAS is clinically excluded in cases who answer "yes" to less than 3 of the 8 questions. When the cut-off level is ≥ 3, the sensitivity of the STOP-Bang questionnaire to predict OSAS (apnea–hypopnea index (AHI) > 5) is 83.9% [11].
OCTA
All eye examinations were performed by the same ophthalmologist. The ophthalmologist was blinded to whether the person examined had OSAS or was a healthy volunteer. The RTVue-XR Avanti OCTA (Optovue, Inc., Fremont, CA) system, which runs the split spectrum amplitude-decorrelation angiography (SSADA) algorithm and has a 70 kHz SD-OCT system was used to determine the superficial and deep capillary plexus densities (With the automatic segmentation of the device, the area between the internal limiting membrane and 9 µm above the internal plexiform layer was determined as the superficial capillary plexus, and the area between 9 µm above the internal plexiform layer and 9 µm below the outer plexiform layer was determined as the deep capillary plexus), FAZ and retinal vascular structures of the subjects. Eyes of all subjects were dilated with 1% tropicamide prior to measurement. Five measurements were run for each individual and the one with the best image quality was selected for statistical analysis. Low-quality and blurred images due to motion were removed. For analysis, fovea-centered 6 × 6 mm scan areas were used [12, 13]. Fovea-centered OCTA images were confirmed by cross-referencing with OCT scans. This was done according to the vascular flow density measured within a parafoveal ring with a diameter of 1–2.5 mm, proportional to the total area. FAZ size was determined in superficial and deep capillary plexus (SCP and DCP) in non-flow mode. The vascular density was determined using the automatic density measurement tool of the device software. The avascular area in the SCP, the FAZ area, the FAZ diameter, the acircularity index of the FAZ (AI; the ratio of the circumference of the FAZ and the circumference of an equal area) and foveal density-300 (vessel density within 300 μm around FAZ), as well as vascular densities in the foveal, parafoveal and perifoveal regions for SCP and DCP were recorded separately for each eye. The peripapillary region was defined as a 700 μm elliptical ring extending from the optic disc boundary. The optical disc boundary was automatically detected by software based on Bruch's membrane opening. In the case of a disc margin detection error, correction of disc margin was performed manually by the observer. Peripapillary and optic nerve head vascular density were defined as the areas covered by the major vessels and microvessels in the peripapillary and optic disc region, respectively.
Choroid measurements were performed using SS-OCT (Triton, Topcon Inc.) by the same physician in all patients. All patients underwent fovea-centered 12 × 12 mm radial line scans. The choroidal thickness was evaluated using the scan in which the choroido-scleral junction was most clearly seen. Choroidal thickness was measured after manually adjusting the external segmentation line in the subfoveal region to the visible choroidoscleral junction (where necessary). The internal segmentation line remained outside the RPE-Bruch’s complex.
Statistical analysis
All statistical analyses were performed using the SPSS software (SPSS version 22, Chicago, IL, US), and p < 0.05 was considered statistically significant. The normality of the data was assessed by the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± standard deviation and discrete variables were presented as frequencies and percentages. The study population was classified as the control group, overall OSAS group (i.e. all patients with OSAS regardless of the disease severity), mild to moderate OSAS group and severe OSAS group. The parameters in all three OSAS groups were compared to those in the control group. Differences were evaluated by Student’s two-tailed t-test for the normally distributed numerical data. The Spearman’s rank correlation coefficient was calculated to determine the relationship between AHI and the time spent (percent of the recording time) with SpO2 below 90% with the FAZ parameters, for all OSAS patients.
Results
The mean age of the 40 patients (27 males, 13 females) in the overall OSAS group was 50.8 ± 9.19 years. Of those, 5 patients had mild, 15 had moderate, and 20 had severe OSAS. The mean AHI was 35.86 ± 24.3 in the overall OSAS group, 17.34 ± 6.21 in the mild-moderate OSAS group and 54.38 ± 21.24 in the severe OSAS group. While the average sleep time spent with SpO2 below 90% in the overall OSAS group was 48.9 ± 74.8 min, in the mild-moderate and the severe OSAS groups these values were 21.2 ± 30.9 and 76.69 ± 94.5 min, respectively. The groups were found to be similar in terms of age and average axial lengths. The descriptive characteristics of the groups are given in Table 1.
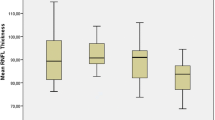
Both the AHI scores and the time spent below 90% SpO2 did not have a statistically significant correlation with the superficial and deep capillary plexus vessel densities (Fig. 1). In the statistical analysis between groups determined according to the severity of the disease shows that superficial capillary plexus vessel densities were similar in all groups. In the deep capillary plexus, the parafoveal region vascular density was significantly decreased in the overall OSAS group. In addition, the mild-moderate OSAS group and severe OSAS group also showed a decrease in vascular density. In the perifoveal region, there was a decrease in vascular densities in the overall OSAS and severe OSAS groups, while in the mild-moderate OSAS group, the vascular densities were similar to the control subjects (Fig. 2). Vascular densities in the other regions were also comparable. Macular choroid thickness was significantly decreased in overall OSAS, mild-moderate OSAS, and severe OSAS groups compared to the control group (Table 2) (Fig. 3).
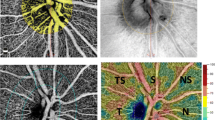
Correlation analysis between AHI scores and time spent below %90 SpO2 and superficial and deep capillary plexus vessel density. a Correlation between SCP parafoveal vessel density and AHI. b Correlation between SCP perifoveal vessel density and AHI. c Correlation between DCP parafoveal vessel density and AHI. d Correlation between DCP perifoveal vessel density and AHI. e Correlation between SCP parafoveal vessel density and time spent %90 SpO2 below. f Correlation between SCP perifoveal vessel density and time spent %90 SpO2 below. g Correlation between DCP parafoveal vessel density and time spent %90 SpO2 below. h Correlation between DCP perifoveal vessel density and time spent %90 SpO2 below
In all three OSAS groups, the FAZ areas in all retinal vascular systems, the FAZ areas in the superficial capillary plexus, the FAZ diameters and the FAZ acircularity indices were similar to the control group. Again, foveal vascular densities and all optic disc vascular densities in all three OSAS groups were found to be similar to the control group. While the peripapillary vascular density was found to be decreased in both overall OSAS and severe OSAS groups, the vascular density in the mild-moderate OSAS group was similar to those in the control subjects (Table 3). It was observed that the time spent with SpO2 below 90% values were positively correlated with the FAZ area (r = 0.33, p = 0.033), but not with the severity of OSAS.
Discussion
This study showed that the intensity of parafoveal and perifoveal vessels in the deep capillary plexus decreased significantly in patients with OSAS when compared to the control group. In addition, it has been demonstrated that the choroid layer is thinned and the peripapillary region vascular density is decreased in OSAS patients. FAZ-related parameters and foveal vessel density were found to be similar to the values in the control group.
Hypoxia and hypercapnic acidosis due to recurrent upper respiratory obstruction during sleep, and consequent sympathetic activation, the release of pro-inflammatory mediators and endothelial dysfunction all play important roles in the pathophysiology of OSAS-associated vascular diseases [14]. Hypoperfusion and decreased retinal vascular density, which occur as the condition gains a chronical characteristic, may lead to partial axonal degeneration and thinning of the retinal nerve fibre layer [15]. In our study, the vessel density in the superficial capillary plexus of all three OSAS groups was similar to that of the control group. However, in the deep capillary plexus, vascular density in the parafoveal and perifoveal region was significantly reduced in the overall OSAS and severe OSAS groups. Coscas et al. reported that vessel density in DCP decreased more than that in SCP after retinal vein occlusion, and this was attributed to higher perfusion pressure and oxygen saturation as a result of the direct relationship between SCP and retinal arterioles [16]. An experimental study using retinal ischemia/reperfusion model in rats showed that the involvement in DCP is greater than in SCP [17]. In another study, macular vascular structures of regular smokers and non-smokers were compared. Similar to our results, they showed that DCP foveal vessel density decreased, while the density of SCP vessel did not change [18]. Our comment of the primary effect of vessel density in DCP; SCP has a high oxygenation and rich metabolic support arising from the central retinal artery and choriocapillaris. In our study, the decrease in DCP vessel density in OSAS patients and the unaffected SCP may be related to the duration of the disease. It may also suggest that the DCP is the first structure to be affected by hypoxia in OSAS patients.
The choroid, which extends from the edge of the optic nerve head to the oro serrata, and has a highly vascularized tissue, may undergo structural changes due to recurrent hypoxia occurring in OSAS patients. Choroidal blood flow is regulated by parasympathetic innervation, in which vasodilator agents are effective. Recurrent hypoxia and reperfusion lead to increased oxidative stress and inflammation, endothelial dysfunction, inadequate response to vasodilators, and increased sympathetic activation. A decrease in choroidal thickness may ultimately occur due to decreased choroidal blood flow [19]. Many studies have shown the thinning of the choroid layer in OSAS patients [20, 21]. According to He et al., choroidal blood flow in mild OSAS patients is adequately maintained, but it is decreased in moderate and severe OSAS patients [20]. Since the number of mild OSAS patients in our study was very low, we could not compare the choroid thickness of these patients with the control group; however, we found that the choroid layer in all three OSAS groups was thinner than the control group. The thinning of the choroid layer in the overall OSAS group may be due to the fact that the majority of the subjects in this group consist of moderate and severe OSAS patients.
The FAZ parameters (area, circumference, acircularity index, and FD-300) can be evaluated by OCTA in the entire retinal vascular area [22]. The photoreceptors reside in FAZ and this region shows a high metabolic activity. FAZ is responsible for central vision. Capillary dropout, which is a prominent feature of retinal vasoconstrictive diseases, causes ischemia, angiogenesis and retinal neovascularization with vision loss. Expansions in FAZ are an important indicator of capillary dropout and can be used to monitor the progression of the disease [22, 23]. Mastropasqua et al. reported that FAZ was found to be expanded in patients with diabetic retinopathy and that this correlated positively with the severity of the disease [24]. Carneveli et al., on the other hand, have found that FAZ area in diabetic patients without retinopathy was similar to that in the healthy control group [25]. One of the most useful parameters of OCTA showing macular perfusion is foveal density. The FD-300 is not affected by the FAZ area, as it measures the vascular density at a distance of 300 µm from the FAZ determined by the device. Inanc et al. reported that compared to the healthy controls, foveal density decreased significantly in children with diabetic retinopathy [26]. In the literature, there is currently no study examining the FAZ parameters and FD-300 in OSAS patients. In our study, we found that FAZ parameters and FD-300 in all three OSAS groups were similar to the values eht ni control group. On the other hand, while the FAZ area showed a positive correlation with the time spent below 90% SpO2, the FAZ area did not correlate with AHI, which determines the severity of OSAS. These findings show that a change related to OSAS has not yet occurred in the FAZ regions of the patients that were included in the study. This may suggest that OSAS causes disruption in FAZ parameters and FD-300 at a later time point than diabetic retinopathy does. Also, the existence and duration of desaturations rather than the severity of OSAS seem to be a more important predictor for the FAZ expansion.
While the vessels in the macula originate only from the central retinal artery, the vascular structures in the peripapillary region originate from the central retinal artery and the posterior ciliary artery [27]. In previous studies, it has been shown that posterior ciliary arteries are more sensitive to high intraocular pressure and hypercapnia than the central retinal artery in glaucoma [28, 29]. Yu et al. reported that peripapillary vascular density decreased significantly in OSAS patients compared to healthy controls, and this decrease was associated with the severity of OSAS [9]. Moyal et al., on the other hand, found the peripapillary vascular densities of OSAS patients and healthy controls similar to each other and attributed this result to the limited number of patients with severe OSAS in their studies. The authors made an intragroup comparison among the OSAS patients and found that the peripapillary vascular density in patients showing desaturation was lower than those who did not [10]. Similarly, in our study, we found that peripapillary vascular densities in both overall OSAS and severe OSAS groups were lower compared to the control group. However, all optic disc vessel densities in these patient groups were found to be similar to those in the control group.
This study had some limitations. First of all, the study was conducted in a single center and with a limited number of patients. In addition, PSG could not be performed in the control group due to its additional cost, and this group was established according to the results of the STOP-BANG survey. In addition, the limited number of mild OSAS patients prevented us from grouping OSAS patients separately according to their severity. All of the patients included in the study were recently been diagnosed with OSAS. Therefore, since it could not be deduced how long these patients have had the disease, studies with longer follow-up will be needed for a confirmation of the clinical importance of our findings in the future. One of the strengths of the study is that this is one of the limited number of studies in which OCTA, which is the most current method showing the retinal vascular system, was used in OSAS patients. In addition, FAZ parameters have been evaluated in OSAS patients for the first time in this study.
In conclusion, our findings indicate that in severe OSAS patients, deep parafoveal, perifoveal and peripapillary vascular densities decrease and the choroid layer becomes thinner. When evaluating a patient with OSAS, it should be kept in mind that there may be eye involvement due to the disease.
References
Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5:136–143. https://doi.org/10.1513/pats.200709-155MG
Jean-Louis G, Zizi F, Brown D, Ogedegbe G, Borer J, McFarlane S (2009) Obstructive sleep apnea and cardiovascular disease: evidence and underlying mechanisms. Minerva Pneumol 48:277–293
Nesper PL, Fawzi AA (2018) Human parafoveal capillary vascular anatomy and connectivity revealed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 59:3858–3867. https://doi.org/10.1167/iovs.18-24710
Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C et al (1999) High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology 106:1009–1012
Archer EL, Pepin S (2013) Obstructive sleep apnea and nonarteritic anterior ischemic optic neuropathy: evidence for an association. J Clin Sleep Med 9:613–618. https://doi.org/10.5664/jcsm.2766
Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN (2007) Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath 11:253–257
Agard E, El Chehab H, Vie A-L, Voirin N, Coste O, Dot C (2018) Retinal vein occlusion and obstructive sleep apnea: a series of 114 patients. Acta Ophthalmol 96:919–925. https://doi.org/10.1111/aos.13798
Spaide RF, Klancnik JMJ, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133:45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616
Yu J, Xiao K, Huang J, Sun X, Jiang C (2017) Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci 58:3506–3512. https://doi.org/10.1167/iovs.17-21414
Moyal L, Blumen-Ohana E, Blumen M, Blatrix C, Chabolle F, Nordmann JP (2018) Parafoveal and optic disc vessel density in patients with obstructive sleep apnea syndrome: an optical coherence tomography angiography study. Graefe’s Arch Clin Exp Ophthalmol 256:1235–1243. https://doi.org/10.1007/s00417-018-3943-7
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S et al (2008) STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108:812–882. https://doi.org/10.1097/ALN.0b013e31816d83e4
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ et al (2012) Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 20:4710–4725. https://doi.org/10.1364/OE.20.004710
Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L et al (2015) Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 133:1045–1052. https://doi.org/10.1001/jamaophthalmol.2015.2225
Mentek M, Aptel F, Godin-Ribuot D, Tamisier R, Pepin J-L, Chiquet C (2018) Diseases of the retina and the optic nerve associated with obstructive sleep apnea. Sleep Med Rev 38:113–130. https://doi.org/10.1016/j.smrv.2017.05.003
Lin P-W, Friedman M, Lin H-C, Chang H-W, Pulver TM, Chin C-H (2011) Decreased retinal nerve fibre layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol 249:585–593
Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M et al (2016) Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol 161:160–162. https://doi.org/10.1016/j.ajo.2015.10.008
Nakahara T, Hoshino M, Hoshino S, Mori A, Sakamoto K, Ishii K (2015) Structural and functional changes in retinal vasculature induced by retinal ischemia-reperfusion in rats. Exp Eye Res 135:134–145. https://doi.org/10.1016/j.exer.2015.02.020
Çiloğlu E, Unal F, Sukgen EA, Kocluk Y, Dogan NC (2020) Evaluation of foveal avascular zone and capillary plexus in smokers using optical coherence tomography angiography. J Curr Ophthalmol 32:53–57. https://doi.org/10.1016/j.joco.2019.09.002
Li C, Fitzgerald MEC, Del MN, Haughey C, Reiner A (2018) Defective choroidal blood flow baroregulation and retinal dysfunction and pathology following sympathetic denervation of choroid. Invest Ophthalmol Vis Sci 59:5032–5044. https://doi.org/10.1167/iovs.18-24954
He M, Han X, Wu H, Huang W (2016) Choroidal thickness changes in obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath 20:369–378. https://doi.org/10.1007/s11325-015-1306-8.6
Karaca EE, Ekici F, Yalçın NG, Çiftçi TU, Özdek Ş (2015) Macular choroidal thickness measurements in patients with obstructive sleep apnea syndrome. Sleep Breath 19:335–341. https://doi.org/10.1007/s11325-014-1025-6
Mo S, Krawitz B, Efstathiadis E, Geyman L, Weitz R, Chui TYP et al (2016) Imaging foveal microvasculature: optical coherence tomography angiography versus adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci 57:130–140. https://doi.org/10.1167/iovs.15-18932
Shen C, Yan S, Du M, Zhao H, Shao L, Hu Y (2018) Assessment of capillary dropout in the superficial retinal capillary plexus by optical coherence tomography angiography in the early stage of diabetic retinopathy. BMC Ophthalmol 18:113. https://doi.org/10.1186/s12886-018-0778-2
Mastropasqua R, Toto L, Mastropasqua A, Aloia R, De Nicola C, Mattei PA et al (2017) Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol 10:1545–1551. https://doi.org/10.18240/ijo.2017
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G et al (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54:695–702. https://doi.org/10.1007/s00592-017-0996-8
Inanc M, Tekin K, Kiziltoprak H, Ozalkak S, Doguizi S, Aycan Z (2019) Changes in retinal microcirculation precede the clinical onset of diabetic retinopathy in children with type 1 diabetes mellitus. Am J Ophthalmol 207:37–44. https://doi.org/10.1016/j.ajo.2019.04.011
Hayreh SS (2001) The blood supply of the optic nerve head and the evaluation of it—myth and reality. Prog Retin Eye Res 20:563–593
Hayreh SS (1972) Optic disc changes in glaucoma. Br J Ophthalmol 56:175–185. https://doi.org/10.1136/bjo.56.3.175
Hosking SL, Harris A, Chung HS, Jonescu-Cuypers CP, Kagemann L, Roff Hilton EJ et al (2004) Ocular haemodynamic responses to induced hypercapnia and hyperoxia in glaucoma. Br J Ophthalmol 88:406–411. https://doi.org/10.1136/bjo.2002.008995
Funding
No Funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çolak, M., Özek, D., Özcan, K.M. et al. Evaluation of retinal vessel density and foveal avascular zone measurements in patients with obstructive sleep apnea syndrome. Int Ophthalmol 41, 1317–1325 (2021). https://doi.org/10.1007/s10792-020-01690-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01690-0