Abstract
Purpose
The purpose of this study is to assess macular choroidal thickness measurements in patients with different severities of obstructive sleep apnea syndrome (OSAS) versus normal controls by using enhanced depth imaging optical coherence tomography (EDI-OCT).
Design
This paper is a descriptive study.
Materials and methods
In this prospective study, the macular area of 74 patients with OSAS and 33 controls were evaluated. All subjects underwent complete ophthalmic examination and macular choroidal thickness (CT) measurements by enhanced depth imaging method of the Spectralis optical coherence tomography system. Choroidal thickness (CT) was measured at the fovea and at 1,000-μm intervals from the foveal center in both temporal and nasal directions by two masked observers.
Results
The mean age was not significantly different between patients with OSAS and controls. Patients were grouped as mild (n = 15), moderate (n = 28), and severe (n = 31) according to apnea-hypopnea index (AHI) scores. The mean subfoveal choroidal thickness (SFCT) was 338.0 ± 85.2 μm in the control group versus 351.3 ± 90, 307.8 ± 65.5, and 325.4 ± 110.2 μm in mild, moderate, and severe groups, respectively (p = 0.416). There was no significant correlation between the severity of OSAS and choroidal thickness.
Conclusions
The patients with OSAS seem to protect the choroidal thickness despite hypoxia. The role of OSAS in the pathophysiology of choroidal blood flow and choroidal structure needs further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a common chronic disorder characterized by recurrent partial or complete upper airway obstruction during sleep. The physiologic consequences of these episodes are repetitive bursts of sympathetic activity, hypoxia, and hypercapnia [1]. Severe hypoxia leads to increase in vascular resistance which, in turn, may compromise retinal perfusion and oxygenation [2, 3]. This vascular resistance may cause structural change on the choroid tissue.
The human retina is supplied by dual vascular structure: the retinal circulation nourishes the innermost retinal layers and the choroid feeds the outermost retinal layers [4]. Maintenance of normal retinal function depends on a continuous supply of oxygen [5]. Retinal circulation and choroidal vasculature have been shown to have a capacity to autoregulate, that is, the ability to maintain constant blood flow with changing ocular perfusion pressure (OPP) and oxygen levels [5–7]. There are conflicting data about the effect of OSAS on the vascular autoregulation and choroidal blood flow (ChBF) response to hypoxia.
Clinical evaluation of the choroid was usually performed with indocyanine green angiography (ICG) or contact B-scan ultrasonography (USG) before the invention of spectral domain optical coherence tomography (SD-OCT) [8]. Accurate in vivo morphologic assessment of the choroid without SD-OCT was quite difficult. The recent development of enhanced depth imaging has made choroidal examination with SD-OCT possible [9]. Enhanced depth imaging optic coherence tomography (EDI-OCT), a relatively new technique, uses light with a longer wavelength, which is more effective for choroidal scanning and provides valuable data about choroidal morphology in normal healthy subjects and in subjects with several diseases [8–14].
Recently, there has been interest in defining a possible link between OSAS and oculovascular health [15]. The aims of our study were to compare choroidal thickness (CT) in patients with OSAS and healthy normal subjects and to investigate how the choroid responds to hypoxia.
Methods
Subjects
Patients were recruited from those who were referred for suspected OSAS to the “sleep unit” of pulmonary diseases clinic (Gazi University Hospital) between March and September 2013. This study was conducted in accordance with the tenets of the Declaration of Helsinki, and written informed consent was obtained from all of the subjects. The study protocol was reviewed and approved by the local Institutional Review Board and Ethics Committee. Patients clinically suspected of having OSAS underwent standard overnight polysomnography (PSG). All patients were referred to ophthalmology clinic from sleep unit in the morning after that overnight PSG. The study included 74 patients with OSAS and 33 control subjects who did not have OSAS on PSG. Because there was no significant difference between right and left eyes, one of them was randomly selected for the study. Diagnosis of OSAS was made by the sleep unit of Gazi University Pulmonary Disease Clinic.
Sleep study (polysomnography)
Overnight PSG was performed in all patients by a computerized system (Somnostar alpha; Sensormedics, USA) and included the following variables: electrooculogram (2 channels), electroencephalogram (4 channels), electromyogram of submental muscles (2 channels), electromyogram of the anterior tibialis muscle of both legs (2 channels), electrocardiogram, and airflow (with a nasal cannula). Chest and abdominal efforts (2 channels) were recorded using inductive plethysmography, and arterial oxyhemoglobin saturation (SaO2, 1 channel) by pulse oximetry with a finger probe. The recordings were conducted at a paper speed of 10 mm/s, and sleep stage was scored according to the standard criteria of Rechtschaffen and Kales [16]. Arousals were scored according to accepted definitions. The apnea-hypopnea index (AHI) was obtained by dividing the total number of apneas and hypopneas by the total sleep time (TST). Apneas were defined as complete cessation of airflow ≥10 s. Hypopneas were defined as reduction of >50 % in one of three respiratory signals, airflow signal, or either respiratory or abdominal signals of respiratory inductance plethysmography, with an associated decrease of ≥3 % in oxygen saturation or an arousal. According to the recently updated International Classification of Sleep Disorders published by the American Academy of Sleep Medicine, a diagnosis of OSAS was made if the AHI is ≥15, independent of occurrence of symptoms, or whenever an AHI >5 is associated with any of the following; (1) sleep attacks; (2) excessive daytime sleepiness (EDS), unrefreshing sleep, fatigue, or insomnia; or (3) witnessed heavy snoring and/or breathing pauses referred by the partner. Patients with an AHI <5 at overnight PSG were included as control subjects. Patients with other sleep disorders such as upper airway resistance syndrome (UARS), periodic leg movements syndrome (PLMs), or narcolepsy were excluded. OSAS severity was classified as mild for an AHI between 5 and 15, moderate for an AHI greater than 15 and less than 30, and severe for an AHI greater than 30 [17]. Minimum SaO2 (LSAT), mean SaO2 (mSO2), and time spent (percent of the recording time) with SaO2 less than 90 % (desaturation index, DESI) were recorded for all cases. Hypoxia was defined as an SaO2 value less than 90 %.
Ophthalmologic examination
All ophthalmologic examinations were done by the same physician blinded to OSAS diagnosis or AHI score. All subjects underwent a thorough ocular examination, including an autorefractometer (RM8900; Topcon), best-corrected visual acuity (BCVA) measurement with a 6-m Snellen eye chart, slit-lamp examination, intraocular pressure measurement, and fundoscopy. Exclusion criteria included high myopia or hyperopia (>+6 or −6 diopters of spherical equivalent), best-corrected visual acuity (BCVA) <20/25, amblyopia, any retinal or choroidal pathology, history of intraocular surgery or injection, evidence of glaucoma, and poor image due to cataract or unstable fixation. Atherosclerotic risk factors likely to affect ocular blood flow such as hypertension (HT) and high blood cholesterol level (HBCL) were investigated in all subjects. Since it was impossible to completely eliminate these factors in both groups, the distribution of these risk factors was evaluated, and it was found similar between groups (chi-square 0.01; p = 0.91). Subjects with systemic diseases or conditions other than HT and HBCL that might affect CT were also excluded, such as those with diabetes mellitus or peripheral vascular diseases.
Choroidal thickness measurements
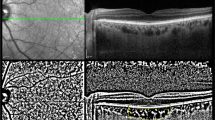
The CT at the macular area of 107 eyes was examined with Heidelberg Spectralis OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). All subjects were examined by using EDI-OCT imaging with pupil dilation. The method of obtaining EDI-OCT images has been reported by Spaide et al. previously [9]. The OCT device was pushed sufficiently close to the eye to obtain an inverted image. Each section was obtained using eye tracking, and 100 B-scans were averaged to improve the signal-to-noise ratio. A 9-mm horizontal image that included the fovea was obtained. Additional hardware or software is unnecessary with EDI-OCT for the EDI technique. The images were reinverted for display purposes in the present study [9, 18]. All EDI-OCT images were obtained by the same experienced technician.
The choroid was independently measured by two masked observers (EEK and NGY) from the external part of the outer highly reflective layer corresponding to the retinal pigment epithelium (RPE)/Bruch reflective complex to the highly reflective layer corresponding to the sclerochoroidal interface. In each image, lines of both RPE and the chorioscleral border were determined manually by the trained observers in a masked fashion. The CT was measured under the foveal center and four different points 1,000-μm apart, starting at the foveal center, in the nasal and temporal directions (Fig. 1).
Statistical analysis
Data analysis was performed by using SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL, USA). Whether the distributions of continuous variables were normal or not was determined by Kolmogorov-Smirnov test. Homogeneity of variances was evaluated by Levene test. Continuous variables were shown as mean ± standard deviation (SD) or median (min-max) where applicable.
The mean differences among groups were analyzed by using one-way ANOVA, and Kruskal-Wallis test was applied for comparisons of the median values. When the p value from one-way ANOVA or Kruskal-Wallis test statistics was statistically significant, post hoc Tukey HSD or Conover’s non-parametric multiple comparison test was used to know which groups differ from which others.
Nominal data were analyzed by Pearson’s chi-square or Fisher’s exact test where applicable. The mean subfoveal choroidal thickness (SFCT) difference between gender groups was compared by unpaired t test. The relationship between continuous variables was evaluated by Spearman’s correlation test.
Whether the mean differences between right- and left-sided clinical measurements were statistically significant or not was evaluated by paired t test; otherwise, Wilcoxon sign rank test was applied for comparisons of the median values.
Bland-Altman plots were used to assess the magnitude of disagreement between observers. The means of agreement differences (i.e., bias) as well as upper and lower limits set at 95 % confidence interval were calculated. Degrees of agreement interobserver were also evaluated by calculating intraclass correlation coefficient (ICC), 95 % confidence intervals, and coefficient of variation (CoV). Bland-Altman plots were performed by using MedCalc, version 11.1.1.0 (MedCalc Software, Broekstraat 52, B-9030 Mariakerke, Belgium). A p value less than 0.05 was considered statistically significant.
Results
One hundred and seventeen subjects underwent PSG exam. Of these, four were excluded because of glaucoma with retinal nerve fiber layer defects, three because of previous ocular surgeries, two because of refractive error, and one for central apnea. A total of 107 subjects were included in the study. In the study group, 15, 28 and 31 patients had mild, moderate and severe OSAS, respectively. Thirty-three subjects with an AHI less than 5 did not have OSAS and were included as normal controls. No significant difference was found between study and control groups for age and gender distribution (p = 0.092 and p = 0.504, respectively). BCVA was 0.00 LogMAR unit (20/20 Snellen equivalent) in all eyes. No significant difference in spherical equivalent (SE) was found among the four OSAS groups. Body mass index (BMI) was significantly lower in both mild and moderate OSAS groups compared to the severe group (p < 0.001). Based on PSG data, significant differences in mSO2, LSAT, DESI, and arousal were observed among groups (p < 0.001). Demographic, polysomnographic, and ophthalmologic data are summarized in Table 1.
The mean SFCT of the right eye was 328.3 ± 89.6 μm, and for the left eye, it was 328.4 ± 79.5 μm. The difference between eyes was not statistically significant (p = 0.985). CT was thinnest 2 mm nasally, reached maximum under the fovea, and decreased slightly at the temporal end of scan in both groups. The mean SFCT in men was 328.7 ± 96.0 μm and that in women was 327.1 ± 70.0 μm; the difference was not significant (p = 0.937). Mean subfoveal choroidal thickness (SFCT) was 338.0 ± 85.2 μm in the control group versus 337.97 ± 85.2 μm in patients with OSAS (p = 0.458), and within OSAS subgroups, mean SFCT were 351.3 ± 90, 307.8 ± 65.5, and 325.4 ± 110.2 μm in mild, moderate, and severe groups, respectively (p = 0.416). There was no significant correlation between controls and OSAS group and among OSAS subgroups as well. Detailed measurements at different locations of the macula are outlined in Table 2.
Correlation coefficients between SFCT and age, BMI, AHI, mSO2, LSAT, and DESI are shown in Table 3. We found a significant negative correlation only between age and SFCT (r = −0.369). Correlations among other parameters were not significant.
Table 4 summarizes interobserver repeatability. All choroidal measurements were highly reproducible. The ICCs showed perfect reliability at all locations (ICCs >0.90), and ICC at the subfoveal region was 0.97 (0.96–0.98). The CoVs obtained from the foveal measurements of observers were 27.29 and 27.08, respectively. Bland-Altman plots of difference against mean CT showed no significant change in variability for the range of CT.
Discussion
In this study, using EDI-OCT, we investigated how choroidal thickness changed in different stages of OSAS and compared with control subjects who did not have OSAS. EDI-OCT is a new technique that allows in vivo measurement of choroidal thickness. Since the first report of EDI-OCT, choroidal OCT imaging has been of interest and EDI-OCT-based choroidal studies are increasing in number [9]. In this study, we aimed to investigate choroidal structure in patients with OSAS by EDI-OCT.
OSAS is characterized by intermittent upper airway obstruction during sleep, with concurrent hypoxia and hypercapnic acidosis. OSAS is known as an important risk factor for vascular diseases. During arousal from sleep, the sympathetic activation may cause arterial hypertension and cardiac arrhythmias by stimulation of vasoconstriction [19]. Additionally, in some studies, autonomic vascular dysregulation has been shown [20]. The retina is one of the most metabolically active tissues in the body, consuming O2 more rapidly than many other tissues, such as the brain [21]. Oxygen demand of the retina is high and a continuous O2 supply is essential for metabolic activity [22]. This supply is delivered by both retinal and choroidal circulations. It is well known that retinal circulation has the capability of autoregulation [4, 23]. Retinal circulation is autoregulated by local metabolic factors, O2, and CO2 levels. Hyperoxia causes vasoconstriction of retinal vessels and reduces blood supply, whereas hypoxia and hypercapnia dilate retinal vessels and increase blood flow [24–26]. However, the influence of O2 and CO2 on choroidal circulation is not fully understood [27]. Some researchers have suggested that autonomic innervation of choroidal circulation is dominant compared to local metabolic control of retinal vasculature [28, 29]. However, Riva and associates showed that breathing 7.5 % CO2 caused a 40 % increase in ChBF measured by laser Doppler flowmetry [30]. Additionally, they found that hyperoxia does not affect foveolar ChBF. Schmetterer and associates measured fundus pulsations in the macula by laser interferometry and they showed that hyperoxia slightly reduces ChBF, but CO2, mixed with either air or oxygen, increases ChBF in the macula [31, 32].
Xin and associates investigated CT of OSAS patients and they found the subfoveal and nasal CT of the severe group was significantly thinner than that of the control, mild, and moderate groups [33]. We also expected to find a decrease of the macular choroidal thickness because of increased sympathetic activity. However, in this study, we did not find significant correlation in the PSG parameters such as AHI, mSO2, LSAT, DESI, arousal, sleep efficiency, and CT among all the OSAS and control groups. This result may be associated with healthy parasympathetic fibers termination on choroid vessels. Choroidal blood flow is regulated by parasympathetic innervations, with fibers rich in the vasodilators vasoactive intestinal polypeptide (VIP) and nitric oxide (NO) [34]. If choroidal blood flow decreases, clearance of debris from RPE cells decreases and pathological changes such as degeneration and atrophy in Bruch’s membrane, RPE, and the retina occur. As a result, a structurally and functionally normal choroid vasculature is essential for the function of retina. Otherwise, abnormal choroidal blood volume and/or compromised flow can result in photoreceptor dysfunction and death [35]. It is obvious that the immediate physiological effects of sleep apnea involve hypoxia, hypercapnia, and inspiratory effort. Early response of choroid to hypoxia is probably mediated by balance between sympathetic and parasympathetic pathways. Our patients are newly diagnosed and they probably have healthy parasympathetic innervations. In accord with the results of our study, we suggest that the choroidal blood flow is preserved in newly diagnosed OSAS patients. On the other hand, patients suffering from OSAS for many years may have dominant sympathetic activity and decrease in choroidal blood flow. In this study, previously diagnosed patients were not included because of mixing effect of the treatment regimen over the results.
CT has been studied in various diseases [36–41]. Maruko and associates reported that the CT was increased in patients with central serous chorioretinopathy and Vogt-Koyanagi-Harada disease; additionally, they showed a decrease in CT after treatment [39, 40]. Margolis and Spaide found a mean SFCT of 287 ± 76 μm in healthy subjects, with a significant negative correlation with age [42]. Manjunath and associates showed that the choroid is thinner at the nasal portion of the macula in normal eyes [43]. In the current study, the mean SFCT of patients with OSAS and the controls were 323.9 ± 91.7 and 337.97 ± 85.2 μm, respectively. Additionally, we found a rapid decrease in nasal direction and reaching 243.9 ± 71 μm in patients with OSAS and 246.7 ± 86.3 μm in controls at 2 mm from the fovea. These findings were compatible with recently published data related to CT [39, 40, 42, 43].
A structurally and functionally normal choroid is essential for retinal health. Whereas choroid may be investigated by B-scan USG and/or ICG, these techniques, however, have major limitations. The former has limited resolution and reproducibility, and the latter is an invasive technique with more qualitative than quantitative results. SD-OCT has the advantage of clear images of the choroid with high reproducibility [10]. Compared with conventional OCT, EDI technique has a higher penetration and, consequently, increased sensitivity for the posterior choroid and the sclera which allows visualization of the chorioscleral interface and microarchitecture [9].
Additionally, we evaluated interobserver variability of manual macular CT measurements in this study. The reproducibility of measurements was quite high. ICCs and CoVs of measurements were ranging between 0.93 and 0.97 and 26 and 33.5, respectively. These are consistent with recently published articles, which analyzed reproducibility and repeatability of manually measured CT by SD-OCT [8, 44, 45]. High variability between observers could influence the validity of the study. Therefore, intraobserver and/or interobserver variability should be documented in the manuscript as additional information.
In conclusion, we did not find any correlation between the severity of apnea (AHI) and CT. This is probably due to intact vascular endothelium in our patients. More severe OSAS may cause sympathetic activation and vascular endothelium damage. Further studies are warranted to clarify the effect of OSAS upon choroidal structure.
References
Bradley TD, Floras JS (2003) Sleep apnea and heart failure: part I: obstructive sleep apnea. Circulation 107(12):1671–1678
Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL (1994) Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol 117(5):603–624
Chervin RD, Guilleminault C (1996) Obstructive sleep apnea and related disorders. Neurol Clin 14(3):583–609
Kergoat H, Marinier JA, Lovasik JV (2005) Effects of transient mild systemic hypoxia on the pulsatile choroidal blood flow in healthy young human adults. Curr Eye Res 30(6):465–470
Lange CA, Bainbridge JW (2012) Oxygen sensing in retinal health and disease. Ophthalmologica 227(3):115–131
Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH (1986) Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci 27(5):722–726
Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M (2003) Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci 44(5):2126–2132
Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E (2012) Macular and peripapillary choroidal thickness in diabetic patients. Retina 32(9):1781–1790
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146(4):496–500
Karaca EE, Ozdek S, Yalcin NG, Ekici F (2014) Reproducibility of choroidal thickness measurements in healthy Turkish subjects. Eur J Ophthalmol 24(2):202–208
Ding X, Li J, Zeng J, Ma W, Liu R, Li T et al (2011) Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci 52(13):9555–9560
Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S, Shiraga F (2012) Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol 56(3):230–235
Coskun E, Gurler B, Pehlivan Y, Kisacik B, Okumus S, Yayuspayi R et al (2013) Enhanced depth imaging optical coherence tomography findings in Behcet disease. Ocul Immunol Inflamm 21(6):440–445
Dhoot DS, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP et al (2013) Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol 97(1):66–69
Dhillon S, Shapiro CM, Flanagan J (2007) Sleep-disordered breathing and effects on ocular health. Can J Ophthalmol 42(2):238–243
Crofts HS, Wilson S, Muggleton NG, Nutt DJ, Scott EA, Pearce PC (2001) Investigation of the sleep electrocorticogram of the common marmoset (Callithrix jacchus) using radiotelemetry. Clin Neurophysiol 112(12):2265–2273
Quan S, Gillin JC, Littner M, Shepard J (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Editorials. Sleep 22(5):662–689
Ikuno Y, Maruko I, Yasuno Y, Miura M, Sekiryu T, Nishida K et al (2011) Reproducibility of retinal and choroidal thickness measurements in enhanced depth imaging and high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci 52(8):5536–5540
Hermida RC, Smolensky MH, Ayala DE et al (2013) Ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals [summary]. Joint recommendations from the International Society for Chronobiology [ISC], American Association of Medical Chronobiology and Chronotherapeutics [AAMCC], Spanish Society of Applied Chronobiology, Chronotherapy, and Vascular Risk [SECAC], Spanish Society of Atherosclerosis [SEA], and Romanian Society of Internal Medicine [RSIM]. Clin Investig Arterioscler 25(2):74–82
Vijayan VK (2012) Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med 6(5):557–566
Wangsa-Wirawan ND, Linsenmeier RA (2003) Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 121(4):547–557
Vanderkooi JM, Erecinska M, Silver IA (1991) Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol 260(6 Pt 1):C1131–C1150
Kergoat H, Herard ME, Lemay M (2006) RGC sensitivity to mild systemic hypoxia. Invest Ophthalmol Vis Sci 47(12):5423–5427
Deutsch TA, Read JS, Ernest JT, Goldstick TK (1983) Effects of oxygen and carbon dioxide on the retinal vasculature in humans. Arch Ophthalmol 101(8):1278–1280
Fallon TJ, Maxwell D, Kohner EM (1985) Retinal vascular autoregulation in conditions of hyperoxia and hypoxia using the blue field entoptic phenomenon. Ophthalmology 92(5):701–705
Pakola SJ, Grunwald JE (1993) Effects of oxygen and carbon dioxide on human retinal circulation. Invest Ophthalmol Vis Sci 34(10):2866–2870
Kergoat H, Faucher C (1999) Effects of oxygen and carbogen breathing on choroidal hemodynamics in humans. Invest Ophthalmol Vis Sci 40(12):2906–2911
Bill A, Sperber GO (1990) Control of retinal and choroidal blood flow. Eye (Lond) 4(Pt 2):319–325
Bill A, Nilsson SF (1985) Control of ocular blood flow. J Cardiovasc Pharmacol 7(Suppl 3):S96–S102
Riva CE, Cranstoun SD, Grunwald JE, Petrig BL (1994) Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci 35(13):4273–4281
Schmetterer L, Wolzt M, Lexer F, Alschinger C, Gouya G, Zanaschka G et al (1995) The effect of hyperoxia and hypercapnia on fundus pulsations in the macular and optic disc region in healthy young men. Exp Eye Res 61(6):685–690
Schmetterer L, Lexer F, Findl O, Graselli U, Eichler HG, Wolzt M (1996) The effect of inhalation of different mixtures of O2 and CO2 on ocular fundus pulsations. Exp Eye Res 63(4):351–355
Xin C, Wang J, Zhang W, Wang L, Peng X (2014) Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye [Lond]. doi:10.1038/eye.2013.307
Stone RA, Kuwayama Y, Laties AM (1987) Regulatory peptides in the eye. Experientia 43(7):791–800
Shiragami C, Shiraga F, Matsuo T, Tsuchida Y, Ohtsuki H (2002) Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 240(6):436–442
Imamura Y, Fujiwara T, Margolis R, Spaide RF (2009) Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29(10):1469–1473
Masuda R, Isoyama T, Bandou T, Toyoshima H, Takemura T (1990) A primary choriocarcinoma of the stomach with a review of 45 cases in Japan. Gan No Rinsho 36(9):1025–1030
Ogasawara M, Maruko I, Sugano Y, Ojima A, Sekiryu T, Iida T (2012) Retinal and choroidal thickness changes following intravitreal ranibizumab injection for exudative age-related macular degeneration. Nihon Ganka Gakkai Zasshi 116(7):643–649
Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T (2011) Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina 31(8):1603–1608
Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T, Fujiwara T et al (2011) Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 31(3):510–517
Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF (2012) Choroidal thickness and visual acuity in highly myopic eyes. Retina 32(7):1229–1236
Margolis R, Spaide RF (2009) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147(5):811–815
Manjunath V, Taha M, Fujimoto JG, Duker JS (2010) Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol 150(3):325–9 e1
Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T (2012) Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci 53(3):1102–1107
Benavente-Perez A, Hosking SL, Logan NS, Bansal D (2010) Reproducibility-repeatability of choroidal thickness calculation using optical coherence tomography. Optom Vis Sci 87(11):867–872
Conflict of interest
The authors report no potential conflict of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaca, E.E., Ekici, F., Yalçın, N.G. et al. Macular choroidal thickness measurements in patients with obstructive sleep apnea syndrome. Sleep Breath 19, 335–341 (2015). https://doi.org/10.1007/s11325-014-1025-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1025-6