Abstract
Purpose To evaluate the long-term safety and efficacy of Mycophenolate Mofetil (MMF) for the control of cystoid macular oedema (CMO) secondary to noninfectious uveitis (NU). Methods The medical records of 19 consecutive patients with inflammatory CMO treated with MMF were retrospectively reviewed. Patient demographics, best corrected visual acuity (BCVA), fluorescein angiography (FA), and optical coherence tomography (OCT) findings were evaluated. Results There were eight females and 11 males with a mean age of 32.9 ± 8.9 years. After a 1-year follow-up, 18/19 patients (31 eyes, 96.9%, P < 0.05) no longer had signs of CMO, as per their FA and OCT findings; the mean central foveal thickness (CFT) was 167.2 ± 12.8 μm. At the last follow-up, only 3/19 patients, all affected by Behçet panuveitis, had recurrences of CMO. Mean BCVA improved from 0.34 ± 0.14 SD at baseline to 0.65 ± 0.2 SD at last follow-up. Conclusions MMF was safe and effective in controlling CMO and in reducing the uveitis relapse rate in patients not responding to traditional immunosuppressants. Further case–controlled studies are mandatory to validate those preliminary results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis is one of the leading causes of visual impairment in ophthalmology; one of its most dangerous sequela is the cystoid macular oedema (CMO) [1]. The prevalence of CMO is estimated in 28–64% of patients with uveitis and leads to permanent visual impairment in 8.5% of the cases [2]. Fluorescein angiography (FA) and, more recently, optical coherence tomography (OCT) [3–5] are used to detect it. Persistent or recurrent CMO could be due to an insufficient control of the inflammation and could be interpreted as a dangerous manifestation of the inflammation itself [6].
Steroids, both local [7] and systemic [8], are the first line of treatment for the control of CMO secondary to uveitis. On the other hand, long-term therapy with steroids could lead to complications such as high blood sugar level, osteoporosis, blood cell abnormalities, cataract, and glaucoma [9].
When the inflammation appears to be steroid dependent, immunosuppression is considered [10].
Several immunosuppressants have been proposed for the control of sight-threatening uveitis. Some of the drugs used are azathioprine [11], methotrexate [12], cyclosporine A [13], and, more recently, FK506 [14]. Considering the role of certain cytokines [15], such as transforming growth factor-β [16], on CMO pathophysiology, and the effect of some drugs on the production of those cytokines [17], other immunosuppressive agents could be considered for the management of uveitic CMO. Mycophenolate mofetil (MMF), another drug recently used for the control of uveitis, has shown promising results in downregulating those cytokines [18] that lead to the oedema.
MMF is a reversible, noncompetitive, selective inhibitor of the de novo pathway of purine synthesis, successfully used in the treatment of rheumatoid arthritis [19], pemphigus vugaris [20], and psoriasis [21]. Several reports have been published on its use for the control of uveitis: in 1998, Kilmartin and co-workers [22] reported a case series of patients, unresponsive to traditional immunosuppressants, successfully treated with MMF. More recently, a larger number of cases has been presented in a retrospective study by Thorne et al. [23] and Siepmann et al. [24], confirming the results previously published.
However, the reports mentioned above did not provide data regarding patients with CMO, and no statistical analyses have been carried out. In addition, we are unaware of any publication on the long-term control of inflammatory CMO using steroid-sparing drugs.
The purpose of this report is to assess the efficacy of MMF in controlling uveitic CMO.
Patients and methods
The study was designed as a retrospective case series.
We reviewed 19 notes of 19 patients affected by noninfectious uveitic (NU) CMO, unresponsive to traditional immunosuppressants, and treated with MMF. Patients have been examined at The Eye Clinic of the Polytechnic University of Marche in Ancona between 2003 and 2007. All patients were examined by one of the authors (PN).
Demographic and clinical variables were collected. Data was entered into an SPSS database (SPSS version 11.0.1, SPSS Inc., Chicago, IL) for the statistical analysis.
International Uveitis Study Group (IUSG) guidelines [25] and the standardization of uveitis nomenclature (SUN) working group [26] were used for the inflammatory scores and uveitis classifications.
An associated systemic disease was diagnosed based on the results of compatible historical, clinical, and/or laboratory data by the appropriate specialist such as a rheumatologist or a dermatologist.
All patients underwent a fluorescein angiography (FA; TRC-50 IX fundus camera, Topcon Corporation, Tokyo, Japan) and an optical coherence tomography (OCT; STRATUS OCT™ Carl Zeiss Meditec Inc., Jena, Germany).
Full blood cell count, liver function, and renal function were tested every 4–6 weeks during the MMF treatment.
Decrease or improvement in visual acuity was defined as a reduction or increase of two or more lines from the initial best corrected visual acuity (BCVA), presented as mean ± standard deviation (SD). An early treatment diabetic retinopathy study (ETDRS) chart was used for the examination.
The uveitis and CMO relapse rates are presented as frequency (number of relapses in a period of 1 year).
The Kaplan–Meyer life-table analysis was used to describe the incidence of CMO resolution and steroid tapering.
The linear correlation between different variables was studied.
The primary end point was the long-term control of CMO secondary to NU with MMF with a dose of steroids <10 mg/day, without OCT signs of intraretinal cysts and/or increase of central foveal thickness, and without FA evidence of CMO. Secondary end points were a reduction in the uveitis relapse rate during the MMF treatment and improvement in BCVA.
Results
The patients had the following diseases (Table 1): idiopathic retinal vascualitis 26.3% (n = 5), Behçet-disease-associated panuveitis 15.8% (n = 3), idiopathic intermediate uveitis 21% (n = 4), sarcoidosis 10.5% (n = 2), birdshot retinochoroidopathy 5.3% (n = 1), ankylosing spondylitis associated uveitis 10.5% (n = 2), and multifocal choroiditis with panuveitis 10.5% (n = 2); 32 eyes were considered. There were eight females and 11 males with a mean age of 32.9 ± 8.9 years.
Thirteen patients (68.4%) had bilateral chronic uveitis with associated CMO; six patients (31.6%) had unilateral chronic uveitis with secondary CMO. All patients changed therapy because of the persistency of CMO. At the beginning of their previous therapy, 17/19 patients (89.5%) received intravenous methylprednisolone followed by oral steroids at the starting dose of 1 mg/kg with an immunosuppressant; 2/19 patients (10.5%) had only oral steroids at the starting dose of 1 mg/kg coupled with an immunosuppressant. The mean duration of previous immunosuppressant was 9.6 ± 2.1 months. Before the introduction of MMF, the mean dose of systemic steroids was 31 ± 8.4 mg/day. All patients with unilateral affection had been treated at least one time with both sub-Tenon and intravitreal injections of triamcinolone acetonide (Kenacort™, Squibb pharma).
Before the introduction of MMF, all patients discontinued previous steroid-sparing drug treatments.
At baseline, mean BCVA was 0.34 ± 0.14 (range: 0.1–0.63); mean follow-up time was 30 ± 6 months (range: 19–38 months). Mean baseline central foveal thickness (CFT) was 441.3 ± 48.6 μm (Table 2).
The uveitis relapse rate (Fig. 1) had a strong correlation with the CMO relapse rate before MMF introduction (R = 0.8123, R 2 = 0.6598, P < 0.0001).
All patients were treated with intravenous methylprednisolone (1 g/day) for 3 days, followed by oral prednisone (1 mg/kg/day) coupled with MMF (1 g twice/day).
At 1-year follow-up, 18/19 patients (31 eyes, 96.9%, P < 0.05) had no signs of CMO, both verified by FA and OCT; the mean CFT was 167.2 ± 12.8 μm (Table 2). After 1 year of follow-up, 18/19 patients (94.7%) had no signs of uveal inflammation and CMO (P < 0.05). At last follow-up, 16/19 patients (28 eyes, 87.5%, P < 0.05) did not experience a CMO relapse, using MMF with a dose of steroid <10 mg/day. After 37 days, the probability of having a regression of OCT and FA signs of CMO was 98.2% (Fig. 2).
The probability of reducing the dose of steroids <10 mg/day was 97% after 25 weeks (Fig. 3); the median time observed for the tapering of oral prednisone <10 mg/day was 19 weeks (range: 9–35 weeks).
Patients 17/28/M and 18/39/F had a CMO relapse after 18 and 16 months, respectively; oral prednisone was increased to 25 mg, and FK506 was then introduced to achieve good control of the inflammation.
In patient 7/29/F, MMF exhibited no control of both uveitis and CMO: uveitis scores were not under control and signs of CMO did not resolve after MMF introduction; MMF was stopped and, after 2 weeks, interferon α 2a (Intron A, Schering-Plough Pharma) was introduced. All patients (3/3) were affected by Behçet panuveitis.
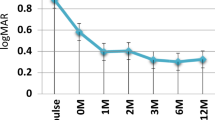
At 1-year follow-up, BCVA improved in 18/19 patients (31 eyes, 96.9%, P < 0.05); the mean BCVA was 0.67 ± 0.18 (range: 0.1–1). There was a strong inverse linear correlation (Fig. 4) between baseline CFT and BCVA after 1 year (R = −0.829, R 2 = 0.6872, P < 0.0001.) At last follow-up, the mean BCVA was 0.65 ± 0.2 (range: 0.1–1).
After 1 year of treatment, 17/19 (89,5%) of patients did not experience uveitis relapse.
The mean uveitis relapse rate decreased from 3.5 ± 0.9/year at baseline to 0.4 ± 0.5/year at last follow-up.
Up to the last follow-up, no severe side effects were recorded during treatment with MMF (Table 3).
Discussion
Cystoid macular oedema is one of the leading causes of visual impairment in patients with uveitis. Steroids are the first step for the control of noninfectious ocular inflammation and for the reduction of associated CMO; knowing their side effects, such as cataract, ocular hypertension. and glaucoma [9], steroid-sparing drugs are mandatory in steroid-dependent inflammations.
Although the role of immunosuppression in ophthalmology has been established [27], there are several unsolved points: the main difficulty probably lies in choosing the right steroid-sparing drug. There is no data regarding CMO control in active uveitis; recently, Deuter and co-workers described the safety and efficacy of interferon-α in patients with quiet uveitis and persistent CMO [28], but no other papers have been published.
Mycophenolate Mofetil is a relatively new immunosuppressive drug, successfully used after solid-organ transplants [29–31]. Recent studies have proven its efficacy with a long-lasting remission in patients affected by Crohn’s disease [32], severe atopic dermatitis [33], Wegener’s granulomatosis, and microscopic polyangioitis [34].
Thorne et al. [23] and Siepman et al. [24] have recently confirmed the satisfactory control of uveitis with MMF in a large cohort of patients.
To our knowledge, there are no studies on MMF for the control of CMO associated with noninfectious uveitis.
Patients treated in our study showed a good response to MMF: 87.5% of eyes treated (P < 0.05) had no recurrence of CMO during the treatment with MMF; the mean BCVA at last follow-up was substantially better than at baseline: 0.67 ± 0.18 at 1-year versus 0.34 ± 0.14 at baseline (P < 0.05).
It is important to stress that, before the treatment, there was a strong linear correlation between the uveitis and CMO relapse rates (R = 0.8123, R 2 = 0.6598, P < 0.0001) meaning that the CMO could be interpreted as the expression of uveitis severity. After MMF introduction, no correlation was observed and the uveitis relapse rate decreased from 3.5 ± 0.9/year at baseline to 0.4 ± 0.5/year at last follow-up; in addition, a strong inverse linear correlation was observed between baseline CFT and last follow-up BCVA (R = −0.829, R 2 = 0.6872, P < 0.0001), meaning that the BCVA outcome would be worse in those patients with a thicker baseline CFT. This correlation could justify an aggressive treatment at the beginning of the protocol to achieve a better BCVA outcome.
The apparently better efficacy of MMF in controlling CMO can be interpreted on the basis of some evidence found in literature: some immunosuppressive drugs, such as cyclosporine A [17], FK506, and sirolimus [35], are known to produce nephrotoxicity [17], inducing the overexpression of soluble mediators that have an important role in CMO pathogenesis [15]. MMF has proven to be effective in reducing such biomechanisms [18], improving arteriolopathy and decreasing the amount of soluble mediators involved in CMO pathophysiology.
In addition, it is important to stress the treatment strategy used to control CMO: the initial high dose of steroids acted to control the inflammation as much as possible, preparing a favorable background for MMF action.
Another key point shown in our study is the reduction of steroids: the probability of reducing the dose of steroids <10 mg/day was 97% after 25 weeks, according to data published previously [23]. In addition, after 1 year, 18/19 patients (31 eyes, 96.9%, P < 0.05) had no recurrence of CMO and 17/19 patients (89.5%) did not have a uveitis relapse.
It is important to stress that patients who had insufficient control of inflammation and CMO were all affected by Behçet panuveitis, thus likely confirming the data published by Adler et al. [36].
The data obtained in our study reflects promising results on the control of both inflammation and associated CMO, supported by evidence of good tolerance of the drug.
It is well known that the balance between the traditional drugs’ efficacy and their side effects can be quite unstable, and MMF has a better profile [37, 38]. The good tolerance to MMF probably derives from its pharmacodynamics: mycophenolic acid has a strong effect on the type II isoform of inosine monophosphate dehydrogenase enzyme, with minor action on the type I expressed in most other cell types, warranting a more potent cytostatic effect on lymphocytes than on other cell types [38]. In addition, another recent publication has highlighted the safety and efficacy of MMF, describing its use in paediatric patients [39]. Our study confirms its good tolerability, emphasizing the need to pay attention to its minor and reversible side effects to the stomach.
In conclusion, the data obtained from this study suggests that MMF is a safe and effective corticosteroid-sparing drug for a substantial portion of patients with uveitic CMO unresponsive to treatment with other immunosuppressants.
This study, however, should be interpreted with the limitations of a retrospective case series. Moreover, due to the difficulty in recruiting large numbers of patients for trials on immunosuppression, multicentric trials are suggested for a larger recruitment, possibly prospective, randomised, and controlled.
Abbreviations
- AZA:
-
Azathioprine
- BPU:
-
Behçet panuveitis
- BS:
-
Birdshot
- CSA:
-
Cyclosporine A
- IIU:
-
Idiopathic intermediate uveitis
- IRV:
-
Idiopathic retinal vasculitis
- IVT:
-
Intravitreal triamcinolone
- STT:
-
Sub-Tenon triamcinolone
- MCP:
-
Multifocal choroiditis with panuveitis
- MMF:
-
Mycophenolate mofetil
- MTX:
-
Methotrexate
- ASAU:
-
Ankylosing spondylitis associated uveitis
- PIA:
-
Previous immunosuppressive agents
- Sarc:
-
Sarcoidosis
References
Nussenblatt RB (1986) Macular alterations secondary to intraocular inflammatory disease. Ophthalmology 93(7):984–988
Malinowski SM, Pulido JS, Folk JC (1993) Long-term visual out-come and complications associated with pars planitis. Ophthalmology 100:818–825
Chang A, Spaide RF, Yannuzzi LA (1999) Post surgical cystoid macular edema. In: Guyer DR, Yannuzzi LA, Chang S et al (eds) Retina, vitreous, macula, vol 1. Saunders, Philadelphia, pp 239–255
Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE (2004) Macular edema. Surv Ophthalmol 49(5):470–490
Catier A, Tadayoni R, Paques M, Erginay A, Haouchine B, Gaudric A, Massin P (2005) Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol 140:200–206
Lardenoye CW, van Kooij B, Rothova A (2006) Impact of macular edema on visual acuity in uveitis. Ophthalmology 113(8):1446–1449
Paolo Ferrante P, Ramsey A, Bunce C, Lightman S (2004) Clinical trial to compare efficacy and side-effects of injection of posterior sub-Tenon triamcinolone versus orbital floor methylprednisolone in the management of posterior uveitis. Clin Exp Ophthalmol 32:563–568
Vitale AT, Foster CS (2002) Corticosteroids. In: Foster CS, Vitale AT (eds) Diagnosis and treatment of uveitis. W.B. Saunders, Philadelphia, pp 142–158
Neri P, Azuara-Blanco A, Forrester JV (2004) Incidence of glaucoma in patients with uveitis. J Glaucoma 13:461–465
Jabs DA, Rosenbaum JT, Foster CS et al (2000) Guidelines for the use of immunosuppressive drugs in patients with intraocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 130:492–513
Greenwood AJ, Stanford MR, Graham EM (1998) The role of azathioprine in the management of retinal vasculitis. Eye 12:783–788
Bom S, Zamiri P, Lightman S (2001) Use of methotrexate in the management of sight-threatening uveitis. Ocul Immunol Inflamm 9:35–40
Dick A, Azim M, Forrester J (1997) Immunosuppressive therapy for chronic uveitis: optimizing therapy with steroids steroids and cyclosporin A. Br J Ophthalmol 81:1107–1112
Kilmartin DJ, Forrester JV, Dick AD (1998) Tacrolimus (FK506) in failed cyclosporin A therapy in endogenous posterior uveitis. Ocul Immunol Inflamm 6:101–109
Kent D, Vinores SA, Campochiaro PA (2000) Macular oedema: the role of soluble mediatore. Br J Ophthalmol 84:542–545
Vinores SA, Chan C-C, Vinores MA et al (1998) Increased vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-beta) in experimental autoimmune uveoretinitis: upregulation of VEGF without neovascularization. J Neuroimmunol 89:43–50
Disel U, Pavdas S, Dogan A, Gulfiliz G, Yavuz S (2004) Effect of colchicine on cyclosporine nephrotoxicity, reduction of TGF-beta overexpression, apoptosis, and oxidative damage: an experimental animal study. Transplant Proc 36(5):1372–1376
Shihab FS, Bennett WM, Yi H, Choi SO, Andoh TF (2003) Mycophenolate mofetil ameliorates arteriolopathy and decreases transforming growth factor-beta1 in chronic cyclosporine nephrotoxicity. Am J Transplant 3(12):1550–1559
Goldblum R (1993) Therapy of reumathoid arthritis with mycophenolate mofetil. Clin Exp Rheumathol 11(suppl 8):S1117–S1119
Enk AH, Knop J (1997) Treatment of pemphigus vulgaris with mycophenolate mofetil. Lancet 350:494
Spatz S, Rudnicka A, McDonald CJ (1978) Mycophenolic acid in psoriasis. Br J Dermatol 98:429–435
Kilmartin DJ, Forrester JV, Dick AD (1998) Rescue therapy with mycophenolate mofetil in refractory uveitis. Lancet 352:35–36
Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP (2005) Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology 112(8):1472–1477
Siepmann K, Huber M, Stübiger N, Deuter C, Zierhut M (2006) Mycophenolate mofetil is a highly effective and safe immunosuppressive agent for the treatment of uveitis. A retrospective analysis of 106 patients. Graefes Arch Clin Exp Ophthalmol 244:788–794
Bloch-Michel E, Nussenblatt RB (1987) International uveitis study group recommendation fro the evaluation of intraocular inflammatory diseases. Am J Ophthalmol 103:234–235
The standardization of uveitis nomenclature (SUN) working group (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 140:509–516
Dick AD (2005) Are we still reluctant to immunosuppress? Clin Exp Ophthalmol 33(1):1–2
Deuter CM, Kotter I, Guenaydin I, Stuebiger N, Zierhut M (2006) Interferon alfa-2a: a new treatment option for long lasting refractory cystoid macular edema in uveitis? A pilot study. Retina 26(7):786–791
Sollinger HW, US Renal Transplant Mycophenolate Mofetil Study Group (1995) Mycophenolate Mofetil for the prevention of acute rejection in primery cadaveric renal allograft recipients. Transplantation 60:225–232
Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group (1996) A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 61:1029–1037
European Mycophenolate Mofetil Cooperative Study Group (1995) Placebo-controlled of mycophenolate mofetil combined wirth cyclosporine and corticosteroids for the prevention of acute rejection. Lancet 345:1321–1325
Miehsler W, Reinisch W, Moser G, Gangl A, Vogelsang H (2001) Is mycophenolate mofetil an effective alternative in azathioprine-intolerant patients with chronic active Crohn’s disease? Am J Gastroenterol 96(3):782–787
Benez A, Fierlbeck G (2001) Successful long-term treatment of severe atopic dermatitis with mycophenolate mofetil. Br J Dermatol 144(3):638–639
Nowack R, Gobel U, Klooker P, Hergesell O, Andrassy K, van der Wounde FJ (1999) Mycophenolate mofetil for maintenance therapy of Wegener’s granulomatosis and microscopic polyangiitis: a pilot study in 11 patients with renal involvement. J Am Soc Nephrol 10(9):1965–1971
Ninova D, Covarrubias M, Rea DJ, Park WD, Grande JP, Stegall MD (2004) Acute nephrotoxicity of tacrolimus and sirolimus in renal isografts: differential intragraft expression of transforming growth factor-beta1 and alpha-smooth muscle actin. Transplantation 78(3):338–344
Adler YD, Mansumann U, Zouboulis CC (2001) Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behçet’s disease. Dermatology 203(4):322–324
Lau CH, Comer M, Lightmen S (2003) Long-term efficacy of mycophenolate mofetil in the control of severe intraocular inflammation. Clin Exp Ophthalmol 31(6):487–491
Allison M, Eugui EM (2000) Mycophenolate mofetil and its mechanism of action. Immunopharmacology 47:85–118
Doycheva D, Deuter C, Stuebiger N, Biester S, Zierhut M (2006) Mycophenolate mofetil in the treatment of uveitis in children. Br J Ophthalmol. Published online 6 Jul 2006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neri, P., Mariotti, C., Cimino, L. et al. Long-term control of cystoid macular oedema in noninfectious uveitis with Mycophenolate Mofetil. Int Ophthalmol 29, 127–133 (2009). https://doi.org/10.1007/s10792-008-9200-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-008-9200-z