Abstract
Atriplex crassifolia (A. crassifolia) is a locally occurring member of Chenopodiaceae family that has been used in folk medicine for the treatment of joint pain and inflammation. The present study was focused to determine the analgesic and anti-inflammatory potential of the plant. n-hexane (ACNH) and methanol (ACM) extracts of A. crassifolia were evaluated for in vitro anti-inflammatory potential using protein denaturation inhibition assay. In vivo anti-inflammatory potential was determined by oral administration of 250, 500, and 1000 mg/kg/day of extracts against carrageenan and formalin-induced paw edema models. Inflammatory mediators such as TNF-α, IL-10, IL-1β, NF-kB, IL-4, and IL-6 were estimated in blood samples of animals subjected to formalin model of inflammation. Analgesic activity was determined using acetic acid-induced writhing and tail flick assay model. Phytochemical profiling was done by GC–mass spectrophotometer. The results of in vitro anti-inflammatory activity revealed that both ACNH and ACM displayed eminent inhibition of protein denaturation in concentration-dependent manner. In acute in vivo carrageenan-induced paw edema model, both extracts reduced inflammation at 5th and 6th hour of study (p < 0.05). A. crassifolia extracts exhibited significant inhibition against formalin-induced inflammation with maximum effect at 1000 mg/kg. ACNH and ACM significantly augmented the inflammatory mediators (p < 0.05). Levels of TNF-α, IL-6, IL-1β, and NF-kB were reduced, while those of IL-4 and IL-10 were upregulated. ACNH displayed maximum analgesic effect at 1000 mg/kg, while ACM showed potent activity at 500 and 1000 mg/kg. The extracts restored the CBC, TLC and CRP toward normal. GC–MS analysis revealed the presence of compounds like n-hexadecanoic acid, Phytol, (9E,11E)-octadecadienoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester, 1-hexacosene, vitamin E, campesterol, stigmasterol, gamma sitosterol in both extracts. These compounds have been reported to suppress inflammation by inhibiting inflammatory cytokines. The current study concludes that A. crassifolia possesses significant anti-nociceptive and anti-inflammatory potential owing to the presence of phytochemicals.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation in the body is concurrently a physiological and a pathological process that is mediated by the intervention of the immune system. It is a contributory process toward healing as the immune system is capable of recognizing and responding to the harmful stimuli in the body through the release of inflammatory mediators; therefore, it is considered as beneficial physiological defense mechanism against infectious agents. However, the dysregulation of the inflammatory process may be harmful (Medzhitov 2008). It may put the body into complicated pathological state. Inflammation mediates various signaling pathways within cell that might lead to cell death followed by organ damage (Bouyahya et al. 2022).
Inflammatory process may be acute or chronic (Tatipamula and Vedula 2017). Chronic inflammation may cause the development of different diseases such as cancer, heart disease, digestive disorders, diabetes, and some neurodegenerative disorders (Bouyahya et al. 2022).
The inflammatory process involves the production of reactive oxygen species (ROS) in the body which increases the permeability of vascular tissues, resulting in changes in blood flow and tissue destruction. This is followed by the release of inflammatory mediators such as IL-6, IL-10, IL-1β, TNF-α, and COX-2 prostaglandins (Anyasor et al. 2019). The inflammatory cytokines along with free radicals also affect some of the internal organs and consequently whole body (Mubashir et al. 2014). The monocytes, T and B cells, and vascular endothelial cells get activated, thereby contributing to the progress of the disease. These inflammatory markers serve as a risk factor in the development of atherosclerosis and other detrimental diseases (Montecucco and Mach 2009). Complications of inflammatory process encompass cellular and vascular outcomes in the form of cardinal signs such as edema, redness, pain, and heat (Shabbir et al. 2014). Pain is a desolating occurrence in the body that involves tissue impairment. It is produced in response to harmful stimuli such as heat, stretch, necrosis, electrical flow, laceration, and inflammation followed by release of cytokines, prostaglandins, histamine, substance P, and serotonin (Al-Sanafi 2018). Inflammatory complications along with pain affect patient’s health in general and exert major burden on communal and health care system. Pervasiveness of pain and inflammatory disorders is increasing day by day. It has been estimated that 30% of adults suffer from pain and inflammatory diseases each year around the globe (Javed et al. 2020). The immense health consequences of inflammatory diseases and pain demand a meticulous understanding of public responses to these health problems to provide them with the best remedies available (Javed et al. 2020).
Inflammatory diseases have been managed over the years by the use of anti-inflammatory drugs, preferably the NSAIDs which serve as attractive therapeutic agents to control inflammation. Despite their targeted control of inflammation, they exert debilitating adverse effects on human health which over the time has raised the quest to search for the natural bioactive compounds for the treatment of inflammatory diseases. Polyphenols, flavonoids, alkaloid, triterpenoids, and other plant secondary metabolites not only serve as anti-inflammatory agents but also as lead for the synthesis of anti-inflammatory and analgesic drugs (Kazemi et al. 2014). Plants exert anti-inflammatory effect by different mechanisms. They hinder the release of inflammatory mediators and obstruct different synthetic pathways such as that of prostaglandin and arachidonic acid (Javed et al. 2020). Over last decade, researchers have ascertained the anti-inflammatory potential of different plant extracts utilizing in vitro and in vivo inflammatory models. The anti-inflammatory molecular mechanism depicted by animal models underpins their probable clinical translation (Maione et al. 2016).
Atriplex crassifolia C.A.Mey is commonly called saltbush or orache belonging to Chenopodiaceae. It is distributed throughout different regions in Pakistan. It is an annual herb that grows well in saline environment and is given the name salt bush due to salt retaining property of its leaves. The powdered leaves are used for the treatment of joint pain, inflammation, and skin allergies (Abbas et al. 2014). However, the anti-nociceptive and anti-inflammatory activity of the plant has not yet been explored (Uttar et al. 2018). So, the current study was designed to establish the scientific evidence of analgesic and anti-inflammatory potential of A. crassifolia.
Materials and methods
Whole herb of A. crassifolia C.A.Mey was collected from District Kasur. The plant was authenticated by the taxonomist Dr. Zaheer-Uddin Khan from Botany department, GC University Lahore with the Voucher Number GC.Herb.Bot.3618. The plant material was carefully subjected to drying, garbling, and pulverization. The powder was stored in air tight glass container.
Preparation of plant extracts
The powdered plant material (1.0 kg) was subjected to successive extraction by maceration with n-hexane and methanol. Plant material was dipped in 2.5 L of n-hexane and methanol separately for 7 days. Plant extracts were obtained by percolation using muslin cloth followed by filtration using Whatman No 1. Solvents were evaporated using rotary evaporator under low pressure using vacuum (Lone et al. 2017). n-hexane extract (ACNH) and methanol extract (ACM) were further dried in oven at 40 ˚C.
Drugs and chemicals
All the organic solvents and other chemicals utilized in this study were of analytical grade purchased from Sigma-Aldrich USA, i.e., n-hexane, methanol, carrageenan, acetic acid, acetonitrile, formic acid, and formalin (Riedel-de Haen, Germany). Diclofenac sodium was arranged from Sami pharmaceuticals.
GC–MS analysis
ACNH and ACM were subjected to GC–MS using Agilent-B-7890 equipped with 5977-B mass spectrometer detector using DB-5 ms column possessing 100% dimethyl polysiloxane (Dimensions: L-30 m, D-0.25 mm, pore size 0.25µm). Injector temperature was set at 250 ˚C and that of the inter phase at 280 ˚C. The equipment was set at scan mode with − 70 eV ionization energy and the temperature of ion source was kept 230 ˚C. Helium at a flow rate of 1 mL/minute was used as carrier gas. The analysis time was 60 min (Fan et al. 2021).
Animal studies
In vivo studies were conducted on healthy male Wistar rats (180–200 g) and albino male mice (25–30 g). Animals were kept in polyacrylic cages and adapted to standard conditions in animal house of Faculty of Pharmacy, University of the Punjab Lahore, Pakistan. The animals were kept in 12 h light and dark cycle with maintained at 26 ± 3 ˚C and 50–55% temperature and humidity, respectively. The animals were given standard pellet diet and water ad libitum (Tatipamula and Vedula 2017). The study protocols were reiterated from Punjab University Institutional Ethics Review Board (No. D/208/FIMS).
Acute oral toxicity study
Safety profile of ACNH and ACM was evaluated by performing acute oral toxicity before conducting in vivo biological evaluation as per OECD guide lines (420). Rats were randomly divided into six groups as per different doses of extracts (100, 250, 500, 750, 1000, and 2000 mg/kg) being administered orally. Extracts were administered for 14 days and the animals were tested for any signs of toxicity. The rats were observed at an interval of 1 h for first 12 h and daily thereafter, for 14 days. After 48 h, the mortality was checked. The animals were evaluated daily for behavioral as well as any other signs of illness such as salivation, lacrimation, tremors, convulsions, and writhing reflex (Amresh et al. 2018).
In vitro anti-inflammatory study
Determination of in vitro anti-inflammatory activity against denaturation of protein albumin
In vitro anti-inflammatory potential of ACNH and ACM was estimated by using inhibition of albumin denaturation assay (Qasim et al. 2021). The reaction mixtures for both extracts (5 mL each) were prepared that contained 2.8 mL of phosphate saline buffer (PH 6.5), 0.2 mL of 90% egg white, and 2 mL of each of the varying concentrations of ACNH and ACM. The control solution was prepared following the same procedure except that 2 ml double distilled water was used in place of the extracts. All the reaction mixtures were then incubated for 15 min at 37 ˚C. Later on, they were heated at 70 ˚C for 5 min duration. They were then measured for absorbance at 660 nm after cooling to room temperature. Percent inhibition of protein denaturation was determined by using the following formula:
Percentage inhibition = (Abs control− Abs sample/Abs control).
In vivo anti-inflammatory studies
Carrageenan-induced paw edema model
Acute anti-inflammatory study was carried out according to previous method (Ratheesh and Halen 2007). Wistar rats were divided into 9 groups (n = 5). Paw sizes of the rats were determined using digital Vernier caliper (Sparkfun USA). Normal and disease group were given vehicle, standard group received diclofenac sodium (10 mg/kg), and treatment groups received ACNH and ACM at 250, 500, and 1000 mg/kg orally, respectively. 0.1 mL of 1% carrageenan suspension in 0.9% NaCl was injected into the subplantar tissue of right hind paw of all experimental animals exactly 30 min after administration of the extracts/standard drug except normal control group. Paw thickness was measured “mm” after carrageenan injection from 1 to 6 h. Percentage reduction in the paw edema over the time determined the anti-inflammatory potentials. Percentage inhibition of inflammation was calculated by using the following formula.
Percentage inhibition = control paw edema− treated paw edema/control paw edema × 100.
Formalin-induced paw edema model
Formalin-induced paw edema model was used to study the anti-inflammatory potential of ACNH and ACM. Animals were divided into 9 groups (n = 5): Group 1: Normal control, Group II: Disease control, Group III: Standard (diclofenac sodium 15 mg/kg, orally), Groups IV–VI: ACNH 250, 500, 1000 mg/kg, orally, and Groups VII–IX: ACM 250, 500, 1000 mg/kg, orally. 0.1 mL of 2% of formaldehyde solution prepared in water was injected into the subplantar area of the hind paw of each rat on 1st and 3rd day of the study except normal control after 1 h of administration of ACNH and ACM (John and Shobana 2012). All the groups followed a 10-day treatment plan as described above. Diameter of paws was taken at days 1, 3, 7, and 10 with the help of digital Vernier caliper. The percentage inhibition in paw edema was estimated. On 10th day, the animals were anesthetized with ketamine and xylazine and blood was withdrawn through cardiac puncture (Asif et al. 2023). Whole blood diagnostic parameters were determined by using Sysmex XP-100 quantitative automated hematology analyzer.
Estimation of inflammatory markers by qRT-PCR
The pro-inflammatory and inflammatory markers including TNF-α, IL-6, IL-4, and IL-10 were analyzed using qPCR (Applied biosystems thermos scientific, SYBR select master mix, 4472903) following the manufacturer’s protocols. Real-Time Expression of targets was performed using SYBR Select Master Mix by taking cDNA as template with relevant primers in duplicates. The relevant Ct’s of samples were compared against controls and control samples containing housekeeping genes (Asif et al. 2023) (Table 1).
Analgesic activity
Acetic acid-induced writhing response
Albino male mice (25–30 g) were selected to determine the analgesic activity of ACNH and ACM. Peripheral analgesia was estimated by using complete randomized block design (CRBD) by using the method described by Saleem et al. The mice were divided into 8 groups of 5 mice in each group. Group I received vehicle, Group II received 15 mg/kg diclofenac sodium, Groups III–V received ACNH at 250, 500, and 1000 mg/kg, and Groups VI–VIII received ACM at 250, 500, and 1000 mg/kg, respectively. 0.1 mL of acetic acid solution (1% in normal saline) was injected into mice through intraperitoneal injection, 1 h after the administration of standard and test doses and writhing effects (abdominal muscle contractions, stretching of hind limbs, twisting of trunk) were counted for 20 min (Saleem et al. 2017). % age inhibition of writhing response in mice was calculated using the following formula.
% inhibition of writhing = (Wc− Wt)/Wc×100,
where Wc = number of writhes in control group. Wt = number of writhes in test group (Rajamanickam and Rajamohan 2020).
Tail flick assay
The anti-nociceptive effects of different doses of A. crassifolia were evaluated using tail flick method. Digital thermostatic water bath (Memmert, Germany) was used as source of heat for the measurement of latency response in albino mice.1–2 cm of mice tails were placed in hot water (50–55 ˚C) to determine the basal flick reaction time. To avoid tail damage, a cut-off time of 10–12 s was imposed. Mice failing to withdraw tail within 5 s were rejected from this study. The normal behavior of each animal was further confirmed by determining 3 basal times at interval of 5 min. Control reaction time was recorded twice with an interval of 20 min. Diclofenac sodium (15 mg/kg) and test doses of extracts (250, 500, and 1000 mg/kg) were then administered orally. Latency response were calculated at different time intervals (30, 60, 90, and 120 min) post-administration of the standard drug and extracts (Rokade and Jharvad 2022). Maximum possible effect (MPE) was determined according to following formula.
MPE (%) = (Post-treatment latency − Pre-treatment latency)/(Cut-off time − Pre-treatment latency) × 100.
Albino male mice were divided into eight experimental groups of 5 animal each. Group I received vehicle, Group II received 15 mg/kg diclofenac sodium, Groups III–V received ACNH at 250, 500, and 1000 mg/kg, and Groups VI–VIII received ACM at 250, 500, and 1000 mg/kg, respectively.
Statistical analysis
The results are represented as mean ± SEM (n = 5). One-way and two-way ANOVA were applied followed by Dunnett’s test using GraphPad prism 8 (San Diego, CA, USA). The results having p-values less than 0.05 were considered to be statistically significant (Niazi et al. 2009).
Results
Acute oral toxicity study
Acute oral toxicity observations in rats with increasing doses of ACNH and ACM extracts of A. crassifolia revealed no toxicity-related mortalities or clinical symptoms. No altered behavior or other illness signs like lacrimation, salivation, or writhing were observed, indicating the safety of plant (Figs. 1, 2).
GC–MS analysis
The results of n-hexane and methanol extracts of A. crassifolia are represented in Tables 2 and 3, respectively.
In vitro anti-inflammatory activity against denaturation of protein albumin
The results of anti-inflammatory activity of n-hexane (ACNH) and methanol (ACM) extracts of A. crassifolia against denaturation of protein albumin are represented in Fig. 3. Both extracts exhibited promising in vitro anti-inflammatory activity in concentration-dependent manner. ACNH displayed mean percentage protein denaturation inhibition of 21.60 ± 0.95, 32.42 ± 1.27, 39.77 ± 0.45, 44.62 ± 0.63, 58.15 ± 0.16, 71.03 ± 0.20, and 74.82 ± 0.49 at concentrations 25, 50, 100, 200, 400, 800, and 1000 µg/ml, ACM demonstrated 27.95 ± 0.30, 30.53 ± 1.71, 39.76 ± 0.44, 47.81 ± 0.32, 60.86 ± 0.63, 71.50 ± 0.48, and 75.31 ± 0.18, and the standard drug diclofenac sodium exhibited a percentage of 36.17 ± 0.35, 39.82 ± 0.50, 43.17 ± 0.81, 52.89 ± 0.73, 63.01 ± 0.81, 73.99 ± 0.33, and 77.42 ± 0.40, respectively. The results of ACNH at concentrations 800 and 1000 and those of ACM at concentrations 400, 800, and 1000 µg/ml were comparable to standard drug (diclofenac sodium).
Graphical representation of % age inhibition of protein denaturation at different concentrations of A. crassifolia extracts. Values are expressed as mean ± SEM. One-way ANOVA is applied to compare means of all values with standard control (diclofenac sodium) at one time interval followed by Dunnett’s post hoc test (p < 0.05). (ACNH: A. crassifolia n-hexane extract, ACM: A. crassifolia methanol extract)
In vivo anti-inflammatory studies
Carrageenan-induced paw edema model
Anti-inflammatory activity of A. crassifolia extracts at 250, 500, and 1000 mg/kg is shown in Table 4. This study revealed a dose-dependent response toward reduction in paw edema. Results were more pronounced at highest dose (1000 mg/kg) of both ACNH and ACM extracts exhibiting 69.28 ± 1.11% and 69.84 ± 0.27% inhibition at 5th hour with a further inhibitory increment in percentage inhibition at 6th hour (76.64 ± 1.06 and 76.75 ± 0.72%) of study, respectively. Further, ACM also showed eminent results at 500 mg dose at 7th hour (75.95 ± 0.40). Both extracts showed minimum inhibition at lowest dose (250 mg/kg). Standard drug (diclofenac sodium) also showed maximum inhibitory effect at 5th and 6th hour of study.
Formalin-induced paw edema model
Inflammation and edema were developed in paw of albino rats after the administration of formaldehyde. As the treatment with extracts continued, there was a steady reduction in paw volume in a dose reliant manner whereby 1000 mg/kg dose of both plants exhibited maximum effect on 7th and 10th day of experiment. Effect of ACM at 500 mg/kg was more marked with peak effect on 10th day, as compared to ACNH at same dose. Both extracts showed minimum inhibition at 250 mg/kg dose. The results of ACNH and ACM at 1000 mg/kg dose at 10th day were comparable to diclofenac sodium at respective days (Table 5).
Estimation of inflammatory markers by qRT-PCR
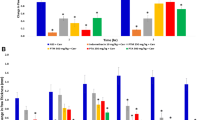
Fold change in gene expressions on inflammatory mediators is presented in Fig. 4. In disease group, there is a reduction in level of IL-4 and IL-10 whereas an increase in expression of TNF-α, IL-6, IL-1β, and NF-kB has been observed. Study groups administered with ACNH at dose of 1000 mg/kg/day and ACM at dose of 500 and 1000 mg/kg/day caused an increase in the level of IL-4 and IL-10 and decrease in TNF-α, IL-6, IL-1β, and NF-kB.
Genetic expression changes in TNF-α (A), IL-4 (B), IL-6 (C), IL-10 (D), IL-1B (E), NF-kB (F) in various treated experimental groups. Two-way ANOVA is applied using post hoc Dunnett’s test. All the groups are compared with disease control at one time interval followed by Dunnett’s post hoc test (p < 0.05). SC: Standard control, ACNH: A. crassifolia n-hexane extract, ACM: A. crassifolia methanol extract (n = 5)
Hematological studies
The results of hematological analysis of study animals are presented in Tables 6 and 7. The results displayed a fluctuated response toward Hb, RBCs, WBCs, and platelet count. A positive shift toward Hb and RBCs level was observed in standard and test extracts as compared to disease control group. There was an increase in total leucocyte and CRP count after administration of formalin and progression of inflammation. The standard and test doses caused a reduction in TLC and CRP as compared to disease control group.
Analgesic activity
Acetic acid-induced writhing response
The results of acetic acid-induced writhing response in mice are presented in Fig. 5. Treatment with ACNH and ACM at 1000 mg/kg showed maximum inhibition of writhing (64.26 ± 0.24, 65.62 ± 0.36). Standard drug (diclofenac sodium displayed the highest %age of inhibition (66.52 ± 0.14).
Values are expressed as mean ± SEM. One-way ANOVA is applied to compare means of all values with standard control at one time interval followed by Dunnett’s post hoc test p < 0.05(***), n = 5. SC: Standard control, ACNH: A. crassifolia n-hexane extract, ACM: A. crassifolia methanol extract.
Tail flick assay in mice
The results of A. crassifolia extracts on pain latency are depicted in Fig. 6. Both ACNH and ACM showed an increase in pain latency time at 500 and 1000 mg/kg/day of extract dose. Maximum possible effect (MPE) was exhibited 90 min post-administration of the extracts and standard drug.
Values are expressed as mean ± SEM. One-way ANOVA is applied to compare means of all values with standard control at one time interval followed by Dunnett’s post hoc test p < 0.05, n = 5. SC: Standard control, ACNH: A. crassifolia n-hexane extract, ACM: A. crassifolia methanol extract.
Discussion
In the present era researchers have been consistently exploring the natural sources to discover the analgesic and anti-inflammatory drugs with lesser side effects and better therapeutic effectiveness (Saleem et al. 2017). The role of A. crassifolia in the management of pain and inflammation was evaluated by performing both in vitro and in vivo tests.
The in vitro anti-inflammatory activity was investigated by employing the protein denaturation inhibition assay that refers to a pathological state in which the structural integrity of body proteins is compromised in response to external stimuli, resulting in diminished biological functionality (Rauf et al. 2015). Denatured proteins act as inflammatory mediators. The generation of autoantigens has a detrimental impact on both the cartilage and the synovial membrane of the joints. This phenomenon subsequently contributes to the progression of inflammatory arthritic conditions (Saleem et al. 2019). The extracts of A. crassifolia, specifically n-hexane and methanol extracts demonstrated a concentration-dependent suppression of protein denaturation assay. This inhibition was found to be comparable to that of the standard drug diclofenac sodium, indicating the plant’s potential for in vitro anti-inflammatory activity.
Inflammation is an intrinsic and indispensable component of the body’s immunological reaction to trauma, illness, or noxious stimuli. The phenomenon is a multifaceted biological process that attempts to protect the body and facilitate the restoration of bodily functions. Inflammation has the potential to manifest in diverse tissues and organs across the entirety of the human body and can occur as an acute or chronic inflammation (Yu et al. 2021). Carrageenan-induced paw edema is well established as a typical acute inflammatory paradigm used to assess the potential anti-inflammatory properties of natural products derived from plants, as well as other non-steroidal anti-inflammatory drugs (Biswas et al. 2011). Carrageenan treatment causes an initial release of histamine and serotonin which is then followed by the release of prostaglandins. Many non-steroidal anti-inflammatory drugs (NSAID’s) have been observed to exert their pharmacological impact through the suppression of prostaglandin synthesis (Saleem et al. 2017). The present investigation showed the inhibitory effects of ACNH and ACM on carrageenan-induced paw edema, potentially attributable to the suppression of prostaglandin synthesis. The in vivo findings are corroborated by the GC–MS analysis of both extracts, which demonstrated the existence of bioactive chemicals with antioxidant and anti-inflammatory properties. The compounds including n-hexadecanoic acid, phytol, (9E, 11E)-octadecadienoic acid, 9-octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl) ethyl ester, 1-hexacosene, vitamin E, campesterol, stigmasterol, and gamma sitosterol might be linked with the anti-inflammatory response. Hexadecenoic acid is a saturated fatty acid and a prostaglandin synthesis inhibitor. It causes inhibition of arachidonic acid pathway (Aparna et al. 2012). Campesterol, stigmasterol, and γ-sitosterol are phytosterols identified in both methanol and n-hexane extract, serve as immune modulators, and decrease inflammation through suppression of pro-inflammatory cytokine production (Silva et al. 2014; Caroprese et al. 2012).
The anti-inflammatory potential of A. crassifolia in the treatment of chronic inflammation was evaluated through formaldehyde-induced paw edema model conducted over a period of 10 days. The clinical pathology of this model has been determined to bear resemblance to human inflammatory state, rendering it a viable selection for investigating the anti-inflammatory properties of newly developed drugs. Pharmacological agents or naturally occurring substances that have inhibitory effects on formaldehyde-induced inflammation function through the dual mechanism of suppressing both the inflammatory and neurogenic stages (Hasan, Alamgeer 2018). In the present study, inflammation was efficiently procured with the administration of ACNH and ACM in a dose-dependent manner and both extracts blocked the release of pro-inflammatory cytokines. Levels of TNF-α, IL-6, IL-1β, and NF-kB were reduced, while those of IL-4 and IL-10 were upregulated. IL-4 serves as a strong macrophage deactivator that prevents the production of inflammatory mediators like TNF-α, IL-10, IL-1β, and NF-kB from monocytes, thereby reducing the inflammatory response. GC–MS analysis of both ACNH and ACM extracts revealed the presence of stigmasterol, β-sitosterol, δ-sitosterol, and campesterol. These phytosteroids have been reported to downregulate the expression of inflammatory mediators (Asif et al. 2023). Effect of ACM at 500 and 1000 mg/kg/day was more pronounced. GC–MS of ACM showed the presence of β-amyrin that also serves as anti-inflammatory agent (Okoye et al. 2014). Hence, it could be suggested that bioactive compounds present in plants exerted anti-inflammatory effect by decreasing oxidative stress and diminishing reactive oxygen species, effects of 5-lipoxygenase, cyclooxygenase -1, cyclooxygenase -2 and by inhibiting synthesis of prostaglandins and other mediators of inflammation (Jin 2012).
Inflammation and pain are closely associated. Several physiological mediators, such as substance P, bradykinin, and prostaglandins, have a role in mediating the pain process. The anti-nociceptive activity of ACNH and ACM was assessed using acetic acid writhing and tail flick assays. Induction of abdominal writhing through the administration of acetic acid serves as a non-selective model for investigating the potential pain-reducing properties of plant extracts and newly developed substances. The administration of acetic acid intraperitoneally induces the release of pro-inflammatory mediators, including TNF-α, IL-10, IL-4, and IL-6. The liberation of these mediators is additionally linked to peripheral pain processes (Nguyen et al. 2020). Findings of acetic acid-induced writhing assay revealed that both extracts demonstrated dose-dependent inhibition of writhing with maximum effect (64.26 ± 0.24, 65.62 ± 0.36 respectively) at the highest dose (1000 mg/kg/day). The tail flick assay is an alternate approach for assessing anti-nociceptive activity, which aids in the identification of pain relief against neuronal and thermally induced algesia. In the tail flick model, the administration of both extracts at doses of 500 and 1000 mg/kg/day resulted in a significant increase in pain latency time after 90 min. Maximum dose of both extracts exhibited an anti-nociceptive effect that was statistically equivalent to the impact of the standard medication, diclofenac sodium. The tail flicking reaction exhibited by animals in response to heat is mediated by spinal reflexes (Wahid et al. 2021). Both ACNH and ACM showed an increase in pain latency time at 500 and 1000 mg/kg/day of extract dose. Maximum possible effect (MPE) was exhibited 90 min post-administration of the extracts and standard drug. The extracts might have exerted analgesic effect by suppressing supraspinal center and spinal reflexes that initiate pain. Analgesic effect of both extracts might be attributed to the antioxidant and anti-inflammatory compounds revealed in GC–MS analysis.
Inflammation is accompanied by thrombocythemia, anemia, and elevated TLC and CRP as extra-articular response (Saleem et al. 2019). Formalin treatment causes a reduction in RBC count and the effects can be observed in disease control that shows decrease in level of Hb and red cells and an increase in leucocyte and CRP levels. The involvement of white blood cells in the body’s defense mechanism is significant, and their concentration increases as inflammation advances (Asif et al. 2023). The disrupted blood parameters resulting from formalin treatment were restored with the administration of plant extracts. The administration of ACNH and ACM effectively ameliorated thrombocythemia and leukocytosis, as seen by a decrease in C-reactive protein levels, suggesting a notable reduction in inflammatory response. The presence of phytosterols, hydrocarbons, terpenoids, and antioxidant chemicals in plants confers certain characteristics that contribute to the pharmacological capabilities of plants.
Conclusion
The current phytochemical and biological investigations confirm the analgesic and anti-inflammatory activity of n-hexane and methanol extracts of A. crassifolia rendering it a promising choice for the management of pain and inflammation. Additional research efforts may be undertaken to gain a deeper understanding of the precise molecular entities responsible for mitigating inflammation.
References
Abbas Q, Khan SW, Khatoon S, Hussain SA, Hassan SN, Hussain A, Qureshi R, Hussain I (2014) Floristic biodiversity and traditional uses of medicinal plants of Haramosh valley Central Karakoram National Park of Gilgit district. Gilgit-Baltistan Pakistan J Bio Env Sci 5:75–86
Al-Snafi AE (2018) Arabian medicinal plants with analgesic and antipyretic effects-plant based review (Part 1). IOSR J Pharm 8(6):81–102
Amresh G, Singh PN, Rao CV (2018) Toxicological screening of traditional medicine Laghupatha (Cissampelos pareira) in experimental animals. J Ethnopharmacol 116(3):454–460. https://doi.org/10.1016/j.jep.2007.12.008
Anyasor GN, Okanlawon AA, Ogunbiyi B (2019) Evaluation of anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clin Phytoscience 5:1–3
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M (2012) Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 80(3):434–439. https://doi.org/10.1111/j.1747-0285.2012.01418.x
Asif A, Ishtiaq S, Kamran SH, Waseem R, Fatima SF, Rehman S (2023) UHPLC–MS and GC–MS phytochemical profiling, amelioration of pain and inflammation with chloroform extract of Funaria hygrometrica Hedw. via modulation of inflammatory biomarkers. Inflammopharmacology 3:1–4
Biswas M, Biswas K, Karan TK, Bhattacharya S, Ghosh AK, Haldar PK (2011) Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J Phytol 3(1):33–38
Bouyahya A, Guaouguaou FE, El Omari N, El Menyiy N, Balahbib A, El-Shazly M, Bakri Y (2022) Anti-inflammatory and analgesic properties of Moroccan medicinal plants: Phytochemistry, in vitro and in vivo investigations, mechanism insights, clinical evidences and perspectives. J Pharm Anal 12(1):35–57. https://doi.org/10.1016/j.jpha.2021.07.004
Caroprese M, Albenzio M, Ciliberti MG, Francavilla M, Sevi A (2012) A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Vet Immunol Immunopathol 150(1–2):27–35. https://doi.org/10.1016/j.vetimm.2012.08.002
Fan X, Jiao X, Liu J, Jia M, Blanchard C, Zhou Z (2021) Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res Int 140:109975. https://doi.org/10.1016/j.foodres.2020.109975
Hasan UH (2018) Antiarthritic efficacy of Clematis orientalis. Bangladesh J Pharmacol 13(2):142–148. https://doi.org/10.3329/bjp.v13i2.33313
Javed F, Jabeen Q, Aslam N, Awan AM (2020) Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. f. J Ethnopharmacol 259:112966
Jin F. (2012) The pharmaceutical potential of compounds from Tasmanian Clematis species. Doctoral dissertation, University of Tasmania
John NA, Shobana G (2012) Anti-inflammatory activity of Talinum fruticosum l. on formalin induced paw edema in albino rats. J Appl Pharm Sci 2(1):123–127
Kazemi S, Shirzad H, Rafieian-Kopaei M (2014) Recent findings in molecular basis of inflammation and anti-inflammatory plants. Curr Pharm Des 24(14):1551–1562. https://doi.org/10.2174/1381612824666180403122003
Lone BA, Chishti MZ, Bhat FA, Tak H, Bandh SA, Khan A (2017) Evaluation of anthelmintic antimicrobial and antioxidant activity of Chenopodium album. Trop Anim Health Prod 49:1597–1605. https://doi.org/10.1007/s11250-017-1364-y
Maione F, Russo R, Khan H, Mascolo N (2016) Medicinal plants with anti-inflammatory activities. Nat Prod Res 30(12):1343–1352
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454(7203):428–435. https://doi.org/10.1038/nature07201
Montecucco F, Mach F (2009) Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology 48(1):11–22. https://doi.org/10.1093/rheumatology/ken395
Mubashir K, Ganai BA, Ghazanfar K, Akbar S (2014) Evaluation of antiarthritic potential of methanolic extract of Gentiana kurroo Royle. Arthritis 2014:1–6. https://doi.org/10.1155/2014/810615
Nguyen T, Chen X, Chai J, Li R, Han X, Chen X, Liu S, Chen M, Xu X (2020) Antipyretic, anti-inflammatory and analgesic activities of Periplaneta americana extract and underlying mechanisms. Biomed Pharmacother 123:109753. https://doi.org/10.1016/j.biopha.2019.109753
Niazi J, Singh P, Bansal Y, Goel RK (2009) Anti-inflammatory, analgesic and antipyretic activity of aqueous extract of fresh leaves of Coccinia indica. Inflammopharmacol 17:239–244. https://doi.org/10.1007/s10787-009-0010-3
Okoye NN, Ajaghaku DL, Okeke HN, Ilodigwe EE, Nworu CS, Okoye FB (2014) Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm Biol 52(11):1478–1486. https://doi.org/10.3109/13880209.2014.898078
Qasim S, Alamgeer SM, Alotaibi NH, Bukhari SN, Alharbi KS, Irfan HM, Anwar R (2021) Appraisal of the antiarthritic potential of prazosin via inhibition of proinflammatory cytokine TNF-α: a key player in rheumatoid arthritis. ACS Omega 6(3):2379–2388. https://doi.org/10.1021/acsomega.0c05698
Rajamanickam M, Rajamohan S (2020) Analgesic activity of flavonoids isolated from Persicaria glabra (wild). Adv Tradit Med 20(1):71–76. https://doi.org/10.1007/s13596-019-00404-x
Ratheesh M, Helen A (2007) Anti-inflammatory activity of Ruta graveolens Linn on carrageenan induced paw edema in wistar male rats. Afr J Biotechnol 6(10). eISSN: 1684–5315
Rauf A, Khan R, Khan H, Tokuda H (2015) Cytotoxic, antitumour-promoting and inhibition of protein denaturation effects of flavonoids, isolated from Potentilla evestita Th. Wolf Nat Prod Res 29(18):1775–1778. https://doi.org/10.1080/14786419.2014.999336
Rokade SA, Jadhav RV (2022) Review on analgesic activity using tail immersion method. Asian J Res Chem 15(6):429–432. https://doi.org/10.52711/0974-4150.2022.00075
Saleem A, Javeed A, Ashraf M, Akhtar MF, Akhtar B, Sharif A, Akhtar K, Raza M, Hamid I, Peerzada S, Ahmad S (2017) Anti-inflammatory, anti-nociceptive and antipyretic potential of Terminalia citrina fruit extracts. Afr J Tradit Complement Altern Med 14(5):24–30. https://doi.org/10.21010/ajtcam.v14i5.4
Saleem A, Saleem M, Akhtar MF, Sharif A, Javaid Z, Sohail K (2019) In vitro and in vivo anti-arthritic evaluation of Polystichum braunii to validate its folkloric claim. Pak J Pharm Sci 3(32):1167–1173
Shabbir A, Shahzad M, Ali A, Zia-ur-Rehman M (2014) Anti-arthritic activity of N′-[(2, 4-dihydroxyphenyl) methylidene]-2-(3, 4-dimethyl-5, 5-dioxidopyrazolo [4, 3-c][1, 2] benzothiazin-1 (4H)-yl) acetohydrazide. Eur J Pharmacol 738:263–272. https://doi.org/10.1016/j.ejphar.2014.05.045
Silva RO, Sousa FB, Damasceno SR, Carvalho NS, Silva VG, Oliveira FR, Sousa DP, Aragão KS, Barbosa AL, Freitas RM, Medeiros JV (2014) Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam Clin Pharmacol 28(4):455–464. https://doi.org/10.1111/fcp.12049
Tatipamula VB, Vedula GS (2017) Anti-inflammatory properties of Dirinaria consimilis extracts in albino rats. J Biomed Sci 4(1):3–8. https://doi.org/10.3126/jbs.v4i1.20572
Uttra AM, Ahsan H, Hasan UH, Chaudhary MA (2018) Traditional medicines of plant origin used for the treatment of inflammatory disorders in Pakistan: a review. J Tradit Chin Med 38(4):636–656. https://doi.org/10.1016/S0254-6272(18)30897-5
Wahid S, Alqahtani A, Khan RA (2021) Analgesic and anti-inflammatory effects and safety profile of Cucurbita maxima and Cucumis sativus seeds. Saudi J Biol Sci 28(8):4334–4341. https://doi.org/10.1016/j.sjbs.2021.04.020
Yu H, Fan J, Shehla N, Qiu Y, Lin Y, Wang Z, Cao L, Li B, Daniyal M, Qin Y, Peng C (2021) Biomimetic hybrid membrane-coated xuetongsu assisted with laser irradiation for efficient rheumatoid arthritis therapy. ACS Nano 16(1):502–521. https://doi.org/10.1021/acsnano.1c07556
Acknowledgements
The authors acknowledged Dr. Saiqa Ishtiaq, Head of department of Pharmacognosy, Punjab University College of Pharmacy, University of the Punjab, Pakistan for providing the lab facilities and guidance throughout the study. The corresponding author also acknowledges Dr. Sairah Hafeez Kamran for helping in biological studies and data compilation and Dr. Muhammad Khalil-ur-Rehman for proof reading the manuscript.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SR performed the experimental work and wrote the manuscript. SI planned the project. SR and SI contributed to elucidation of the GC–MS and results. SHK was involved in biological studies and data compilation. MKR did proof reading. SR and SI performed manuscript formatting. All authors carefully read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Data availability
All data generated or investigated in the study are included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rehman, S., Ishtiaq, S., Khalil-Ur-Rehman, M. et al. Ameliorative effects of Atriplex crassifolia (C.A.Mey) on pain and inflammation through modulation of inflammatory biomarkers and GC–MS-based metabolite profiling. Inflammopharmacol 32, 1187–1201 (2024). https://doi.org/10.1007/s10787-024-01430-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01430-1