Abstract
The present experimental study conducted to determine the four flavonoids (1–4) isolated from extracts of Persicaria glabra (Polygonaceae) and was tested for its potential central and peripheral analgesic activity. All animals were lowered on a hot plate (55 ± 0.5 °C), the observations were made before and after administration of respective drugs at 30, 60, and at the end of 90 min. In writhing method, acetic acid is administered intraperitoneal to the experimental animals to create pain sensation. The writhing movements were observed and counted for 30 min after acetic acid administration. The results showed that the compound quercetin (1) and its glycosides (3) at a dose of 100 and 200 mg/kg body weight significant analgesic activity (P < 0.05) in both central and peripheral models of analgesia used. The same doses, compounds 2 and 4 did not show any analgesic effects. Among them, quercetin (1) was the most potent in both models tested. Also due to structural difference of four compounds, a number of hydroxyl groups, substitution of –OCH3 and glycosylation, they will exhibit different analgesic responses. The P. glabra leaf extracts containing mixture of quercetin, isorhamnetin and their glycosides were effective in both the central and peripheral models of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain is the most common symptom for which patients seek medical attention (Hasselstrom et al. 2002) and is a major cause of sick leave (Mantyselka et al. 2001). The chemical structures, positions and the degree of hydroxylation of flavonoids are important for their biochemical and pharmacological activities (Heim et al. 2002). For centuries, preparations that contain flavonoids as the principal physiologically active constituents have been used by physicians and lay healers in attempts to treat human diseases (Havsteen 1983).Increasingly, flavonoids are becoming the subject of medical research. They have been reported to possess many useful properties, including anti-inflammatory activity, oestrogenic activity, enzyme inhibition, antimicrobial activity (Harborne and Baxter 1999), antiallergic activity, antioxidant activity (Middleton and Chithan 1993), vascular activity and cytotoxic antitumor activity (Harborne and Williams 2000). It has been suggested that because flavonoids are widely distributed in edible plants and beverages and have previously been used in traditional medicine, they are likely to have minimal toxicity (Skibola and Smith 2000).
The genus Polygonum (Polygonaceae) comprises 65 species found primarily in northern temperate countries; few are tropical or grow in the southern hemisphere (Boulos et al. 1984). Polygonum species exhibit a variety of interesting biological activities (Lin et al. 2010; Mittal et al. 2011). Polygonum glabrum commonly known as attalaree or sivappu kumbakodaali in Tamil and neeru kanagilu in Mangalore, it is mainly distributed in South, North and North-East India. So far 13 species were reported from South India (Gamble and Fischer 1957). It has been used as a folk medicine and as an ingredient in various Ayurvedic preparations. A decoction of the plant leaves has been used as a foot and leg soak in the treatment of rheumatism (Shiddamallayya et al. 2010). Traditionally rootstock of P. glabra used in pneumonia, consumption, jaundice, fevers and leaves are used as antispasmodic, astringent, diuretic, rubefacient and vermifuge. The whole plant was much more used in traditional as well as in modern era. The findings show that Polygonum glabrum extract is clinically effective as the anti-inflammatory drug and works by the mechanism of action similar to that of NSAIDs (Muddathir et al. 1987). Our previous study on the chemical constituents has resulted in the isolation of flavonoids are demonstrated to contribute to the anti-microbial and anti-inflammatory activities of crude extracts of Persicaria glabra. In the present study we further investigated the analgesic activity of four flavonoids tested using hot-plate and acetic acid-induced pain model.
Materials and methods
Plant materials
The leaves of P. glabra (2.30 kg) was collected in April 2014 from the river basin of Cauvery in Thanjavur District, Tamilnadu (India) and authenticated by Prof. N. Ramakrishnan, (Department of Botany) and voucher specimens (GACBOT-160) was deposited at the Herbarium of the same department for future reference. The collected leaves were air dried at room temperature for 2 weeks. The dried leaves of P. glabra were crushed to fine powder.
Extraction and isolation
The powdered leaves of P. glabra (2.30 kg) were soaked in 95% methanol for 1 week. The methanol extract was concentrated under vacuum at 40 °C, using a rotary evaporator under reduced pressure. This extract was suspended in water (1000 mL) and successively partitioned with benzene, petroleum ether and ethyl acetate to obtain crude extracts. The petroleum ether fraction (10.8 g) was separated over a silica gel column to yield compound 1 (5.2 g) [n-BuOH/AcOH/H2O (4:1:5, organic phase)] and to yield compound 2 (3.1 g) [CHCl3/AcOEt/MeOH (14:3:3)]. Similarly, the EtOAc fraction (19.6 g) was subjected to column chromatographic methods with EtOAc–MeOH–H2O (98:1:1) and EtOAc/HCOOH/H2O (10:2:3) as eluent to afford compounds 3 (8.3 g) and 4 (6.2 g). There was no sign of realizing any crystalline material from the benzene fraction (Manivannan and Shopna 2015).
Animals
Albino mice (25–30 g) of both sexes were used for assessing analgesic studies. The animals were kept in polypropylene cages under standard laboratory conditions maintained at 26 ± 2 °C, relative humidity of 60–65%, and with 12 h dark and 12 h light cycle. All the animals have fed with standard rodent pellet diet, and water ad libitum. The experimental protocol was subjected to the scrutiny of the Institutional Animal Ethics Committee (IAEC), Bharathidasan University, Trichirappalli, Tamilnadu, India (Approval No. BDU/IAEC/2011/31/29.03.2011). Oral administration was used for the isolated flavonoids, vehicle, and the standard drug solutions.
Experimental design
The experimental animals were divided into ten groups, each consisting of six animals which were fasted overnight prior to the experiments. Control group animals treated with Tween 80 (1% w/v in water) at a volume of 10 ml/kg; positive controls animals received subcutaneous morphine (5 mg/kg) administered intraperitoneal (i.p.) for hot plates test and aspirin (50 mg/kg body weight) administered orally for acetic acid-induced writhing test and different doses of isolated flavonoids (1–4) from P. glabra (100, and 200 mg/kg body weight) were dissolved in normal saline by using 0.1% Tween-80 (10 ml/kg).
Acute toxicity tests
The LD50 value (dose of the compounds producing mortality in 50% of the experimental animals) was determined using the graphical method (Litchfield and Wilcoxon 1949) in mice. The test groups (n = 6) received the compounds per orally at the doses of 100, 200, 1000 and 2000 mg/kg body weight were administered i.p. Animals were critically observed for possible behavioral changes, allergic reactions, and mortality at least once during the first 30 min of dosing followed by occasional observation for the first 24 h and continued for 72 h for the recording. Confirmatory test was carried out and the LD50 was calculated from the graph of percent mortality against profit log dose of the test compounds.
Analgesic activity
Hot plate method (thermal stimulation)
Evaluation of analgesic activity of the isolated compounds from plant extract was carried out using hot plate method (Eddy and Leimback 1953). Experimental animals of either sex were randomly selected and divided into ten groups consisting of six mice in each group for control, standard and test groups respectively. The control group was treated with Tween 80 (1% w/v in water) at a volume of 10 ml/kg; test groups were treated at a dose of 100 and 200 mg/kg body weight; standard was treated with morphine (5 mg/kg body weight) administered intraperitoneal (i.p.) route. All animals were lowered (individually) on a hot plate (55 ± 0.5 °C) enclosed with cylindrical glass and the time between the placement of the animal on the hot plate and for the animal to licking of the hind paws or jumping off from the surface was noted as the reaction time. A cutoff period of 30 s was observed to avoid damage to the paw. The observations were made before and after administration of respective drugs at 30 min, 60 min, and at the end of 90 min. The reaction time of the test and standard groups were compared with the control.
Acetic acid induced writhing test (chemical stimulation)
The analgesic activity of the test samples was evaluated using acetic acid induced writhing method (Koster et al. 1959). The experimental albino mice (25–30 g) were divided into ten groups each consisting of six animals. The first group treated as control, served as Tween 80 (1% v/v in water) administered orally at a volume of 10 ml/kg body weight. The second group served as standard (received aspirin 50 mg/kg body weight), while the third to tenth groups received isolated flavonoids (1–4) were treated at doses of 100 and 200 mg/kg body weight orally (dissolved in normal saline by using 0.1% Tween-80). In this method, acetic acid is administered intraperitoneal to the experimental animals to create pain sensation. Writhing in animals was produced by i.p. administration of 300 mg/kg acetic acid (3%) solution. The writhing movements were observed and counted for 30 min after acetic acid administration. The number of writhes of test groups at different dose levels and standard were compared with the control. The percent inhibition of writhing count of the treated group was calculated from the mean writhing count of the control group.
Percentage inhibition was calculated using the following formula:
where Wc no. of writhes in control group; Wt no. of writhes in test group.
Statistical analysis
The experimental results were expressed in multiple comparisons of Mean ± SEM and were carried out by one-way analysis of variance (ANOVA) followed by Dunnet Multiple Comparisons Test using SPSS version 11 and statistical significance was defined as P < 0.05.
Results
Chemical studies
The P. glabra leaves extracts was subjected to column chromatography to isolate and identified as known flavonoids: quercetin (1), isorhamnetin (2), avicularin (3) and new one isorhamnetin-3-O-α-l-(6′′-E-p-coumaroyl)-rhamnoside (4). These flavonoids were identified previously by HPLC, UV, IR, 1H-NMR, and 13C-NMR data (Manivannan and Shopna 2015). The structure of flavonoids 1–4 were identified by spectral data consistent with those of the compounds reported in previous studies (Mabry et al. 1970; Agarwal 1989; Byoung et al. 2012; Kim et al. 2006; Markham and Geiger, 1994). The structures of four compounds (1–4) are shown in Fig. 1.
Analgesic activity
Many methods are available for evaluation of analgesic effect. In all the methods, one or other type of stimulus is applied to produce pain reaction. The methods can be classified based on the type of stimulus used such as thermal, electrical, mechanical and chemical changes. Of the methods available, the chemically induced writhing tests are used for the evaluation of the analgesic activity of peripherally acting drugs and the hot plate methods could be employed for screening of centrally acting analgesics.
The results showed that both doses of four isolated compounds (100 and 200 mg/kg; body weight, p.o.) from P. glabra leaf had good analgesic effects on heat-induced pain in mice when compared to control and standard morphine (P < 0.05). Latency time was noted at 0, 30, 60 and 90 min after the administration of control, standard, isolated compounds. Treatment of animal with compound 1 (quercetin) at dose of 100 and 200 mg/kg body weight was observed significant increase in latency time (8.12 ± 0.61 and 9.16 ± 0.52) even at 30 min following and the maximum effect (15.32 ± 0.45 and 18.78 ± 0.49) at 90 min prior and administration of compound 3 (quercetin glycosides) at doses of 100 and 200 mg/kg body weight noted maximum latency time (12.33 ± 0.46 and 15.07 ± 0.42) at 90 min prior respectively (Table 1). The results also demonstrated that the compounds 2 and 4 showed (12.97 ± 0.87 and 10.09 ± 0.22) at doses of 200 mg/kg body weight, which was lesser than compounds 1 and 3 and where this was compared to the effect of standard morphine sulphate was (19.52 ± 0.47).
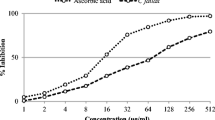
Analgesic activities of the four flavonoids (1–4) were assessed by the acetic acid induced writhing test. Animals were treated with isolated compounds 1–4 (100 and 200 mg/kg bodyweight, p.o.) suspension in Tween 80 (0.5 ml of 1% solution). The severity of writhing and the inhibition of pain were noted for 30 min (Kulkarni 1999). Aspirin (50 mg/kg body weight, p.o.) showed maximum inhibition of writhing (78.41%), whereas compounds 1 and 3 at a dose of 200 mg/kg body weight produced 74.18% and 61.73%; while compound 2 and 4 at a dose of 200 mg/kg body weight showed 59.41 and 50.97% of writhing inhibition respectively (Table 2).
Discussion
Analgesic drugs commonly prescribed for arthritis target a variety of pain mechanisms, but may fail to provide adequate pain relief, or may be discontinued due to adverse events (Schnitzer 2006). Morphine used as standard in hot-plate method was more effective in inflamed tissues than normal tissue (Barr 1999). The increase in latency was dose dependant. From our results, the flavonoids were more active than glycosides. From literature, the anti-inflammatory effects of flavonoids could be attributed to their chemical structures. Furthermore, phenolic hydroxyl groups are able to donate a hydrogen atom or an electron to a free radical lead to inhibit the release of inflammatory mediators, also ortho-dihydroxy has the best electron donating properties, which confers the higher stability to the radical form and participates in electron delocalization (Dai and Mumper 2010).
The acetic acid-induced writhing response is thought to be mediated by peritoneal mast cells (Ronaldo et al. 2000) acid sensing ion channels (Hossain et al. 2006) and the prostaglandin. The writhing movements such as an extension of the hind limb, abdominal constriction, and trunk twisting were observed and counted for 30 min. Aspirin was used as a standard drug. Aspirin leads to a relief from pain by suppressing the formation of pain inducing substances in the peripheral tissues (Jain et al. 2007). The test compounds significantly (P < 0.05) inhibited acetic acid-induced pain sensation in a dose-dependent manner.
Quercetin (1) had the highest analgesic activity among the four compounds. When the glycosylation at 3-OH on the C ring (compound 3), both central and peripheral analgesic activity reduced. Meanwhile, isorhamnetin (2) and its glycosides (4) displayed lower analgesic activity than with quercetin (1) and quercetin glycosides (3). From literature evidence, this could be attributed to the fact that several structural requirements and the substitution pattern of hydroxyl groups, that is, the –OH in 3, 5 and 7th position on the A ring possessed high biological activity and the presence of ortho-dihydroxy groups on the B ring in the compounds (1 and 3) structure, which are important for biological activity. The ortho-dihydroxy has the best electron donating properties, which confers the higher stability to the radical form and participates in electron delocalization (Dai and Mumper 2010). In addition; the 2, 3-double bond with a 4-oxo function in the C ring is responsible for electron delocalization from the B ring, which is essential for the maximum biological efficacy. Also, the reduced analgesic activity in compounds 2 and 4 may be due to –OCH3 in the 3′ position of the B ring (Rastelli et al. 2000; Kumarasamy et al. 2005). Therefore, it can be assumed that flavonoids of the plant possess biologically active secondary metabolites that intermediate with the release of noisome stimulants and thus produced a significant antinociceptive effect. Furthermore, the authors previously reported that anti-inflammatory effect may be due to P. glabra leaf extracts containing a mixture of above mentioned four flavonoids (Manivannan and Shopna 2015).
Conclusion
In conclusion, the present study evidently indicates that the flavonoids (1 and 3) isolated from P. glabra leaf exhibited significant analgesic activity both central and peripheral model of pain. Also, the results obtained, quercetin (1) showed highest analgesic activity among them by testing both model pain may be due to chemical structure and substitution pattern. P. glabra leaf could become useful supplements for pharmaceutical products as a potential source of new analgesics from natural products. This finding offers a theoretical basis for the further study of bioactive compounds from P. glabra leaves.
References
Agarwal PK (1989) Carbon-13 NMR of flavonoids. Elsevier, New York, pp 95–175
Barr GA (1999) Antinociceptive effects of locally administered morphine in infant rats. Pain 81:155–161
Boulos L, Hadidi MN, Gohary M (1984) The weed flora of Egypt. American University in Cairo Press, Cairo
Byoung JP, Tomohiko M, Tsutomu K, Cheol HP, Kwang JC, Michio O (2012) Phenolic compounds from the leaves of Psidium guajava II. Quercetin and its glycosides. Chem Nat Comp 48(3):477–479
Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352
Eddy NB, Leimback D (1953) Synthetic analgesic. II. Dithienyl butenylanddithienyl butyl amines. J Pharmacol Exper Therap 107:385–393
Gamble JS, Fischer CEC (1957) Flora of the presidency of madras. Newman and Adlard, London (Reprint ed. Vol. II. Botanical Survey of India, Calcutta) 1925, p 1244
Harborne JB, Baxter H (1999) The handbook of natural flavonoids, vol 1,2. Wiley, Chichester
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Hasselstrom J, Liu-Palmgren J, Rasjo-Wraak G (2002) Prevalence of pain in general practice. Eur J Pain 6:375–385
Havsteen B (1983) Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol 32:1141–1148
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem 13:572–584
Hossain MM, Ali MS, Saha A, Alimuzzaman M (2006) Antinociceptive activity of whole plant extracts of Paederiafoetida. Dhaka Univ J Pharm Sci 5:67–69
Jain PS, Mallipedi S, Belsare DP, Mandal SC, Pal SC, Badgujar VB (2007) Analgesic activity of stem bark of Kigelia pinnata Linn. Indian Drugs 44:63
Kim GB, Shin KS, Kim CM, Kwon YS (2006) Flavonoids from the leaves of Rhododendron schlipenbachi. Kor J Pharmacog 37:177–183
Koster R, Anderson M, De Beer EJ (1959) Acetic acid for analgesics screening. Fed Proc 18:412–417
Kulkarni SK (1999) Handbook of experimental pharmacology, 2nd edn. Vallabh Prakashan, Delhi, pp 123–128
Kumarasamy Y, Nahar L, Byres M, Delazar A, Sarker SD (2005) The assessment of biological activities associated with the major constituents of the methanol extracts of wild carrot (Daucus carota L) seeds. J Herb Pharmacother 5(1):61–72
Lin HT, Nah SL, Huang YY, Wu SC (2010) Potential antioxidant components and characteristics of fresh Polygonum multiflorum. J Food Drug Anal 18:120–127
Litchfield JT, Wilcoxon F (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exper Therap 96:99–133
Mabry TJ, Markham KR, Thomas MB (1970) The systematic identification of flavonoids. Springer, Berlin
Manivannan R, Shopna R (2015) Isolation of quercetin and isorhamnetin derivatives and evaluation of anti-microbial and anti-inflammatory activities of Persicaria glabra. Nat Prod Sci 21(3):170–175
Mantyselka P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamaki H, Halonen P, Takala J (2001) Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 89:175–180
Markham KR, Geiger H (1994) 1H nuclear magnetic resonance spectroscopy of flavonoids and their glycosides in hexadeutero dimethyl sulfoxide. In: Harborne JB (ed) Advances in research since 1986. Chapman and Hall, London, pp 441–497
Middleton JE, Chithan K (1993) The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne JB (ed) The flavonoids: advances in research since 1986. Chapman and Hall, London
Mittal DK, Joshi D, Shukla S (2011) Hepatoprotective effects of Polygonum bistorta (Linn.) and its active compound against acetaminophen-induced toxicity in rats. Toxicol Lett 205:S237
Muddathir AK, Balansard G, Timon-David P, Babadjamian A, Yagoub AK, Julien MJ (1987) Anthelmintic properties of Polygonum glabrum. J Pharm Pharmacol 39(4):296–300
Rastelli G, Antolini L, Benvenuti S, Constantino L (2000) Structural bases for the inhibition of aldose reductase by phenolic compounds. Bioorg Med Chem 8:1151–1158
Ronaldo AR, Mariana LV, Sara MT, Adriana BPP, Steve P (2000) Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387:111–118
Schnitzer TJ (2006) Update on guidelines for the treatment of chronic musculoskeletal pain. Clin Rheumatol 25(Suppl 1):229
Shiddamallayya N, Yasmeen Azra, Gopakumar K (2010) Medico-botanical survey of kumar parvatha kukke subramanya, Mangalore, Karnataka. Indian J Tradit Knowl 9(1):96–99
Skibola CF, Smith MT (2000) Potential health impacts of excessive flavonoid intake. Free Radic Biol Med 29:375–383
Acknowledgements
Authors are thankful to Department of Chemistry, Botany and Zoology, Government Arts College (Autonomous), Kumbakonam for providing all the required research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The welfare of animals used for analgesic activity (research) of the test compounds was evaluated in male albino mice (25–30 g) approved by Institutional Animal Ethics Committee (IAEC), Bharathidasan University, Trichirappalli, Tamilnadu, India (Approval No. BDU/IAEC/2011/31/29.03.2011).
Conflict of interest
Manivannan Rajamanickam has no conflict of interest. Shopna Rajamohan has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajamanickam, M., Rajamohan, S. Analgesic activity of flavonoids isolated from Persicaria glabra (wild). ADV TRADIT MED (ADTM) 20, 71–76 (2020). https://doi.org/10.1007/s13596-019-00404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-019-00404-x