Abstract
Asphodelus tenuifolius is traditionally used in the management of rheumatic pain and inflamed body parts. The current study validated its traditional use as an anti-arthritic and anti-inflammatory agent using a series of in vivo models. Carrageenan and histamine-induced acute oedema models were employed to study the effects of n-hexane (n-HeAT) and ethanolic (EeAT) extracts on acute inflammatory mediators and were found to inhibit oedema formation in a dose-dependent manner. Formalin and complete Freund’s adjuvant (CFA) were injected into the hind paw of rats for the induction of arthritis. In the formalin model both n-HeAT and EeAT showed significantly better (p < 0.05) anti-oedema effects from day 6 onward. In CFA model rats were treated on 8th day of induction with extracts at the doses of 250, 500, and 750 mg/kg respectively. Piroxicam (10 mg/kg) and normal saline (10 mL/kg) were used as positive and negative controls respectively. Both n-HeAT and EeAT significantly (p < 0.05) decreased arthritis development in a time-dependent manner and at 28th day extent of inflammation was even less than that observed at day 8. The arthritic score was measured at day 12, 16, 20, 24, and 28 and was observed to be significantly less (p < 0.05) in animals treated with 750 mg/kg of n-HeAT and EeAT, respectively. Joint inflammation (p < 0.01), bone erosion (p < 0.001) and, pannus formation (p < 0.01) were significantly declined in A. tenuifolius treatment groups. Radiographic evaluations (X-ray) were conducted to check bone integrity and extent of inflammation and were observed to be diminished at day 28 in A. tenuifolius extracts treated groups. HPLC was performed to screen the phytochemical profile of n-HeAT and EeAT and were found to contain flavonoids and phenolic compounds. Quantitative real‐time polymerase chain reaction (qPCR) was performed to detect effects of n-HeAT and EeAT treatments on inflammatory markers i.e., IL-4, IL-6, IL-10, COX-2, NF-κB, and I-κB using blood samples. ELISA assays were performed for the detection of levels of C-reactive proteins, respectively. Significant downregulation of TNF-α, IL-4, IL-6, IL-1β, COX-2, NF-κB with simultaneous upregulation of IL-10 and I-κB was observed in n-HeAT and EeAT treatment groups. ELISA assays also showed significant (p < 0.05) down-modulation in the serum levels of CRP and TNF-α. Both extracts showed relatively weak antioxidant activities as compared with ascorbic acid in in vitro assay. Based on findings of the current study it is concluded that A. tenuifolius has anti-inflammatory and anti-arthritic effects and thus has potential to be used as an adjunct to standard NSAIDs therapy.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a symmetric polyarticular disorder having characteristic patterns of synovial inflammation, hyperplasia, polyarthritis, and bone/cartilage destruction. RA has multiple etiologies but frequently associated with autoantibodies like rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) (Quinonez-Flores et al. 2016). Numerous epigenetic mutations and environmental agents (cigarette smoke, silica crystals, bacterial/viral infections) also tend to increase the risk of RA that ultimately causes systemic diseases of cardiovascular, pulmonary, and musculoskeletal origin (Anić and Mayer 2014; Shabbir et al. 2018). American College of Rheumatology estimated that females have high mean values of swollen joints count (4.5), tender joints count (6.9), and erythrocyte sedimentation rate (30) as compared with males (Sokka et al. 2009). Moreover, the World Health Organization (WHO) has reported that RA prevalence varies between 0.3 and 1% globally and appears more in women than men. It tends to strike during the most productive years of adulthood i.e., between ages 20–40 years (Naqvi et al. 2019). The exact pathological mechanism of RA remains to be elucidated, however, the contribution of lymphocytes, macrophages, fibroblasts, chondrocytes, and dendritic cells during the triphasic stage (inflammation, synovial hyperplasia, and irreversible joint damage) of RA development is highlighted by multiple research groups (Livshits et al. 2018). Both oxidative stress and inflammation are interlinked (Rani et al. 2006). Under physiological conditions, oxidants are required to catalyze redox reactions as well as to boost cellular signaling, cytoskeletal growth, and apoptosis (Fonseca et al. 2019). However, oxidative stress or deficiency of antioxidants (enzymatic or non-enzymatic) induce repetitive cycles of re-oxygenation thus, promote hypoxia leading to inflammation development. Furthermore, these free radicals cause molecular damage and trigger NF-κB associated transcription of pro-inflammatory cytokines (proteases, IL-1β, and TNF-α) culminating in synovitis and cartilage destruction (Quinonez-Flores et al. 2016). Therefore, antioxidants level, ROS, and C-reactive protein are considered to be potent biomarkers of RA associated systemic inflammation. Current symptomatic treatment of rheumatoid arthritis involving NSAIDs and DMARD’s is ubiquitous because of their anti-inflammatory and analgesic properties (Uttra 2017). The clinical effects of NSAIDs are related to inhibition of COX-2 which turndowns prostaglandins (PGs) release at the inflammatory site while repression of COX-1 causes NSAIDs related adverse effects (Crofford 2013). In the recent years traditional medicines have regained their popularity as an alternative source to relieve pain in RA patients as well as to minimize drawbacks associated with standard drugs (Yuan et al. 2016). Zingiber officinale has showed anti-inflammatory and anti-rheumatic effects by inhibiting PGs and leukotrienes biosynthesis (Hajja and Bahlouli 2018). Ashwagandha is a potent anti-inflammatory agent that exerts its effects by suppressing IL-6, IL-5, IL-4, and TNF-α levels systemically. Arctium lappa has been shown to produce magnificent activity against rheumatoid disorders and chronic inflammatory conditions via inhibition of nuclear signalling pathway (NF-κB) (Kadhim et al. 2016). Glycyrrhiza glabra is a well known anti-rheumatic and anti-inflammatory herbal medicine. Licorice (Glycyrrhiza glabra) demonstrated biological activities by targeting cycloxygenase-2 enzyme which is the primary participant of tumor and RA pathogenesis (Huang et al. 2016).
Asphodelus tenuifolius (Onion weed or Piazzi) belongs to family Asphodelaceae and is indigenous to the Mediterranean region (Eddine et al. 2015), in Pakistan, it is found in district Bahawalpur, Gujarat, Jhang, Lahore, Muzaffargarh, Sargodha, Sahiwal, and Sheikhupura (Saeed et al. 1979). Literature review including the world checklist of selected plant families (WCSP) has claimed folklore use of A.tenuifolius as anti-rheumatic, anti-inflammatory, diuretic, anti-pyretic, and anti-ulcerative agent (Dangi et al. 2013). Triterpenoids (Asphorin-A and Asphorin-B), amino acids, carbohydrates, fatty acids, and flavonoids are proposed to be accountable for its pharmacological actions (Malmir et al. 2018). To best of our knowledge, no scientific data is available on in vivo anti-inflammatory and anti-rheumatic activities of A. tenuifolius. Therefore, the present study was designed to evaluate the anti-arthritic and anti-inflammatory activities of Asphodelus tenuifolius at both cellular and molecular levels.
Materials and methods
Collection and preparation of plant extract
A. tenuifolius was collected in April 2019, and identified by Professor Dr. Zaheer-u-Din Khan, Government College University, Lahore, Punjab, Pakistan and was assigned voucher no. 3582 for future reference. Coarsely grinded plant material was dried, crushed, and soaked into n-hexane and ethanol separately for 5–6 days at room temperature. Afterwards, filtration was done through muslin cloth and Whatman filter paper (grade-1) respectively. The filterate was dried in a rotary evaporator. The resultant extracts were stored at 16–20 °C in separate containers and labeled as n-HeAT and EeAT respectively. 250, 500 and 750 mg doses of both extracts were used in the current study to evaluate the anti-inflammatory and anti-arthritic activities of A. tenuifolius.
Animals
Sprague Dawley rats (100–230 g) of both sex were procured from the University of Veterinary and Animal Sciences (UVAS), Lahore, and kept in stainless steel cages under standard housing conditions i.e., temperature (25 ± 2 °C) and humidity (45–56%) with 12 h light/dark cycle. All animals were provided free access to diet and water. All the animal protocols were approved by the Institutional Review Board of Government College University Faisalabad (study number 19571 and Ref. no. GCUF/ERC/1971).
Non-pharmacological testing
Solubility analysis
To select suitable solvent for in vivo models, solubility of n-HeAT and EeAT (1 mg) was assessed in 1 mL of water, water with tween 20 and 80 (5%), ethanol (10%, 50%, and 100%) and water with gum acacia (5%) respectively (Asif et al. 2020).
Phytochemical screening
Qualitative analysis of phytochemicals (primary or secondary metabolites) in n-HeAT and EeAT was done adopting reported methods (Asif et al. 2020).
Quantitative determination of phytochemicals
Reported high-performance liquid chromatography (HPLC) method was adopted to identify major phytochemicals in n-HeAT and EeAT. The procedure was executed using shim pack HPLC (Shimadzu, Japan) column CLC-ODS. Then detection of compounds was made on a UV–visible detector at 280 nm. Retention time at which sample give peak was measured and results are represented in ppm (Yaseen et al. 2020).
Free radical scavenging assay
Antioxidant potential of n-HeAT and EeAT was evaluated in 2,2-diphenyl-1-picrylhydrazyl (DPPH) antioxidant assay adopting reported procedure (Yaseen et al. 2020).
Pharmacological testing
In vivo anti-inflammatory and anti-arthritic models
Animals were divided into nine groups (n = 5) for evaluating anti-inflammatory and anti-arthritic effects of n-HeAT and EeAT as:
Group 1 (disease control): Untreated inflammation-induced group.
Group 2 (control): Standard pellet diet and normal saline were given to rats.
Group 3 (standard): Received diclofenac sodium (10 mg/kg) orally.
Group 4, 5 and 6 (n-HeAT treated): 250, 500 and 750 mg/kg n-HeAT was administered orally.
Group 7, 8, and 9 (EeAT treated): 250, 500, and 750 mg/kg EeAT was administered orally.
Acute inflammatory models
To minimize number of animals used in the experiments, same animals were resused after executing one acute inflammatory model. Before use in next model, washout period of two weeks was given to experimental rats and only those animals having no signs of inflammation/necrosis or any physical disability were reused.
Carrageenan and histamine-induced paw oedema
Rats were pretreated with specified doses of n-HeAT, EeAT and diclofenac sodium orally. After half hour, inflammation was induced by injecting 0.1 mL of 1% freshly prepared carrageenan and histamine solution in sub-planter region of right hind paw of all groups except the control group. After 1, 2, 3 and 4 h, paw size was measured with digital vernier caliper and percentage inhibition of inflammation development was calculated and represented as mean ± SD (Asif et al. 2020).
Chronic inflammatory models
Formaldehyde-induced arthritis
Rats were pretreated with different doses of n-HeAT, EeAT and diclofenac sodium for 10 days. Thirty minutes after administration of vehicle/drug/extracts, arthritis was induced by injecting 0.1 mL of 2% formaldehyde in sub-plantar area of rats hind paws on day 1st and 3rd respectively. Increase in paw volume was measured on consecutive days for 10 days with digital vernier caliper. Results are presented as mean ± SD of percentage inhibition of paw oedema (Saleem et al. 2020b).
Complete Freund's adjuvant-induced arthritis (CFA)
CFA-induced rat arthritis is extensively used model because of its similar pathological characteristics as seen in rheumatic patients (Ouyang et al. 2019). On day 0, paw size of all rats was measured with plethysmometer. Then 0.1 mL of CFA containing 10 mg/mL of heat-killed M. tuberculosis was injected into sub-plantar region of right hind paws of all rats except the control group. Treatment with n-HeAT, EeAT and diclofenac sodium was initiated on 8th day and continued for 20 days. On 0, 12th, 16th, 20th, 24th and 28th day paw volume was measured (Saleem et al. 2020a). On 28th day, animals were anaesthetized and blood was withdrawn for examination of biochemical and hematological parameters. Then animals were sacrificed and rat paws were dissected and preserved in 37% formalin solution. Tissues were further fixed in paraffin wax and a small section (5 µm) of each tissue was cutted with microtome for staining with eosin and hematoxylin (H&E) dye to evaluate bone/cartilage erosion, pannus formation, inflammation and plasma cell infiltration. Arthritic index (erythema, redness and swelling) and histopathological scoring was done by semi-quantitative scoring method which ranged from 0 to 4 (Bancroft and Gamble 2008).
Quantitative real-time polymerase chain reaction (qPCR)
To quantify gene expression of pro-inflammatory biomarkers i.e., IL-4, IL-6, COX-2, IL-10, IL-1β, NF-κB and TNF-α, real-time polymerase chain reaction (qPCR) was performed using blood samples obtained after 28 days of treatment in CFA-induced arthritis model. For isolation of high-quality RNA, Invitrogen Pure link™ RNA Mini Kit (Thermo Fisher Scientific®, USA) was used. The synthesis of complementary DNA (cDNA) was done by priming with oligo-dT12-18 using Reverse Transcriptase III (Thermo Fisher Scientific®, USA) following instructions of the kit maker (Super Script™ VILO™® cDNA Synthesis Kit) (Die et al. 2016). This first strand of cDNA was stored at − 20 °C. Moreover, for amplification and quantification qPCR kit manufacturer’s protocol (2 × SYBER qPCR Master Mix Gene All®, Korea) was followed. In short, a total of 20 µL of PCR reactions mixture was transferred to microplate having a composition as Master mix (10 μL), reverse and forward primers (150–400 nM), cDNA template + RNase-free water (as per requirement). Then the plate was placed in qPCR instrument (Thermo Fisher Scientific®, USA) and the thermal cycler temperature was set at 90 °C (2 min) for 40 cycles of denaturation, for annealing at 60 °C (15 s) and extension at 72 °C (1 min). Finally, threshold cycles (CT) and relative quantification (ΔΔCT) were calculated (Saleem et al. 2020a). Details of primers used in the current study are given in Table 1.
Estimation of TNF-α in serum
Enzyme-linked immunosorbent assay (ELISA) was carried out to evaluate rat serum levels of TNF-α by adopting kit manufacturer’s protocol (Nanjing Pars Biochem Co LTD®, China). Firstly, 100 µL of all 9 serum samples were added in micro-plate (3 wells/sample) and incubated overnight at room temperature. After 24 h, the liquid sample was removed and plate was washed with wash buffer and diluted biotinylated detection Ab working solution was added into each well. Then the plate was covered with sealing tape and incubated at 37 °C temperature for 1 h. Then, the solution was aspirated and washed with wash buffer (3 times). Then HRP conjugate solution (100 µL/well) was added and the plat was incubated for 30 min. After aspiration, 90 µL of substrate reagent was added into each well and incubated for 15 min at 37 °C in the dark place. Finally, a 50 µL of stop solution was added in each well, and plate was read at 450 nm using ELSIA reader. The results are represented as mean ± SD of TNF-α levels in three experiements (Yaseen et al. 2020).
Statistical analysis
Results are presented as mean ± SD of three independent experiments (n = 3). Two way and One-way ANOVA followed by Post Tukey’s test were applied for comparison of quantitative variables and p value < 0.05 was considered statistically significant.
Results
Solubility testing
n-HeAT was found to be soluble just in water containg 5% tween 20 and 80 while EeAT was soluble in distilled water and ethanol respectively.
Qualitative phytochemical analysis
Qualitative phytochemical screening of n-HeAT and EeAT indicated the presence of various secondary metabolites such as saponins, flavonoids, steroids, glycosides, and phenols.
Quantitative phytochemical analysis
HPLC analysis revealed the presence of eleven compounds in n-HeAT and EeAT such as quercetin, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, synergic acid, p-coumaric acid, m-coumaric acid, cinnamic acid, ferulic acid, and sinapic acid respectively (Table 2, Figs. 1, 2).
DPPH radical scavenging assay
Data of antioxidant assay demonstrated relatively weak free radical scavenging potentials of n-HeAT and EeAT as compared with ascorbic acid. IC50 values of n-HeAT, EeAT, and ascorbic acid were calculated to be 56.2, 77.7, and 6 µg/ml respectively (Fig. 2).
Acute oedema models
Carrageenan and histamine-induced paw oedema models
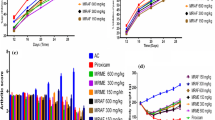
Data in Table 3 represent that in carrageenan-induced acute inflammatory model n-HeAT and EeAT showed dose-dependent anti-inflammatory effects. When comparison made among all n-HeAT and EeAT treated groups (250 vs 500 mg/kg) and (500 vs 750 mg/kg) significant (p < 0.05) reduction in paw oedema was observed in higher dose treated groups starting from 1st to 24th h. Diclofenac sodium (50 mg/kg) exhibited better anti-inflammatory effects (p < 0.05) than higher doses (750 mg/kg) of n-HeAT and EeAT during the whole study (Table 3, Fig. 3).
In histamine-induced model, paw oedema induced by histamine was significantly (p < 0.05) reduced by both n-HeAT and EeAT (250, 500 and 750 mg/kg) from 0 to 5th h. When comparison was done between n-HeAT and EeAT treated groups (250 vs 500 mg/kg) as well as (500 vs 750 mg/kg) dose-dependent anti-inflammatory effects (p < 0.05) were observed. Moreover, indomethacin (25 mg/kg) treated animals also demonstrated significant (p < 0.05) reduction in paw oedema and effects were comparable with 750 mg/kg doses of n-HeAT and EeAT (Table 3, Fig. 3).
Anti-inflammatory effects of n-HeAT and EeAT (250, 500 and 750 mg/kg) in acute and chronic oedema models. Values shown are mean ± SD of percentage inhibition of paw oedema (n = 6). * = p < 0.05, ** = p < 0.01, *** = p < 0.001 and ns = p > 0.05. Capped line having black color star indicates comparison between n-HeAT, EeAT (250, 500 and 750 mg/kg). While green and red color stars indicate comparison of 750 mg/kg of n-HeAT, EeAT with standard treatment group
Chronic inflammatory models
Formaldehyde-induced arthritis
Data in Table 4 exhibit that in formalin-induced inflammation model maximum inhibition of paw oedema was observed at 6th, 8th, and 10th day after treatment with n-HeAT and EeAT (250, 500 and 750 mg/kg). Diclofenac sodium (50 mg/kg) showed comparatively better effects (60.09 ± 1.98) as compared to 750 mg/kg of n-HeAT (51.01 ± 0.78) and EeAT (53.7 ± 1.34) respectively.
CFA-induced arthritis
Arthritis was found to be severe in the arthritic control group than other treatment groups. On 16th, 20th, 24th and 28th day, when comparisons of n-HeAT and EeAT treatments groups was done (250 vs 500 mg/kg) and (500 vs 750 mg/kg) significant (p < 0.05) reduction in poly-arthritic phase was observed as compared to the arthritic control group. Furthermore, piroxicam (10 mg/kg) produced significantly better (p < 0.05) anti-arthritic activity than the higher doses of n-HeAT and EeAT from 16 to 28th day (Table 4, Fig. 3).
Arthritic index
The arthritic index was found to be higher in the arthritic control group over 28 days in the CFA model. On 20th, 24th and 28th day, significant (p < 0.05) reduction in the arthritic index was observed in n-HeAT, EeAT and piroxicam treated animals as compared with arthritic control (Fig. 4).
Effect of n-HeAT and EeAT on arthritic index on 12th, 16th, 20th, 24th and 28th day. Values shown are mean ± SD of arthritic score in CFA-induced model (n = 6). * = p ˂ 0.05, ** = p ˂ 0.01, *** = p ˂ 0.001 and ns = p > 0.05. Capped line having blue, violet and green color stars indicate comparison of n-HeAT, EeAT and piroxicam treatment groups with arthritic control
Histopathological evaluations
Histopathological evaluation at end of CFA model indicated that groups treated with 750 mg/kg of n-HeAT and EeAT showed significantly less bone erosion, decreased pannus formation and paw inflammation with reduced infiltration of mononuclear cells (Figs. 5, 6).
Microscopic Examination of rats paws. Slides were analyzed illustrated that paw of normal control group did not show cartilage erosion, pannus formation and inflammation (a) while severe pannus formation and edematous condition was seen in arthritic control group (b). n-HeAT treated rats (250, 500 and 750 mg/kg) showed mild pannus formation and bone erosion (c–e) whereas, EeAT treated animals (250, 500 and 750 mg/kg) demonstrated minimal infiltration of mononuclear cells and bone erosion (f–h) respectively. Piroxicam treated rat exhibited less pannus formation, no inflammation and bone erosion (i)
Radiographic scoring
Results of radiographic scoring in CFA control, n-HeAT, EeAT and piroxicam treated groups were (5 ± 0.0), (2 ± 0.0), (2 ± 0.0) and (1.33 ± 0.577) respectively. Both extracts and standard (piroxicam-10 mg/kg) treated groups showed less soft-tissue oedema, joint space, and degenerative joints as compared with arthritic control group.
Effect of n-HeAT and EeAT treatments on serum C-reactive protein (CRP) and TNF-α
CRP values in arthritic control group was 72.91 ± 4.42 ng/mL and was found to be significantly reduced (p < 0.05) in n-HeAT (38.23 ± 1.43 ng/mL), EeAT (33.32 ± 1.12 ng/mL) and piroxicam (27.67 ± 0.70 ng/mL) treatment groups respectively. Serum TNF-α levels in arthritic control group (4.22 ± 0.52 ng/mL) was significantly high (p < 0.05) as compared with n-HeAT (1.97 ± 0.33 ng/mL), EeAT (1.53 ± 0.35 ng/mL) and piroxicam (1.02 ± 0.12 ng/mL) treatment groups respectively (Fig. 7).
Effect of n-HeAT and EeAT on mRNA expression of inflammatory biomarkers
Findings of real-time polymerase chain reaction (qRT-PCT) revealed dose-dependent effects of n-HeAT and EeAT on inflammatory biomarkers. Data showed that the levels of IL-4 (pleiotropic anti-inflammatory) was statistically increased (p < 0.001) in treatment groups (n-HeAT, EeAT, and piroxicam) as compared to arthritic control. Alternatively, significant (p < 0.001) elevation in the concentration of IL-6 was observed in the disease control group as compared to treatment groups. Moreover, down-regulation in the expression of IL-10 (p < 0.05) and escalation in the levels of IL-1β was found to be statistically significant (p < 0.01) in arthritic animals than n-HeAT and EeAT and piroxicam treated animals. While mRNA transcription of I-kB was better (p < 0.0001) in extracts and standard treatment groups than arthritic rats, however, there was a significant decrease (p < 0.001) in levels of COX-2 in n-HeAT, EeAT and piroxicam treatment groups as compared to disease control. Furthermore, significant up-regulation in expression of NF-kB (p < 0.05) and TNF-α (p < 0.01) was observed in the arthritic control group in contrast to treated groups (Table 5 and Fig. 8).
Effects of n-HeAT and EeAT on mRNA expression of inflammatory biomarkers. Values shown are Mean ± SD of inflammatory cytokines in CFA-induced model (n = 6). Where * = p < 0.05, ** = p < 0.01, *** = p < 0.001 and ns = p > 0.05. A capped line having blue, violet and green color stars indicate a comparison of n-HeAT, EeAT and piroxicam treatment groups with the arthritic control. Where, COX-2 (cyclooxygenase-2); TNF-α (tumor necrosis factor); NF-kB (nuclear factor kappa-B) and 1-kB (inhibitor of kappa-B)
Estimation of total antioxidant (T-AOC) activity
Data of ELISA assays showed high T-AOC activity in n-HeAT (100.51 ± 3.18 U/mL), EeAT (108.14 ± 1.40 U/mL) and piroxicam (19.26 ± 2.96 U/mL) treated groups as compared with disease control (8.47 ± 5.34 U/mL).
Discussion
Traditionally, A.tenuifolius is reported to have an anti-inflammatory and antioxidant properties (Malmir et al. 2018). Therefore, the current study was carried out to explore in vivo anti-inflammatory and anti-arthritic activities of n-HeAT and EeAT. It is reported that plant-derived therapeutic moieties demonstrate high medicinal properties when extracted using organic solvents (Madane et al. 2013) which formed the logical basis of using two different organic solvents for extraction. A literature survey has suggested that plants contain rich contents of phytoconstituents which play a central role in their biological effects. Flavonoids possess strong antioxidant capacity as well as have magnificent inhibitory effects on prostaglandins synthesizing enzymes like cyclooxygenases, protein tyrosine kinase and phospholipase A2. Moreover, phenols and glycosides may perform anti-inflammatory actions by reducing expression of ROS and iNOS pathways (Owolabi et al. 2018). Saponins exhibits reduction in oedema rate via discouraging vascular infiltration caused by swelling agents. Steroids inhibit recruitment of pro-inflammatory cytokines (e.g. iNOS, TNF-α and COX-2) at wound site thus support anti-oedemtous response (Andreicut et al. 2018). Findings of qualitative phytochemical screening revealed that n-HeAT and EeAT were having these bioactive compounds which are proposed to contribute towards the anti-inflammatory and anti-arthritic effects of both extracts. Reactive oxygen species act as signalling molecules in inflammatory disorders and may lead to endothelial dysfunctioning and tissue injury (Mittal et al. 2014). Moreover, antioxidants help to reduce inflammation and protect cells from injury by counteracting oxidative stress. In the antiradical assay, decoloration of DPPH (violet to yellow) suggested radicals scavenging potential of test drugs which provide the basis for oxidative stress in RA (Kedare and Singh 2011). Results obtained from DPPH are in agreement of phenolic contents of extracts. Oedema induced by carrageenan is reproducible and classically involves two phases characterizing histamine and serotonin release (phase-I) along with prostaglandins secretions (phase-II). Carrageenan or histamine-induced oedema lacks systemic effects and glucocorticoids or prostaglandins antagonist are mainstay of therapy (Patil et al. 2019). In the present study, n-HeAT and EeAT showed positive inhibitory responses against pro-inflammatory mediators secretions which are attributed to their acute anti-inflammatory effect.
Formaldehyde and CFA induce oedema in rats is an accurate model for estimation of anti-inflammatory effects of test drugs in chronic inflammation because of their pathological resemblance with human arthritis. Mainly biphasic responses are induced by formalin named as neurogenic phase (secretion of bradykinins and substance-P) that can be reverted by opioids. Subsequently, inflammatory phase (release of histamine, 5-HT, prostaglandins) which can be treated by anti-inflammatory drugs (Boddawar et al. 2016). Results of the present study indicated that n-HeAT and EeAT possess both opioid and NSAIDs like actions thus reduced oedema produced by formaldehyde. On the other side, in Freund's adjuvant arthritis (CFA) model local oedema reaction occurs in first ten days. Afterward, chronic systemic effects start which can persist for months (Patil et al. 2019). In the current study, animals treated with both extracts exhibited a significant reduction in arthritic index after twenty-eight days of treatment as compared to the arthritic control group that suggested decrease in disease progression. Moreover, estimation of serum biochemical and hematological parameters along with inflammatory biomarkers (CRP, interleukins, COX-2, NF-kB, and TNF-α) classify inflammatory condition of the patient (Brenner et al. 2014). Higher levels of alkaline phosphate, ALT, and AST cause hypophosphatemia and hepatocellular injury respectively (Olago-Rakuomi et al. 2017). In the current study, levels of WBCs, RBCs, platelets, CRP, AST ALP, and ALT were not altered in extract and standard treated animals thus it provided the least supportive environment for the inflammatory process. However, WBCs and platelets were monitored to be high in the arthritic group which may indicate immune responses induction against the pathogens (Foyet et al. 2015). IL-4 and IL-10 suppress Th-1 cell activity via inhibiting IFN-γ thus directly inhibit macrophages activity in synovium of arthritic patients (Lubberts and van den Berg 2013). The present study exhibited upsurge in IL-4 and IL-10 levels in both extract-treated and standard groups indicating their strong inhibitory effects on pro-inflammatory cytokines. Furthermore, neutrophils secrete protease enzymes and reactive oxygen intermediates which promote severe joint devastation and inflammation, hypercoagulability and hypoalbuminemia. Findings of the CFA model showed a high level of IL-6 in the diseases control group while in treatment groups levels were normalized which attributed to the anti-arthritic actions of n-HeAT, EeAT, and piroxicam. IL-1β plays a fundamental ole in cartilage destruction and T-cell activation via the release of reactive oxygen species and proteolytic enzymes while nuclear factor-kappa (I-kB) causes inflammatory cytokines release following toll like receptor (TLR) pathway (Mateen et al. 2017). Outcomes of the present study exhibited retrograding of IL-1β and I-kB levels after treatment with both extracts and standard which evidenced their inhibitory effects on pro-inflammatory mediators release. COX-2 activates prostaglandins (PGE2) synthesis which then promotes TNF-α and IL-1β release from chondrocytes thus evoking inflammation and pain in RA. In the present study qPCR expression analysis indicated that n-HeAT, EeAT, and standard restored levels of COX-2 thus decrease symptoms of polygenetic arthritis. Multiple pathological stimuli like oxidative stress, cytokines and growth factors spur NF-kB activation thus mobilize the inflammatory environment (Makarov and Therapy 2001). A similar mechanism was analyzed in the synovial lining of CFA-induced arthritic control rats. While re-establishment of normal levels of NF-kB was seen in both extracts treated and standard groups respectively.
TNF‐α is a pro-inflammatory cytokine secreted from monocytes, macrophages, and fibroblasts. This cytokine functions as autocrine as well as paracrine inducer for other inflammatory mediators (interleukin‐1, IL‐6, IL‐8, GM‐CSF) and adhesion molecules (ICAM‐1) thus developing rheumatoid synovitis (Vasanthi et al. 2007). Data of the present report showed a similar response in arthritic control group however, a marked decrease in TNF-α expression was observed in both extracts and piroxicam treated groups. The non-specific and acute inflammatory phase detector is C-reactive protein and its normal limit range is 0–8 mg/L while, overexpression of CRP (100 mg/L) indicates active rheumatoid arthritis (Finney and Thwaites 2010). The present study demonstrates normal levels of CRP in treatment groups (n-HeAT, EeAT, and piroxicam) which are comparable with anti-inflammatory attributes of n-HeAT, EeAT. Histopathological scoring and microscopic examination of rats paws indicated minimal bone erosion and inflammation in both extracts treated groups. X-Rays examination helps to monitor joint deterioration and punctured holes (Finney and Thwaites 2010) which are found to be at least level as compared to standard consequently supporting anti-oedematous effects of both extracts. Quantitative phytochemical analysis showed presence of multiple flavonoids and phenolic compounds in n-HeAT and EeAT which highlight their role in rheumatic and inflammatory disorders as described in the literature.
Conclusion
To conclude, data of multiple in vitro and in vivo studies showed that both extracts of A. tenuifolius possess remarkable anti-inflammatory and anti-arthritic potentials making it a suitable candiadte for possible future therapy of inflammation. Moreover, A. tenuifolius has ability to down-regulate expression of inflammatory cytokines e.g. TNF-α, IL-6, COX-2, IL-1B, and NF-kB as well as up-regulate IL-4 and IL-10 biomarkers. Therefore, further experimentations are required to establsih the safety profile and standardise the chemical composotion of A. tenuifolius extracts before commencement of clinical trials.
References
Andreicut A-D et al (2018) Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid Med Cell Longev 2018
Anić B, Mayer MJR (2014) Pathogenesis of rheumatoid arthritis. Reumatizam 61:19–23
Asif M et al (2020) Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology 1–16
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences
Boddawar GD, Dhawale SC, Shaikh SS (2016) Assessment of anti-inflammatory potential of Sesbania bispinosa Linn. leaf extracts and fractions by acute and chronic models. Alex J Med 52:289–293
Brenner DR et al (2014) A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Prev Biomark 23:1729–1751
Crofford LJ (2013) Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther 15:S2
Dangi A, Aparna MS, Yadav J, Arora D, Chaudhary UJJ (2013) Antimicrobial potential of asphodelus tunifolius (CAV). J Evolut Med Dent Sci 12:5663–5667
Die JV, Roman B, Flores F, Rowland LJ (2016) Design and sampling plan optimization for RT-qPCR experiments in plants: a case study in blueberry. Front Plant Sci 7:271
Eddine LS, Segni L, Ridha OM (2015) In vitro assays of the antibacterial and antioxidant properties of extracts from Asphodelus tenuifolius Cav and its main constituents: a comparative study. Int J Pharm Clin Res 7:119–125
Finney A, Thwaites CJ (2010) Rheumatoid arthritis. 1: Background, symptoms and ensuring prompt diagnosis and treatment. Nurs Times 106:22–24
Fonseca LJS, Nunes-Souza V, Goulart MOF, Rabelo LA (2019) Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Oxid Med Cell Longev
Foyet HS, Tsala DE, Bodo JZE, Carine AN, Heroyne LT, Oben EK (2015) Anti-inflammatory and anti-arthritic activity of a methanol extract from Vitellaria paradoxa stem bark. Pharmacogn Res 7:367
Hajja G, Bahlouli A (2018) Medicinal plants in the prevention and treatment of rheumatoid arthritis. MOJ Bioequiv Availab 5:00084
Huang Q-C, Wang M-J, Chen X-M, Yu W-L, Chu Y-L, He X-H, Huang R-Y (2016) Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget 7:1193
Kadhim M, Kaizal A, Hameed I (2016) Medicinal plants used for treatment of rheumatoid arthritis: a review. Int J Pharm Clin Res 8:1685–1694
Kedare SB, Singh RP (2011) Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 48:412–422
Livshits G, Kalinkovich A (2018) Hierarchical, imbalanced pro-inflammatory cytokine networks govern the pathogenesis of chronic arthropathies. Osteoarthr Cartil 26:7–17
Lubberts E, van den Berg WB (2013) Cytokines in the pathogenesis of rheumatoid arthritis and collagen-induced arthritis. In: Madame Curie Bioscience Database [Internet]. Landes Bioscience
Madane A, Kamble S, Patil B, Aparadh V (2013) Assessment of solvent solubility by using phytochemical screen tests of some Euphorbiaceae members. Asian J Pharm Res 3:53–55
Makarov SS (2001) NF-κB in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res Ther 3:200
Malmir M, Serrano R, Caniça M, Silva-Lima B, Silva OJP (2018) A comprehensive review on the medicinal plants from the genus Asphodelus. Plants 7:20
Mateen S, Moin S, Shahzad S, Khan AQ (2017) Level of inflammatory cytokines in rheumatoid arthritis patients: correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 12:e0178879
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Naqvi AA, Hassali MA, Aftab MT (2019) Epidemiology of rheumatoid arthritis, clinical aspects and socio-economic determinants in Pakistani patients: a systematic review and meta-analysis. JPMA 69:389–398
Olago-Rakuomi A, Oyoo G, Kamau E, Genga E, Okalebo F, Ogutu E (2017) Prevalence of abnormal liver function tests in rheumatoid arthritis. University of Nairobi
Ouyang L et al (2019) Effect of umbelliferone on adjuvant-induced arthritis in rats by MaPK/nF-κB pathway. Drug Des Dev Ther 13:1163
Owolabi OO, James DB, Sani I, Andongma BT, Fasanya OO, Kure B (2018) Phytochemical analysis, antioxidant and anti-inflammatory potential of Feretia apodanthera root bark extracts. BMC Complement Altern Med 18:12
Patil KR et al (2019) Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int J Mol Sci 20:4367
Quinonez-Flores CM, Gonzalez-Chavez SA, Del Rio Najera D, Pacheco-Tena C (2016) Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. BioMed Res Int 2016
Rani HS, Madhavi G, Srikanth B, Jharna P, Rao U, Jyothy A (2006) Serum ADA and C-reactive protein in rheumatoid arthritis. Int J Hum Genet 6:195–198
Saeed SA, Ahmad AN, Sadiq M (1979) Density and frequency of weeds in wheat fields of the Punjab Province (Pakistan). Pak J Agric Sci 16:85–90
Saleem A, Saleem M, Akhtar MF, Shahzad M, Jahan S (2020a) Polystichum braunii extracts inhibit Complete Freund’s adjuvant-induced arthritis via upregulation of I-κB, IL-4, and IL-10, downregulation of COX-2, PGE2, IL-1β, IL-6, NF-κB, and TNF-α, and subsiding oxidative stress. Inflammopharmacology 1–16
Saleem M et al (2020b) Investigation of in vivo anti-inflammatory and anti-angiogenic attributes of coumarin-rich ethanolic extract of Melilotus indicus. Inflammopharmacology
Shabbir A et al (2018) Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed Pharmacother 97:1710–1721
Sokka T et al (2009) Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 11:R7
Uttra AM (2017) Assessment of anti-arthritic potential of Ephedra gerardiana by in vitro and in vivo methods. Bangladesh J Pharmacol 12:403–409
Vasanthi P, Nalini G, Rajasekhar G (2007) Role of tumor necrosis factor-alpha in rheumatoid arthritis: a review. APLAR J Rheumatol 10:270–274
Yaseen H et al (2020) Methanolic extract of Ephedra ciliata promotes wound healing and arrests inflammatory cascade in vivo through downregulation of TNF-α. Inflammopharmacology
Yuan H, Ma Q, Ye L, Piao G (2016) The traditional medicine and modern medicine from natural products. Molecules 21:559
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, M., Iftikhar, A., Asif, M. et al. Asphodelus tenuifolius extracts arrested inflammation and arthritis through modulation of TNF-α, NF-κB, ILs, and COX-2 activities in in vivo models. Inflammopharmacol 29, 483–497 (2021). https://doi.org/10.1007/s10787-020-00761-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00761-z