Abstract
Tragia involucrata Linn. (T. involucrata) belongs to the family of Euphorbiaceae found in the subtropical regions. Traditionally, the plant parts are used to treat inflammation, wounds and skin infection by people of the Western Ghats, India. Few studies on the acute anti-inflammatory activity of T. involucrata extracts were reported earlier. The present study aims to identify the bioactive fraction of T. involucrata and to evaluate its mechanism in Complete Freund’s Adjuvant-induced arthritic rat model. The leaf extract was highly effective among the methanolic leaf and root extracts. The hexane (HF) and a methanolic fraction (MF) of the leaf extract of T involucrata were further identified as a bioactive fraction evaluated through protein denaturation assay. The HF and MF were further studied for their anti-inflammatory potential in a chronic inflammatory model, and their mechanism of action was explored further. Arthritis was induced by administering 0.1 ml of CFA intradermally. The treatment was started the next day with HF (100 and 250 mg/kg/day) and MF (100 and 250 mg/kg/day), while the HF and MF alone group served as the drug control, Indomethacin-treated group served as the positive control. On the 25th day, the animals were euthanized, and their body weight, paw thickness, arthritic score, spleen and thymus weight, haematological parameters, biochemical parameters, radiographs and histopathology were analyzed. Results showed that the MF-treated animals maintained dry weight, reduced paw thickness, arthritic scores, and haematological and biological parameters compared to the HF-treated and CFA-induced arthritic rats. Both radiological and histopathological analyses of the joints revealed that the MF-treated groups restored bone architecture without any erosion and normal tissue architecture with nil signs of active inflammation. Western blot analysis revealed that MF has effectively inhibited the protein expression levels of MMP-3, MMP-9, and NF-κB in the synovial tissues compared to that of CFA-induced arthritic rats. Besides, HPLC analysis revealed the presence of flavonoids, including gallic acid, rutin and Quercetin, in the MF of T. involucrata, which had shown to have potent anti-inflammatory potential. Thus, it can be emphasized that T. involucrata could be a potential therapeutic candidate for treating inflammatory diseases, which needs further experimental studies to confirm its safety and efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tragia involucrata Linn. (Euphorbiaceae) is a small shrub distributed throughout the tropical and subtropical regions. It is an annual herb commonly available as a weed plant in India (particularly in Punjab, Himalayas, Assam, Meghalaya and Tamil Nadu), Sri Lanka, and Asia. It is popularly known as “Indian stinging nettle” in English and “Chenthatti” in Tamil. The tribes in the Western Ghats of India use different parts of this plant to treat inflammation, wounds and skin infections. It has also been effective in treating pain and bronchitis (Kirtikar and Basu 1987). Various secondary metabolites were reported in the leaves of T. involucrata—hydrocarbon esters like shellsol, vinyl hexyl ether, 2, 4-dimethyl hexane, 2-methylnonane and 2, 6- dimethyl heptane (Samy et al. 2006, 2013). One of the phytoconstituent from the methanolic extract of the leaves of T. involucrata 5-hydroxy-1-methylpiperidin-2-one was reported as a potent muscle relaxant, bronchodilator and anti-allergic on histamine (Alagar Yadav et al. 2015). Five different compounds isolated from ethyl acetate extract of the roots of T. involucrata were 10, 13-dimethoxy-17-(6- methylheptan-2-yl)-2, 3, 4, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17- tetradecahydro-1H- cyclopenta [a]phenanthrene, stigmasterol, Quercetin, rutin and 3-(2,4-dimethoxyphenyl)-6,7- dimethoxy-2,3- dihydrochro-men-4-one (Panda et al. 2012).
The anti-inflammatory activity of T.involucrata aqueous and methanolic extracts was investigated in albino rats with carrageenan-induced hind paw oedema and cotton pellet granuloma models, as well as its effect on acute and subacute inflammation (Dhara et al. 2000; Samy et al. 2006). Furthermore, the same author reported that the analgesic activity of T. involucrata root, mediated by the inhibition of prostaglandins, which sensitized pain receptors at the site of inflammation (Dhara et al. 2000). The hydrocarbon esters such as shellsol, vinyl hexyl ether and 2,4-dimethyl hexane isolated from T. involucrata showed a potent antimicrobial and anti-inflammatory activity which controls the growth of food-borne and food spoilage pathogens (Samy et al. 2013).
Although T. involucrata extracts have been shown to have anti-inflammatory efficacy in several animal models, their bioactive components still need to be thoroughly investigated. Therefore, the current study aims to identify the bioactive fraction of T. involucrata that exhibits anti-inflammatory activity and to study its efficacy and mechanism of action in an in vivo model.
Materials and methods
Plant collection and authentication
The plant Tragia involucrata was collected from Suriyur, Tiruchirappalli district (Tamil Nadu). It was authenticated by Dr.S.Soosairaj, Department of Botany, St. Joseph's College, Tiruchirappalli. The voucher specimen Number is 2799.
Reference standards
Rutin (98%), quercetin (96%), and gallic acid (92%) were obtained from Natural remedies Pvt Ltd, Bangalore and Sami labs limited, Bangalore. Indomethacin was purchased from Sigma.
Preparation of extracts
The leaves and roots of T. involucrata were cleaned and shade dried for about 2 weeks. Then, they were powdered and passed through sieve no.14. The prepared materials were stored in an airtight container for further use. Powdered leaves (350 g) and powdered root (50 g) of T. involucrata were weighed and subjected to reflux extraction using methanol at 65 °C for 2 h. The methanolic leaf (MLE) and methanolic root (MRE) extract was filtered and the filtrate was concentrated under reduced pressure using a rotary vacuum evaporator. The obtained yield of the dried MLE and MRE was found to be 19.53% w/w and 29.27% w/w, respectively.
Fractionation of the leaf extract of T. involucrata
The methanolic leaf extract (39 g) was further fractionated using four different solvents like hexane, chloroform, ethyl acetate and methanol, as shown in Fig. 1.
In vitro anti-inflammatory assay
Inhibition of protein denaturation
The reaction mix (0.5 mL) consisted of 450 μL of 5% aqueous bovine serum albumin and 50 μl of various concentrations (50, 100, 250, 500 and 1000 µg mL−1) of extracts and fractions of T. involucrata. The pH of the reaction mix was adjusted to 6.3 using 1N hydrochloric acid (HCl) and was incubated at 37 °C for 20 min, followed by heating at 57 °C for 3 min. This mixture was then brought to room temperature and 2.5 ml phosphate buffer saline (pH 6.3) was added to each tube. Turbidity was measured at a wavelength of 660 nm. 50 μl distilled water was used in place of extracts and fractions as a control and Aceclofenac was used as the standard. The percentage inhibition of protein denaturation was calculated. The control represents 100% protein denaturation. The experiment was performed in duplicate (Mizushima and Kobayashi 1968; Sakat et al. 2010).
HPLC analysis of MF and HF
The methanolic fraction of T. involucrata was standardized with respect to the reference standard rutin, gallic acid and quercetin as per Nisar et al. 2017, while the hexane fraction was standardized with respect to lupeol, β-sitosterol and oleanolic acid as per Ekambaram et al. 2017 using HPLC. The Agilent 1260 Infinity series HPLC was equipped with an Agilent Zorbax Eclipse C-18 column of dimension 250 X 4.6 mm, 5 µm. The mobile phase containing acetonitrile: methanol: 3% formic acid (50/50/0.3, v/v/v) with a flow rate of 0.5 mL/min was used for the standardization of methanolic fraction, while a linear gradient solvent system of acetonitrile (solvent A) and 0.1% aqueous formic acid (solvent B) with the following profile: 75–50% A until 50 min, followed by 95–5% A over 30 min; maintaining the 5% A condition for 15 min, then 5% A over 5 min, and finally reconditioning the column with 95% A isocratic for 10 min was used for separating the hexane fraction and the flowrate was set as 1 mL/min at 27 °C. Finally, the characteristic peaks were detected using a UV–Vis detector at 254 nm.
In vivo studies
Animals used
Male Wistar albino rats were purchased from Sri Venkateshwara Enterprises, Bangalore and then placed in the animal house of the University College of Engineering, Anna University BIT campus, Tiruchirappalli. Animals were kept under a 12 h dark/light cycle at 25 ± 2 °C and 55 ± 5% humidity in cages. The study was approved by the Institutional Animal Ethical Committee (IAEC) (AUBIT/CAF/6 IECM/0012/2020 Dt. 12. 02. 2020).
Acute toxicity studies
The plant fractions (HF and MF) were evaluated for acute toxicity as per the organization for economic cooperation and development (OECD) guidelines 423. The single oral dose of 5000 mg/kg of plant fraction was administered to overnight fasted rats and observations were continuously recorded for behavioural profiles for 2 h and mortality up to 24 h.
In vivo anti-arthritic study in CFA-induced rats
Experimental design
The rats were allocated into nine groups, with six animals in each group. Group I served as a standard control group (N), Group II as a negative control group (NC), Group III as a positive control group, Groups IV, V, VI, and VII, received HF I & II, and MF I & II at a dose level of 100, 250 mg/kg/day, respectively, and Group VIII and IX served as drug control (HF and MF). Arthritis was induced by intradermal injection of 0.1 ml CFA emulsion (consisting of heat-killed Mycobacterium tuberculosis suspended in mineral oil acquired from Sigma-Aldrich) in the sub-plantar area of the right hind paw on the first day to every animal, excluding normal and drug control. Treatments were started by oral gavage following CFA administration and continued till the 25th day. The plant fractions and Indomethacin, the positive control, were dissolved in carboxymethyl cellulose (0.5%) prior to administration.
Body weight
The body weight was recorded during the experimental period using a weighing balance just before CFA injections on the 1st day and subsequently at various time (weekly once) intervals until the 25th day.
Paw thickness evaluation
The right paw thickness was measured every 5 days at time intervals using a vernier calliper. The paw thickness alterations were considered the difference between the final and initial paw thickness. The visual arthritis scoring systems were used to assess the severity of arthritis, as described previously (Kumar et al. 2017). The arthritis score ranged from 0 to 4, graded as follows: 0 = normal paw; 1 = mild swelling and erythema; 2 = swelling and erythema; 3 = severe swelling and erythema; 4 = gross deformity and inability.
Determination of the spleen and thymus weights
At the end of the experimental studies, the rats were anesthetized and euthanized by administering thiopentone sodium (40 mg/kg/i.p). Then, the thymus and spleens of all rats were removed and weights were recorded.
Haematology assessment
The animals were euthanized on the 25th day, and blood was collected by cardiac puncture and subjected to evaluation of haematological parameters such as red blood cells (RBC), white blood cells (WBC), haemoglobin (Hb), platelet, lymphocytes, neutrophils, eosinophils, basophils, monocytes. Samples were analyzed at Doctors Diagnostic Centre, Tiruchirappalli.
Biochemical estimation
On the 25th day, the blood samples were withdrawn from the rats by cardiac puncture. As per the manufacturer’s instructions, serum alkaline phosphatase (ALP) was estimated. C-reactive protein (CRP) and rheumatoid factor (RF) were estimated by immunoturbidimetry method analyzed at Doctors Diagnostic Centre, Tiruchirappalli.
Radiological assessment
On the 25th day, the animals were anaesthetized and radiographs of CFA-injected hind paws were recorded using X-ray. The X-ray image was interpreted for the radiographic changes.
Histopathological assessment
On the 25th day, anaesthetized rats were euthanized. Ankle joints were detached and submitted for histopathological assessment.
Western blot
On the 25th day, anaesthetized rats were sacrificed and synovial tissues were collected. The total protein content was quantified using a bicinchoninic acid assay kit (BCA) (Thermofisher Scientific). Then, the quantified proteins were separated using 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad). The membrane was incubated with primary antibodies of anti-rat MMP3 (1:1000) and MMP9 (1:1000) from Abclonal, pNF-κBp65 (1:1000) and NF-κBp65 (1:1000) from Cell Signalling Technology for 12 h at 4 °C. Afterwards, the HPR-conjugated Goat anti-Rabbit secondary antibody (1:2000) from Abclonal was added to the membranes and washed with PBST buffer. The immunoreactive bands were visualized using a chemiluminescent reader (Bio-Rad), and the intensity of the bands was measured using ImageJ software.
Statistical evaluation
Mean ± standard deviation (SD) was used to express the results of the study. For statistical analysis, multiple data comparisons were carried out using analysis of variance (ANOVA), and then a post-test of Tukey was used for post hoc analysis. Significance was statistically acceptable at a level of p < 0.05. Software Graph pad prism (8.0) was used for all data analysis.
Results
In vitro anti-inflammatory assay
Inhibition of protein denaturation
The percentage inhibition of protein denaturation of samples obtained from T. involucrata at various concentrations was determined. The IC50 value for MLE and MRE was 219.53 µg/ml and 479.32 µg/ml, respectively. From the results, MLE had shown effective inhibition of protein denaturation compared to MRE, as shown in Fig. 2a. Thus, the MLE of T. involucrata Linn was considered for fractionation using different solvents.
Preliminary anti-inflammatory assay to identify the effective fractions
Inhibition of protein denaturation assay
The IC50 values for MF and HF were 230.58 µg/ml and 252.10 µg/ml, respectively. The results showed that the percentage inhibition of protein denaturation was above 80% in the MF and HF, as shown in Fig. 2b. It was observed that MF and HF were found to be the bioactive fractions. The HF and MF were evaluated for anti-arthritic activity (in vivo model) based on the obtained results.
HPLC
The methanolic fraction of T. involucrata was standardized with respect to the reference standard gallic acid, rutin, and quercetin (Table 1). In contrast, the hexane fraction was standardized with respect to lupeol, β-sitosterol and oleanolic acid using HPLC. HPLC analysis revealed the quantity of the aforementioned phytoconstituents in the methanolic fraction as 0.07 ± 0.21%, 28.31 ± 0.09% and 0.106 ± 0.13% with the retention time of 3.680 min, 6.490 min and 13.929 min, respectively, as shown in Fig. 3a. In comparison, the phytoconstituents in the hexane fraction showed the presence of 0.07 ± 0.21%, 28.31 ± 0.09% and 0.106 ± 0.13% with the retention time of 3.680 min, 6.490 min and 13.929 min, respectively, as shown in Fig. 3a.
In vivo evaluation
Acute toxicity studies
The single dose 5000 mg/kg/p.o administration of MF and HF did not show any mortality in the rats. No lethal effects or behavioural changes were noted throughout the observation period.
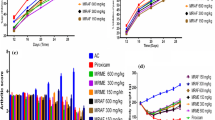
Effect of MF and HF on body weight in CFA-induced rats
In the CFA-treated arthritis control rats, there was a substantial reduction in body weight (p < 0.05) compared to normal control rats. In contrast, the rats treated with the standard (Indomethacin) and plant fractions showed a marked increase in body weight from the third week. Notably, the fractions were found to restore and maintain the body weight of the animals gradually, as shown in Fig. 4a. In general, the body weight of the CFA-induced rats was found to be well maintained by both MF and HF fractions.
Effect of MF and HF on paw thickness in CFA-induced rats
CFA was administered on the 1st day, which resulted in a progressive increase in paw thickness. The treatment with standard (Indomethacin) and plant fractions (MF and HF) was given from day 1 to 25. As presented in Fig. 4b, it can be seen that both the standard as well as fractions caused significant abatement of paw thickness which was noticed from day 19 to day 25.
Effect of MF and HF fractions on the arthritic score in CFA-induced rats
The arthritic score of the right hind paws was noted on different days from the 1st to the 25th day. The clinical arthritic score was increased in the negative control, whereas indomethacin, MF & HF groups have reduced the swelling and redness of the right hind paws. The results exhibited that Indomethacin, HF & MF groups had shown minimal arthritic scores compared to the negative control, as represented in Fig. 4c. The macroscopic images of the right hind paw are shown in Fig. 5.
Effect of MF and HF on spleen and thymus weight in CFA-induced rats
Immunological functions are related to the thymus and spleen weights. On the 25th day, the rats were sacrificed and thymus and spleen weights were determined. As presented in Fig. 6a, b, the spleen and thymus weights of drug-treated group animals were significantly lower than the arthritic control group (p < 0.05). Further, it was also observed that MF I and II were much better than HF I and II in reducing the thymus and spleen weights of arthritic rats Table 1.
Effect of MF and HF fractions on haematological parameters in CFA-induced rats
In arthritic control rats, WBC, neutrophils, eosinophils and platelets were significantly increased compared to normal rats. In contrast, the above-said parameters were significantly decreased in the drug-treated and positive control groups, as shown in Table 2.
Effect of MF and HF on biochemical parameters in CFA-induced rats
The effect of fractions (HF and MF) treatment on specific serum biochemical parameters such as alkaline phosphatase, C-reactive protein and rheumatoid factor in CFA rats were studied and the results are shown in Table 3. In negative control rats, alkaline phosphatase, C-reactive protein and rheumatoid factor were significantly increased compared to normal rats. Treatment with fractions reverted the condition at all the dose levels with p < 0.05 level of significance. Furthermore, the effect of fractions was also found to be dose-dependent.
Effect of MF and HF fractions on radiological evaluation of hind paws in CFA-induced rats
Radiological evaluation of the hind limbs of the animals in all groups was recorded on day 25 and the results are shown in Fig. 7. As per the radiological micrographs, the normal control rats did not show any abnormalities in the architecture, while the CFA-induced arthritic rats showed swelling and erosion of the joints. Indomethacin-treated positive control showed typical bone architecture similar to that of control rats. The experimental groups treated with fractions showed marked recovery of the joint bone with reduced swelling, reverting the effects of CFA in a dose-dependent manner.
Radiological assessment of Hexane and methanolic fractions of T. involucrata L. on CFA—induced arthritic rats. a control; b negative control; c positive control (CFA + indomethacin); d HF (100 mg/kg/day); e HF (250 mg/kg/day); f MF (100 mg/kg/day); g MF (250 mg/kg/day); h HF alone; i MF alone. The red arrow indicates swelling of joints and soft tissues (color figure online)
Histopathology
On the 25th day, the rats were sacrificed, and the organs were collected and subjected to histopathological analysis, as shown in Fig. 8. Tissues from the control (Fig. 8a) showed viable Osteocartilagenous tissue with no active inflammation or necrosis, whereas the negative control (Fig. 8b) showed chronic inflammatory granulation tissue. The positive control treated with CFA and Indomethacin (Fig. 8c) showed focal disruption of muscle fibres with no evidence of active inflammation or necrosis. In contrast, the experimental groups treated with 100 mg/kg/day (Fig. 8d) and 250 mg/kg/day (Fig. 8e) of HF showed mild restoration of the inflammatory granulation tissue with respect to increasing concentration. However, the groups treated with 100 mg/kg/day (Fig. 8d) and 250 mg/kg/day (Fig. 8e) of MF showed no sign of inflammation without any tissue necrosis. Besides, HF alone (Fig. 8h) and MF alone (Fig. 8i) showed no signs of necrosis or disruption of muscles with no active inflammation. Histopathology results signified that MF had shown a potential anti-inflammatory effect in restoring the normal tissue architecture in the CFA-induced arthritic rats.
Effect of Hexane and methanolic fractions of Tragia involucrata L. on CFA—induced arthritic rats. a Control; b negative control; c positive control (CFA + indomethacin); d HF (100 mg/kg/day); e HF (250 mg/kg/day); f MF (100 mg/kg/day); g MF (250 mg/kg/day); h HF alone; i MF alone. The red arrow indicates inflammatory infiltration, whereas the yellow arrow indicates synovium (color figure online)
Methanolic fraction of T. involucrata inhibits the protein expression levels of MMPs and NF-κBp65
The protein expression levels of MMP-3, MMP-9, p-NF-κBp65 and NF-κBp65 were assessed using western blot assay. Results showed 2.75-fold, threefold and a threefold increase in the protein expression levels of MMP-3, MMP-9, and p-NF-κBp65/ NF-κBp65 in the CFA alone treated groups, as shown in Fig. 9a and Fig. 9b, respectively. In contrast, the MF-treated groups showed a decrease in the protein expression levels by 2.5-fold, 1.5-fold and 1.25-fold, respectively, exhibiting the inhibition of MMP-3 (Fig. 9c), MMP-9 (Fig. 9d), and NF-κBp65 (Fig. 9e) in CFA-induced arthritic rats.
Effect of MF on the protein expression levels of MMPs and NF-κB in the FCA induced arthritic rats. a Expression of MMP-3 and MMP-9. b Expression of pNF-κBp65 and NF-κBp65 c relative expression of MMP-3 with respect to β-actin d relative expression of MMP-9 with respect to β-actin e relative expression of pNF-κBp65/NF-κBp65with respect to β-actin
Discussion
T. involucrata plant parts are widely used in traditional medicine to treat various inflammation (Mallik et al. 2012; Appian et al. 2013; Uprety et al. 2016). Experimental validation of the anti-inflammatory activity of T. involucrata plant parts was reported in various acute and sub-acute animal models (Dhara et al. 2000; Samy et al. 2006, 2013). However, there are no reported studies exploring the chronic anti-inflammatory activity of T. involucrata, as well as the mechanism of action and the responsible phytochemicals for its anti-inflammatory activity. The present study focussed on identifying the bioactive fraction from T. involucrata leaves and its mechanism of anti-arthritic action.
The methanolic extract of leaves and roots of T.involucrata were prepared and screened for preliminary in vitro anti-inflammatory activity and found that leaf extract MLE was highly effective in inhibiting the protein denaturation rather than the root extract MRE (Fig. 2a). From the literature, it is revealed that both the leaves and roots of T.involucrata were reported for significant oral anti-inflammatory activities in animal models (Dhara et al. 2000; Samy et al. 2006). However, in the present comparative study, the MLE was highly active as an anti-inflammatory agent. Thus, MLE was further subjected to solvent fractionation to yield various fractions, in which the hexane (HF) and methanol fractions (MF) were found to show higher inhibition of protein denaturation. A similar kind of study on in vitro anti-arthritic activity by inhibiting protein denaturation of T.involucrata leaf extracts showed higher activity for chloroform extract followed by petroleum ether extract (Velu and Malipeddi 2015). In the present study, both the MF and HF were standardized with respect to the reference standards, gallic acid, rutin, Quercetin and lupeol, β-sitosterol, and oleanolic acid, respectively. HPLC analysis showed the presence of phenols and flavonoids, including gallic acid, rutin and Quercetin in the MF, which have been shown to have potent multifaceted pharmacological properties such as anti-inflammatory, anti-arthritic, anti-cancer activities (Alam et al. 2017; Salehi et al. 2020). Similarly, HF comprises triterpenoids, including lupeol (Rathinavel et al. 2021), β-sitosterol (Zhang et al. 2020) and oleanolic acid (Sen 2020), exhibiting anti-inflammatory potential.
In the current study, the highly active non-polar hexane and polar methanol fractions were taken for further in vivo evaluation of anti-inflammatory activity in Freund's complete adjuvant-induced arthritic Wistar albino rats. To the best of our knowledge, this is the first report on the efficacy of T. involucrata leaf extract on Freund's adjuvant-induced arthritis in Wistar albino rats.
CFA-induced arthritis is one of the most common, appropriate and widely recognized methods for inducing RA in animals, as it shares numerous similarities with RA in humans. In the present study, the CFA-induced arthritic rats exhibited a significant decrease in body weight gain increase in paw swelling, redness and paw oedema as well as joint deformation, signifying the progress of impulsive inflammation as stated in earlier reports (Gohil et al. 2018; Patel et al. 2021). The change in body weight in the animals induced with CFA is a significant factor for assessing the duration of the disease towards inflammation as well as the response of a drug (Naik and Wala 2014). The animals induced with arthritis were found to display high arthritic index scores and paw swelling (Zhang et al. 2017). Paw swelling is an effective indication of measuring the rate of inflammation in CFA-induced rats. The respective reduction in paw swelling after drug treatment indicates the anti-arthritic activity of the drug (Rajendran and Krishnakumar 2010). Treatment with HF and MF at 100 and 250 mg/kg significantly suppressed the increased arthritic score, paw oedema, and swelling in the treated RA rats, indicating its significant anti-arthritic activity.
The spleen and thymus are essential organs in which the cells and antibodies for immunological actions are formed (Choudhary et al. 2014). Hence, the weight of the organs was increased in the CFA-induced arthritic rats due to the infiltration of inflammatory cells. However, upon treatment with HF and MF, the weight of the spleen and thymus was reduced compared to that of CFA-induced experimental animals. During the arthritic condition, the haematological levels of red blood cells, white blood cells, lymphocytes, monocytes, neutrophils, eosinophils, basophils, hemoglobin and platelets were found to be altered and increased (Li et al. 2018). Nevertheless, upon treatment with HF and MF, especially MF has drastically reduced their levels compared to CFA-induced arthritic rats.
Alkaline phosphatase (ALP) is found to be increased in arthritic conditions and is a good index for assessing kidney impairment and inflammation (Mbiantcha et al. 2017). Serum C Reactive protein (CRP) is a biomarker for systemic inflammation found to be increased in arthritic patients. In contrast, Rheumatoid factor Rf is an immunoglobulin molecule that cannot elicit an immune reaction, but its levels were found to be increased in the arthritic condition (Kim et al. 2015; Mbiantcha et al. 2017; Pope and Choy 2021). The serum levels of ALP, CRP, and Rf were reduced in the MF-treated experimental groups compared to that of HF and CFA-induced arthritic rats. In addition, the radiographs showed deterioration of bone architecture and increased erosion in the CFA-induced rats, whereas the bone architecture was restored to normal bone architecture in the HF and MF-treated animals and especially in MF, they reverted in par with the untreated controls. Similarly, the histopathology results revealed that in CFA-induced rats, increased chronic inflammatory tissue was found with active signs of inflammation and tissue necrosis, whereas the MF-treated animals exhibited normal tissue architecture without signs of active inflammation, such as infiltration of inflammatory cells. Hence, results indicate the anti-inflammatory effect of MF in CFA-induced arthritic rats.
MMP3 and MMP-9 are matrix metalloproteases, bone-degrading enzymes playing a critical role in synovial inflammation, significantly degrading the bone matrix in the arthritic condition, which has been implicated in chronic inflammatory conditions (Sun et al. 2014; Xue et al. 2014). Besides, NF-κB is regarded as a master regulator of inflammation implicated in multiple diseases, including arthritis and cancer, exhibiting multifaceted physiologic functions such as cell survival, proliferation, and death (Liu et al. 2017). The expression levels of MMP3, MMP-9 and NF-κB were upregulated in the synovial tissue of the CFA-induced arthritic rats. In contrast, the expression levels of MMP3, MMP-9 and NF-κB were significantly reduced in the MF-treated groups compared to that of HF-treated groups implicating the potent anti-inflammatory property of MF of T.involucrata.
Conclusion
In the present study, the bioactive fraction of T. involucrata Linn was identified with respect to the anti-inflammatory property. From the in vitro anti-inflammatory assay, the methanolic leaf extract was found to be highly effective when compared to root extract. Thus, the leaf extract was considered for bioactive fractionation using successive solvent fractionation techniques. From the in vitro anti-inflammatory assay, the HF and MF fractions were found to be more effective. Thus, these two fractions were further subjected to in vivo studies. Complete Freund’s adjuvant-induced arthritis model was attained in Wistar male rats. MF and HF treatment at two gradual doses (100, 250 mg/kg) was started on daily administration for up to 25 days. Body weight, food intake, and increase in hind paw size were monitored on a weekly basis. Both the fractions (HF and MF) have shown marked reduction in paw thickness, arthritic score, serum parameters and, finally, evidenced by radiological assessment. However, MF was found to be highly effective when compared to HF. Radiological and histopathological studies showed that MF restored normal bone and tissue architecture in the experimental rats. Besides, western blot analysis revealed the inhibition of MMP3, MMP-9 and NF-κB in MF-treated groups compared to FCA-treated animals. Flavonoids and phenolic compounds like Quercetin, rutin and gallic acid in MF might contribute to its significant anti-inflammatory properties. Hence, it can be emphasized that T. involucrata is a potent therapeutic candidate for treating inflammatory and related diseases.
Data availability
Data supporting findings are presented within the manuscript. Enquiries about data availability should be directed to the authors.
References
Alagar Yadav S, Ramalingam S, Jabamalai Raj A, Subban R (2015) Antihistamine from Tragia involucrata L. leaves. J Complement Integr Medi 12:217–226. https://doi.org/10.1515/jcim-2015-0015
Alam P, Parvez MK, Arbab AH, Al-Dosari MS (2017) Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP- and NP-HPTLC methods for quality control of anti-HBV active extract of Guiera senegalensis. Pharm Biol 55:1317–1323. https://doi.org/10.1080/13880209.2017.1300175
Appian S, Madhavachandran V, Appukuttannair G (2013) Medicinal plants in the treatment of arthritis. Ann Phytomedicine 2:3–36
Choudhary M, Kumar V, Gupta P, Singh S (2014) Investigation of antiarthritic potential of Plumeria alba L. leaves in acute and chronic models of arthritis. Biomed Res Int 2014:474616. https://doi.org/10.1155/2014/474616
Dhara AK, Suba V, Sen T et al (2000) Preliminary studies on the anti-inflammatory and analgesic activity of the methanolic fraction of the root extract of Tragia involucrata Linn. J Ethnopharmacol 72:265–268. https://doi.org/10.1016/S0378-8741(00)00166-5
Ekambaram SP, Perumal SS, Pavadai S (2017) Anti-inflammatory effect of Naravelia zeylanica DC via suppression of inflammatory mediators in carrageenan-induced abdominal oedema in zebrafish model. Inflammopharmacology 25:147–158. https://doi.org/10.1007/s10787-016-0303-2
Gohil P, Patel V, Deshpande S et al (2018) Anti-arthritic activity of cell wall content of Lactobacillus plantarum in freund’s adjuvant-induced arthritic rats: involvement of cellular inflammatory mediators and other biomarkers. Inflammopharmacology 26:171–181. https://doi.org/10.1007/s10787-017-0370-z
Kim K-W, Kim B-M, Moon H-W et al (2015) Role of C-reactive protein in osteoclastogenesis in rheumatoid arthritis. Arthritis Res Ther 17:41. https://doi.org/10.1186/s13075-015-0563-z
Kirtikar KR, Basu BD (1987) Indian medicinal plants, 2nd edn. International book distributors, Dehradun
Kumar BS, Suneetha P, Mohan A, Kumar DP, Sarma KVS (2017) Comparison of Disease Activity Score in 28 joints with ESR (DAS28), Clinical Disease Activity Index (CDAI), Health Assessment Questionnaire Disability Index (HAQ-DI) & Routine Assessment of Patient Index Data with 3 measures (RAPID3) for assessing disease activity in patients with rheumatoid arthritis at initial presentation. Indian J Med Res 146:S57–S62. https://doi.org/10.4103/ijmr.IJMR_701_15
Li Y, Kakkar R, Wang J (2018) In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Front Pharmacol 9:1341
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Mallik BK, Panda T, Padhy RN (2012) Traditional herbal practices by the ethnic people of Kalahandi district of Odisha, India. Asian Pac J Trop Biomed 2:S988–S994. https://doi.org/10.1016/S2221-1691(12)60349-9
Mbiantcha M, Almas J, Shabana SU et al (2017) Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement Altern Med 17:111. https://doi.org/10.1186/s12906-017-1623-5
Mizushima Y, Kobayashi M (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 20:169–173. https://doi.org/10.1111/j.2042-7158.1968.tb09718.x
Naik S, Wala S (2014) Arthritis, a complex connective and synovial joint destructive autoimmune disease: animal models of arthritis with varied etiopathology and their significance. J Postgrad Med 60:309–317. https://doi.org/10.4103/0022-3859.138799
Nisar A, Mamat A, Mohamed Dzahir MIH et al (2017) Identification of flavonoids (quercetin, gallic acid and rutin) from Catharanthus Roseus plant parts using deep eutectic solvent. Recent Adv Biol Med 3:1–6. https://doi.org/10.18639/RABM.2016.02.347628
Panda D, Dash SK, Dash GK (2012) Phytochemical examination and antimicrobial activity of various solvent extracts and the selected isolated compounds from roots of Tragia involucrata Linn. Int J Pharm Sci Drug Res 4:44–48
Patel R, Kadri S, Gohil P et al (2021) Amelioration of complete Freund’s adjuvant-induced arthritis by Calotropis procera latex in rats. Futur J Pharm Sci 7:213. https://doi.org/10.1186/s43094-021-00361-w
Pope JE, Choy EH (2021) C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum 51:219–229. https://doi.org/10.1016/j.semarthrit.2020.11.005
Rajendran R, Krishnakumar E (2010) Anti-arthritic activity of Premna serratifolia Linn., wood against adjuvant induced arthritis. Avicenna J Med Biotechnol 2:101–106
Rathinavel T, Ammashi S, Shanmugam G (2021) Analgesic and anti-inflammatory potential of lupeol isolated from Indian traditional medicinal plant Crateva adansonii screened through in vivo and in silico approaches. J Genet Eng Biotechnol 19:62. https://doi.org/10.1186/s43141-021-00167-6
Sakat S, Juvekar A, Gambhire M (2010) In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci 2:146–155
Salehi B, Machin L, Monzote L et al (2020) Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega 5:11849–11872. https://doi.org/10.1021/acsomega.0c01818
Samy RP, Gopalakrishnakone P, Houghton P et al (2006) Effect of aqueous extract of Tragia involucrata Linn. on acute and subacute inflammation. Phyther Res 20:310–312. https://doi.org/10.1002/ptr.1845
Samy PR, Sethi G, Chow TKV, Stiles GB (2013) Plant-based hydrocarbon esters from Tragia involucrata possess antimicrobial and anti-inflammatory activities. Infect Disord–drug Targets 13:141–153
Sen A (2020) Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J Clin Cases 8:1767–1792. https://doi.org/10.12998/wjcc.v8.i10.1767
Sun S, Bay-Jensen A-C, Karsdal MA et al (2014) The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet Disord 15:93. https://doi.org/10.1186/1471-2474-15-93
Uprety Y, Poudel RC, Gurung J et al (2016) Traditional use and management of NTFPs in Kangchenjunga landscape: implications for conservation and livelihoods. J Ethnobiol Ethnomed 12:19. https://doi.org/10.1186/s13002-016-0089-8
Velu V, Malipeddi H (2015) In vitro anti-arthritic and hemolytic activity of leaf extracts of Tragia involucrate. IntJ Pharm Tech Res 8:46–50
Xue M, McKelvey K, Shen K et al (2014) Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 53:2270–2279. https://doi.org/10.1093/rheumatology/keu254
Zhang X, Dong Y, Dong H et al (2017) Investigation of the effect of phlomisoside F on complete Freund’s adjuvant-induced arthritis. Exp Ther Med 13:710–716. https://doi.org/10.3892/etm.2016.3995
Zhang F, Liu Z, He X et al (2020) β-sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund’s adjuvant-induced arthritis in rats: involvement of NF-кB and HO-1/Nrf-2 pathway. Drug Deliv 27:1329–1341. https://doi.org/10.1080/10717544.2020.1818883
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vigneshwaran, S., Maharani, K., Sivasakthi, P. et al. Bioactive fraction of Tragia involucrata Linn leaves attenuates inflammation in Freund’s complete adjuvant-induced arthritis in Wistar albino rats via inhibiting NF-κB. Inflammopharmacol 31, 967–981 (2023). https://doi.org/10.1007/s10787-023-01154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01154-8