Abstract
Hepatocellular carcinoma is emerging as one of the most common forms of cancer resulting in thousands of death worldwide. The purpose of this study was to screen nimesulide for anticancer activity in chemically induced hepatocellular carcinoma in Wistar rats as well as in BEL 7402 and HEP G2 cell lines. HCC in rats was induced by administering a single dose of diethyl nitrosamine (150 mg/kg) intraperitoneally. Duration of the in vivo study was 12 weeks and the anticancer potential was further confirmed by in vitro cell line study. Administration of DENA in Wistar rats significantly elevated the levels of serum biochemical parameters and α-feto protein. Treatment with different dose of nimesulide significantly decreased the markedly raised serum levels of biochemical parameters as well as maintained the histology of the liver tissues nearly similar to the normal. Further study of hepatocytes enzymes showed that treatment with nimesulide also improved the antioxidant enzyme levels. Our study also examined the cytotoxicity and DNA synthesis inhibition by nimesulide in BEL 7402 and Hep G2 cell lines. Cell viability was assessed by [3H]-thymidine uptake procedure. The results obtained by in vitro cell line study, histopathological and biochemical data concluded that nimesulide, a preferential COX-2 inhibitor, has anticancer activity, which is by first reducing the formation of reactive oxygen species and second by inhibiting the PGE2 effect via Wnt signaling pathway (cell invasion, angiogenesis, and cell proliferation).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the primary hepatic malignancy which has gradually emerged as the most common cancer in the world ranking fifth in male mortality and eighth in case of females (Ghouri et al. 2017). Diethylnitrosamine (DENA) has been identified to cause severe liver toxicity, and in many cases, it caused HCC by releasing free radicals (Thirunavukkarasu and Sakthisekaran 2003). DENA has also been found responsible for ravaging various enzymes which are responsible for the repair mechanism of DNA. Therefore, nowadays, it is commonly employed as an established model in chemical-induced liver cancer in animals (El-Serag and Mason 1999). Nimesulide, a well-known drug to counter inflammatory process, is a preferential cyclooxygenase-2 (COX-2) inhibitor extensively used in Asia, Africa, and whole of Europe to get relief from inflammatory conditions. There are two most common isoforms of fatty acid cyclooxygenase enzymes, COX-2 and COX-1, which are held responsible for limiting the rate of reaction for the synthesis of prostaglandins from arachidonic acid. COX-2 is responsible for producing inflammation and is auto-induced in the inflammatory cells during cell injury. It forms many inflammatory mediators by catalyzing two molecules of oxygen in the arachidonic acid molecule including pro-inflammatory bioactive molecule Prostaglandin E2 (PGE2), which again possesses pro-angiogenic activities (Subbaramaiah and Dannenberg 2003). Factors of growth like fibroblast-growth factor [FGF-2], vascular-endothelial growth factor [VEGF], endothelial growth factor [EGF], cytokines such as tumor necrotic factor-α [TNF-α], interleukin-1β [IL-1β] and interleukin-α [IL-α], and tumor promoters such as v-Ha-ras, v-src, Wnt., and HER-2/neu induce a massive increase in COX-2 level. Overexpression of COX-2 was reported in various human cancers including colon, prostate, breast, skin, and lung (Subbaramaiah and Dannenberg 2003). Contrastingly, overexpression of COX-2 is not seen in the vasculature of normal tissues. Novel nimesulide analogs have been reported to regulate the aromatase expression selectively in breast cells (Buckman et al. 1998; Koki and Masferre 2002). Several research studies have suggested that in vitro COX-2 inhibition by nimesulide has been associated with induction of apoptosis in cancer cell as well as the seizure of angiogenic process (Fodera et al. 2004; Hida et al. 2000). Moreover, it prevents in vivo metastasis and tumor development by suppressing telomerase activity in cancerous cells. The concept of COX-2 possessing anti-carcinogenic potentials was conceived because prostaglandins, which are subsequently responsible for tumor growth, are synthesized by COX-2, and its inhibition terminates the entire cascade which leads to the tumor cell growth (Boland et al. 2004; Keller and Giardiello 2003). According to Liang et al. and Esteves-Souza et al., nimesulide has anticancer activity due to its dual action: first, it stops the cancer cell proliferation via COX inhibition; and second, it stimulates the immune responses of the cancerous cell which makes it to respond to the apoptotic stimuli (Liang et al. 2009; Esteves-Souza et al. 2006). These findings motivated us to undertake the study, it main aim was to screen nimesulide for anti-cancerous activity together with the evaluation of cytotoxicity in BEL 7402 and HepG2.
Methods
Drugs and chemicals
Nimesulide in pure powder form was purchased form Himgiri traders Dehradun, Uttarakhand; DENA was obtained from St. Louis, USA, through Sigma-Aldrich Chemicals Co. Ltd. Kits for the estimation of biochemical parameters were procured from Nicholas India Private, Limited. All reagents and chemicals procured were of the standard analytical grade.
Animals
Adult, healthy, male and female Wistar albino rats that weigh around 100–125 g were obtained from Siddhartha Institute of Pharmacy, Dehradun. The animals were randomly grouped and kept in standard cages under controlled conditions of IAEC. The temperature was set at (22 ± 3 °C) and light (14:10 h dark and light cycle) was provided along with standard quality pallet diet and water ad libitum. This research study was approved by the Institutional Animal Ethical Committee (IAEC) of Siddhartha Institute of Pharmacy (Ethical approval no. SIP/IAEC/PCOL/O7/2016 on 02 April 2016). The protocol was carried out as per the guidelines of the CPCSEA, India. All institutional and national guidelines for the care and use of laboratory animals were followed.
Initiation of hepatocellular carcinoma
HCC was induced using an established oncogenic dose of DENA, i.e., 200 mg/kg body weight administered (i.p.). Animals were maintained on a schedule of fasting and then refeeding for the administration of aforementioned dose of DENA (Alwahaibi et al. 2010; Muzio et al. 1999; Xu-yinga et al. 2009).
Research methodology
In vivo study
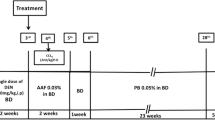
Animals were acclimatized and randomly divided into four groups (n = 6). These groups were given different treatment for a period of 12 weeks. Group-I: Normal Control group (NC), rats were treated with saline water. Group-II: DENA Control group (Disease control), a single dose of 200 mg/kg DENA was administered. Group-III: DENA + nimesulide (2 mg/kg), rats were administered with DENA (200 mg/kg) on day 1, and after the 7th day, they were given nimesulide (2 mg/kg) until the day before their sacrifice. Group-IV: DENA + nimesulide (6 mg/kg), the rats were given single dose (200 mg/kg) of DENA on day 1 and after the 7th day administered with nimesulide (6 mg/kg) until the day before the sacrifice of animals. Group-V: DENA + nimesulide (9 mg/kg), the rats were given single dose (200 mg/kg) of DENA on day 1 and after the 7th day administered with nimesulide (9 mg/kg) until the day before the sacrifice of animals. Group-VI: Wistar rats were doctored only with nimesulide at a dose of 6 mg/kg and served as nimesulide only group. The duration of the entire study was 12 weeks. Effective dose fixation study was also performed for the selection of dose. Induction of HCC was confirmed by the estimation of AFP a week after DENA administration.
Estimation of biochemical parameters
Whole blood was collected from the retro-orbital plexus of the rats under the influence of light anesthesia. On the termination day of the experiment, whole blood was left to stand for 30 min at normal room temperature without any anticoagulant. Then, it was centrifuged for 10 mins at 2500 rpm to separate the serum. The obtained serum was stored at low temperature (2–4 °C) for further estimation of various biochemical parameters. Estimation of serum glutamate pyruvate transaminase (SGPT), serum glutamate oxalate transaminase (SGOT), total cholesterol (TC), high-density lipoprotein (HDL), triglycerides (TG), alkaline phosphatase (ALP), blood glucose, albumin (ALB), total protein (TPR), and total bilirubin (TBR) was carried out with regular kits procured from Nicholas India Private Limited using semi-auto analyzer (Nicholas India Private Limited). Serum level of α-feto protein (AFP) in serum was also assessed.
Histopathological examination
Isolated liver samples preserved in formalin solution with 10% phosphate-buffer and subsequently fixed in paraffin were utilized for histopathology. The liver sample was sliced in sections with 5 µm thickness and was abridged; paraffin was removed and then tainted with eosin and hematoxylin. The segments were scrutinized for any cell swelling, changed hepatic architecture, ballooning, tubular dilatation, and edema of interstitium and necrosis of cell in all treated groups.
In vitro study
Cell culture
BEL7402 and HepG2 cell lines were used for the study. These cell lines were cultured in Dulbecco’s Eagle medium (DMEM) at optimum temperature (37 °C), further appended with bovine serum (10%), which was sought from Life Technologies India Pvt Ltd. in addition with 100 U/mL of penicillin and l00 μg/mL of streptomycin (Himgiri Traders, Dehradun) in a dampened incubator with CO2 (5%) and passed every 3 days. Mycoplasma contamination was routinely examined.
Preparation of nimesulide in different dilutions
Nimesulide was freshly prepared by dissolving it in DMSO at different dilutions. After obtaining the anticipated strength of drug in solution, it was mixed with media and applied to the growing cells. The last strength of DMSO was about 0.1%, the vehicle concentration was also maintained (0.1% v/v) for all. Different strengths of nimesulide ranging from 0.1 to 50 µm were chosen for the in vitro study (Ding et al. 2005).
Cell viability assay
MTT assay was carried out to examine the cell viability. The principle is based on the MTT reduction via enzyme mitochondrial dehydrogenase of integral cells to a purple color product. 525 cells were scattered into 60 mm plates of 100 mL volumes in a 96-well culture plates. After 24 h of development, the old medium was replaced with a newly formed medium having nimesulide at different strengths ranging from 0.1 to 50 µm and the cells were left to grow for 4 days. Every group enclosed within 6 wells arranged parallel and every strength stated above was observed as 1 treated group. There was no nimesulide in the control group. Afterwards, MTT (20 μL) as a working solution was mixed in cultured wells and then incubated for 4 h. The colored insoluble crystals of formazan were formed during the period for which the sample was incubated. It was then solubilized by adding a solubilizing agent to each well (Zhang et al. 2007) and measured by spectrophotometry at 570 nm.
[3H]-thymidine uptake
3H-thymidine treatment in cell cultures is used to access the proportionality of DNA synthesis. 21 × 105 cells were scattered into the plates of 24 wells. These cell cultures were then given with various dilutions of nimesulide. After 0, 24, and 48 h of incubation, 1 μCi [3H]-thymidine was adjoined to every plate and then incubated for another 6 h at 37 °C, and the cultured cells were then nurtured onto a glass fiber filter mat by a cell harvester. Incorporation of 3H-thymidine was calculated with TM scintillation counter (1450 Microbeta). The assays were repeated two times and mean CPM values were analyzed (Rumi et al. 2001).
Statistical analysis
The final data were calculated as mean ± SEM (standard error mean). The significance of results among more than two groups was examined via one-way ANOVA and then Student’s t test was applied using software (Graph Pad, Prism 5.0). The differences of (p < 0.05) were taken to be significant statistically.
Results
Animal weight
The groups which received DENA showed significant drop in their body weight as compared to NC animals. In DENA + nimesulide group and nimesulide only treated group, the body weights were increased considerably when compared to disease control animals (Table 1).
Blood glucose
Animals of disease control group displayed considerable lowering of blood glucose level in comparison to NC animals. Such major alterations were not found in the blood glucose levels of DENA + nimesulide and nimesulide only groups when compared to DENA-treated animals (Table 1).
Albumin (ALB)
In DENA-treated animals, the level of albumin declined drastically (p < 0.001) as compared to NC. However, DENA + nimesulide groups of different dose showed slight rise in the albumin levels. In the nimesulide only treated rats, there was no alteration in ALB level as compared to NC (Table 1).
Total protein (TPR)
Disease control groups revealed a significant reduction in TPR levels when assessed against NC. Nimesulide increased the level of TPR dose dependently as compared to the DENA Control animals. Group-IV displayed a significant decline in TPR levels when compared to NC (Table 1).
Alanine transaminase/serum glutamate pyruvate transaminase (ALT/SGPT)
DENA-treated animals revealed substantial elevation in the levels of ALT when assessed against NC animals, while DENA + nimesulide group animals with increased dose showed remarkable normalization of SGPT levels in comparison to DENA control animals. SGPT levels of nimesulide only treated group remained near the normal value (Table 2).
Serum glutamate oxaloacetate transaminase/aspartate transaminase (SGOT/AST)
Levels of serum AST were significantly elevated (p < 0.001) in diseased animals when contrasted with NC. DENA + nimesulide and nimesulide only treated groups exhibited depression in SGOT levels towards normal value when compared to the diseased animals (Table 2).
Serum alkaline phosphatase (ALP)
There was a substantial decline in ALP levels in DENA + nimesulide group animals with increased dose when analyzed against DENA control while the levels remained unaltered in the nimesulide only treated rats (Table 2).
Gamma-glutamyltranspeptidase (GGTP)
No significant alteration was found in GGTP levels in the disease control when equaled to NC. In DENA + nimesulide group animals at the different dose, the amount of GGTP substantially improved when matched to disease control. There were no major changes observed in the level of GGTP in the nimesulide only treated rats as compared to NC (Table 2).
Total bilirubin (TBR)
TBR levels remained unaltered in every group (Table 2).
Serum α-feto protein (AFP)
DENA-controlled animals exhibited remarkable elevation in the serum level of AFP (p < 0.05) when compared to NC. The concentration of serum AFP in DENA + nimesulide groups was around the normal values (Table 2).
Lipid profile
In DENA control animals, the levels of TC and TG were significantly elevated whereas it was decreased in case of HDL as compared to NC. These parameters were maintained towards the normal levels by different treatment groups of nimesulide dose dependently and there were no alterations found the lipid profile of nimesulide only treated rats (Table 3).
Histopathological study
The sections of hepatic tissues of NC group exhibited typical histology with discreet central veins; no evidence of hepatocellular injury, fibrosis, or malignancy was detected. In DENA control animals, massive necrosis and inflammatory insinuate were encircling the central veins, revealing the clusters of the necrotic liver cell along with portal tract having pronounced atypia and overgrowth of the bile duct. The pleomorphic behavior of hepatic cells was seen and they were clustered in a group of 2–8 cells; broad trabeculae were separated with sinusoidal spaces which in turn were layered by endothelial cells. Dilation in blood vessels was seen in the different DENA + nimesulide groups. The slight proliferation of bile duct was witnessed along with intrusion of intra-acinar inflammatory cells. The section of liver tissue from the nimesulide only treated group was spotted with normal structural design and without necrosis (Fig. 1).
Histopathological studies showing normal (a), DENA-treated (b), DENA + nimesulide 2 mg/kg (c), DENA + nimesulide 6 mg/kg (d), DENA + nimesulide 9 mg/kg (e), and nimesulide control (f). *Arrows indicating the centrilobular necrosis, hepatocyte ballooning, and infiltration of inflammatory cells, respectively, in DENA-treated rats
Antioxidant estimation
Superoxide dismutase (SOD)
In the diseased group, the SOD level was tremendously declined when equated with NC. While in the different groups treated dose dependently with nimesulide and nimesulide only treated animals, the SOD concentration was remarkably increased when compared to DENA-treated animals (Table 4).
Catalase
The DENA-administered animals exhibited major decline in the levels of catalase in comparison to NC, while the dose-dependent treatment of DENA + nimesulide group animals showed significant restoration of catalase levels when compared to DENA-treated animals. The concentration of catalase in nimesulide only treated group remained maintained towards the normal value (Table 4).
Lipid peroxidation (LPO)
In DENA-treated animals, the amount of LPO was remarkably increased as compared to the NC. While DENA + nimesulide group animals exhibited a significant decrease in LPO concentration when equated with DENA control animals (Table 4).
Glutathione peroxidase (GPx)
Diseased animals where DENA was administered showed a remarkable drop in the GPx level when compared to NC. However, DENA + nimesulide groups as well as nimesulide only administered animals depicted a significant increase in GPx level as compared with disease control group (Table 4).
Glutathione (GSH)
GSH was (p < 0.001) decreased drastically in DENA-treated rats as compared to NC. DENA + nimesulide group animals at different dose showed remarkable elevation (p < 0.001) in GSH concentration, while the only drug-treated animals exhibited stagnant concentration as in the NC (Table 4).
Nimesulide-induced cell growth inhibition on HCC cells
The cytotoxic effect of nimesulide on HCC cells was investigated on BEL-7402 and HepG-2 cells which show overexpression of COX-2 (Rahman et al. 2000). These were cell lines which were treated with different strengths of nimesulide for a period of 0, 24, and 48 h. Nimesulide (over 1 µm) exhibited remarkable anticancer effects on these cells by diminishing the growth size. Cell viability was reduced significantly when the cells were treated with 10 µm nimesulide for 48 h (Fig. 2).
Cell growth inhibition detected by MTT assay. After cells were treated with different concentrations of nimesulide, MTT assay was used to detect cell growth inhibition as described in Materials and methods. The inhibitory rate at 50 mM is much higher than that of lower concentrations of nimesulide (p < 0.01)
Nimesulide inhibits DNA synthesis
DNA synthesis inhibition induced by nimesulide was confirmed by the 3H-thymidine administration in every cancer cell lines after half an hour of drug incorporation. A major decline was observed in a dose-dependent manner after introduction of 3H-thymidine at all concentration in Hep-G2 and Bel-7402 cells. The data revealed that drug at a dose of 50 µm could hamper the production of DNA more significantly than 10 µm nimesulide affecting DNA synthesis. Observations and obtained data advocate an idea that nimesulide plays a potential role in curbing the uncontrolled proliferation of hepatocellular carcinoma cells and has the cytotoxic effect (Fig. 3).
Discussion
Among the cancer incidences, liver carcinogenesis especially (HCC) has been ranked as the third most conventional cause of mortality due to cancer (Parkin et al. 2001). DENA, a compound with potential to damage liver cells, is commonly used hepatotoxic for various animal models. Damage to the liver tissue is then confirmed by assessing the serum markers (such as AFP, ALP, ALT, and AST, etc.) in the DENA-administered animals. Raised level of AFP is indicative of cancerous condition which is further confirmed by histopathological studies (Sivaramakrishnan et al. 2008; Barbisan et al. 2003; Bansal et al. 2005; Yadav and Bhatnagar 2007).
The present protocol mentions significant alterations in the levels of enzymes: AFP, AST, ALT, and bilirubin, etc. in Wistar rats. Many researchers through extensive research studies have established that raised level of liver enzymes like ALP, AFP, GGT, AST, bilirubin, and other are detected only in conditions like hepatocellular damage (Plaa and Hewitt 1989; Al-Rejaie et al. 2009; Ghorab et al. 2009). The gradual drop in the level of glucose is also an indicative of liver impairment and a possible process of cancer development, as cancer cells require more glucose as the currency for survival (Afzal et al. 2012). In the current research, concentration of glucose declined tremendously over time especially in disease control as compared to NC. It implies that the liver of the disease control group is necrotic and cell proliferation might have begun. While DENA + nimesulide groups at different dose displayed a significant elevation in the glucose level.
An increase in SGOT activity causes resorption of GSH by pre-neoplastic foci that augments cell multiplication and thus increases tumor production. Alpha feto proteins have been utilized by various investigators to confirm the occurrence of hepatocellular carcinoma in their early diagnosis (Ghorab et al. 2009). Nimesulide with increased dose tends to reinstate the concentrations of various liver enzymes including AFP significantly towards the normal controls and GGTP were also reduced at a substantial level (p < 0.01), suggesting that nimesulide possesses the ability to be a remedy for liver damage. These findings reveal the hidden potency of nimesulide that it can be utilized to develop the chemotherapeutic treatment. The level of cholesterol increases remarkably in rapidly dividing cells (Bansal et al. 2005; Kaplan 1993). Recent literature also reveals that hiked level of cholesterol and TG are associated with the incidence of DENA-induced HCC in Wistar rats (Kaplan 1993; Jiang et al. 2006). An elevated level of cholesterol is often accompanied with the fluidity of membrane, regulation of its permeability, and alteration in the core viscidness of the cell along with its chemical constitution (Abel et al. 2009). Our current research reveals the major elevation in the serum TG and cholesterol level after the administration of DENA in disease control group, DENA + nimesulide with different dose, and prophylactic group. The transformation may be due to the peroxidation of unsaturated lipids in the cell membrane through reactive oxygen species (Yadav and Bhatnagar 2007). Nimesulide (2 mg/kg)-treated groups revealed the remarkable decline in the serum level of TG and cholesterol in DENA-administered HCC rats, clearly suggesting the chemopreventive role of nimesulide.
Disease-controlled animals exhibited a significant elevation in the levels of Direct Bilirubin when compared with NC. However, TBR concentration was considerably lowered when treated with nimesulide dose dependently, as compared to NC. Histopathological study in disease control group shows remarkable necrosis as well as extensive inflammation around central veins intervening the cells. The tumor cells which resemble to the liver cells exhibit pleomorphism. The area is surrounded with the 2–8 cells with varied trabeculae that are segregated by lined endothelium with sinusoidal spaces. However, in therapeutic-treated group which exhibited mild bile duct proliferation with no central veins, observation in photograph suggests tumor suppressing nature of nimesulide with increased dose.
Literature data reveal that COX-2 signaling is associated with hepato-carcinogenesis and the drugs that inhibit the activity of COX-2 enzyme impede tumor growth in both in vitro as well as in the in vivo model. This finding paves a strategic breakthrough mechanism to counter and seize the tumor signaling as the drug targets directly COX-2 derived prostaglandin which in turn is responsible for the promotion of cell growth via Wnt-catenin pathway. The researchers also have claimed it to be the most effective approach to cut down the tumor burden. Chemopreventive activities of nimesulide are also confirmed by the inhibition of COX-2 signaling in conjunction with added growth monitoring alleyways like Met, EGFR, VEGF, iNOS, and polyunsaturated fatty acids. These pathways are anticipated to stipulate essential chemotherapeutic connotations (Wu 2006). The researchers have also informed the unique role of nimesulide in impeding PD-L1 surface manifestation observed in various breast cancer cell lines (Liang et al. 2009).
The free radicals like oxide anion [O], hydrogen peroxide [H2O2], and hydroxyl ion [OH] are produced by DENA through monooxygenase utilizing CYP450 are mainly responsible for damaging liver tissue. Increase in the level of ROS has been clearly linked with the activation of c-Myc oncogene manifestation (Vafa et al. 2002). The raised free radicals are generally countered by the available antioxidants, a natural defense mechanism of the body. The list includes catalase, sodium dismutase, GPx, and glutathione (GSH/GST). Antioxidants levels that should be at the optimum level to be active defense mechanism are the major requirement to balance the toxic-free radical and related injuries. In our study, these levels were restored in the treatment groups as compared to NC (Shinya et al. 1995). Lipid peroxidation and its products are known to be involved in carcinogenesis. DENA induction may also decrease the level of stearic acid, arachidonic, linoleic acid, and also palmitoleic and oleic acids; triacylglycerol could elevate the generation of reactive oxygen species (Muzio et al. 1999). The level of LPO was found to be elevated in disease control animals, while its level was found to be significantly decreased in therapeutic group confirming the scavenging activity of nimesulide.
The potential for nimesulide as an anticancer agent has been established in hepatocellular carcinoma cell lines. There is an ample evidence to confirm the crucial function of COX-2 in carcinogenesis (Liang et al. 2009), that briskly provoke and attract various cytokines and mutagens. Likewise, the availability of COX-2 can be easily deducted in various cancer cell lines. Based on this finding, we can say that COX-2 and its derived prostaglandins add to tumor growth in animal models by prompting DNA synthesis that endures tumor cell viability and development.
To tryout this hypothesis, we had assessed the inhibition of DNA synthesis within hepatocellular carcinoma BEL 7402 and Hep G2 cell lines by nimesulide with 3H-thymidine incorporation. Both models develop a significant hepatocellular carcinoma when the tumors get to an extent larger than 1.5 cm in range. We found that nimesulide, having in vitro selectivity for COX-2, was a very potent inhibitor of DNA synthesis. Nimesulide inhibits tumor growth and size in a dose-dependent manner. The result confirms the effectiveness of the COX-2 blocker in hampering the synthesis of cancerous cells. It is worth mentioning that a comparable growth inhibitory effect of nimesulide could be achievable at a much low concentration (50 µM) in HCC cell lines.
The anticancer effect of nimesulide detected in both animal models in vitro and in vivo ameliorates their utilization in cancer treatment.
Conclusion
The above observations suggest that nimesulide, the inhibitor of COX-2, hampers the multiplication of several ranges of cancer cells which are reliant on COX-2-mediated signaling for progression of tumor growth and make it a worthy compound for the development of the anticancer drug. Nimesulide with increased dose curb the tumor growth and reduce the biochemical markers which are raised in hepatocellular carcinoma. This will certainly bring new hopes and major outlooks in the field of cancer that nimesulide, commonly available drug at the low price, is a chemo preventive drug to avert, slow down, or heal the incidence of HCC. In vitro results delivered potential mechanism for nimesulide provoked anticancer effect in HCC, revealing that COX-2 inhibitor nimesulide may be an effective and potential tool as an adjuvant for the remedy of liver cancer and as an immunosuppressant. The further investigation is required for confirming and establishing the mechanism of anticancer and for its clinical trials.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- ALP:
-
Alkaline phosphatase
- TBi:
-
Total bilirubin
- AFP:
-
α-Feto protein
References
Abel S, DeKock M, van Schalkwyk DJ, Swanevelder S, Kew MC, Gelderblom WC (2009) Altered lipid profile, oxidative status and hepatitis B virus interactions in human hepatocellular carcinoma. Prost Leukot Essent Fatty Acids 81:391–399
Afzal M, Kazmi I, Gupta G, Rahman M, Kimothi V, Anwar F (2012) Preventive effect of metformin against N-nitrosodiethylamine-initiated hepatocellular carcinoma in rats. Saudi Pharm J 20:365–370
Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, Fatani AG, Al-Shabanah OA, Sayed-Ahmed MM (2009) Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol 151:373–380
Alwahaibi N, Mohamed J, Alhamadani A (2010) Supplementation of selenium reduces chemical hepatocarcinogenesis in male Sprague-Dawley rats. J Trace Elem Med Biol 24:119–123
Bansal AK, Bansal M, Soni G, Bhatnagar D (2005) Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact 156:101–111
Barbisan LF, Scolastici C, Miyamoto M, Salvadori DM, Ribeiro LR, da Eira AF, de Camargo JL (2003) Effects of crude extracts of Agaricus blazei on DNA damage and on rat liver carcinogenesis induced by diethylnitrosamine. Gen Mol Res 2:295–308
Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ (2004) COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer 90:423–429
Buckman SY, Gresham A, Hale P (1998) COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis 19:723–729
Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE (2005) Anticancer activity of the antibiotic clioquinol. Cancer Res 65:3389–3395
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340:745–750
Esteves-Souza K, Pissinate MG, Nascimento NF, Grynberg A, Echevarria A (2006) Synthesis, cytotoxicity, and DNA-topoisomerase activity of new asymmetric ureas and thioureas. J Med Chem 14:492–499
Fodera DD, Alessandro N, Cusimano A, Poma P, Notarbartolo M, Lampiasi N, Montalto G, Cervello M (2004) Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Ann N Y Acad 1028:440–449
Ghorab MM, Ragab FA, Hamed MM (2009) Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem 44:4211–4217
Ghouri YA, Mian I, Rowe JH (2017) Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog 16:1
Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T (2000) Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 6:2006–2011
Jiang J, Nilsson-Ehle P, Xu N (2006) Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis 5:4
Kaplan MM (1993) Laboratory tests. In: Schiff L, Schiff ER (eds) Diseases of the liver, 7th edn. J B Lippincott, Philadelphia, pp 108–144
Keller JJ, Giardiello FM (2003) Chemoprevention strategies using NSAIDs and COX- 2 inhibitors. Cancer Biol Ther 2:140–149
Koki AT, Masferre JL (2002) Celecoxib: a specific cox-2 inhibitor with anticancer properties. Cancer Control 9:28–35
Liang M, Yang H, Fu J (2009) Nimesulide inhibits IFN-c-induced programmed death-1-ligand 1surface expression in breast cancer cells by COX-2 and PGE2 independent mechanisms. Cancer Lett 276:47–52
Muzio G, Marengo B, Salvo R, Semeraro A, Canuto RA, Tessitore L (1999) Liver cancer is induced by a subnecrogenic dose of DENA when associated with fasting/refeeding: role of glutathione-transferase and lipid peroxidation. Free Rad Biol Med 26:1314–1320
Parkin DM, Bray F, Ferlay J (2001) Estimating the world cancer burden: globocan 2000. Int J Cancer 94:153–156
Plaa GL, Hewitt WR (1989) Detection and evaluation of chemically induced liver injury. In: Wallace Hayes A (ed) Principles and methods of toxicology, 2nd edn. Raven, New York, pp 399–428
Rahman MA, Dhar DK, Masunaga R, Yamanoi A, Kohno H, Nagasue N (2000) Sulindac and exisulind exhibit a significant antiproliferative effect and induce apoptosis in human hepatocellular carcinoma cell lines. Cancer Res 60:2085–2089
Rumi MA, Sato H, Ishihara S, Kawashima K, Kazumori SHH, Okuyama T, Fukuda R, Nagasue N, Kinoshita Y (2001) Peroxisome proliferator activated receptor gamma ligand induced growth inhibition of human hepatocellular carcinoma. Br J Cancer 84:1640–1647
Shinya TP, Keisei O, Junji Y, Hiroshi H (1995) Persistent oxidative stress in cancer. FEBS Lett 13:358
Sivaramakrishnan V, Shilpa PN, Kumar VRP, Devarajm SN (2008) Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel flavonol Morin. Chem Biol Interact 171:79–88
Subbaramaiah K, Dannenberg AJ (2003) Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 24:96–102
Thirunavukkarasu C, Sakthisekaran D (2003) Sodium selenite modulates tumour marker indices in N-nitrosodiethylamine initiated and phenobarbital-promoted rat liver carcinogenesis. Cell Biochem Funct 21:147–153
Vafa O, Wade M, Kern S (2002) C-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced geneticin stability. Mol Cell 9:1031–1044
Wu T (2006) Cyclooxy genase-2 in hepatocellular carcinoma. Cancer Treat Rev 32:28–44
Xu-yinga W, Minga L, Xiao-dongb L, Ping H (2009) Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact 181:15–19
Yadav AS, Bhatnagar D (2007) Chemo-preventive effect of staranise in N-nitrosodiethylamine initiated and phenobarbital promoted hepato-carcinogenesis. Chem Biol Interact 169:207–214
Zhang JF, Liu JJ, Lu MQ, Cai CJ, Yangm Y, Li H, Xu C, Chen GH (2007) Rapamycin inhibits cell growth by induction of apoptosis on hepatocellular carcinoma cells in vitro. Transpl Immunol 17:162–168
Acknowledgements
This research work was not funded by any organization.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that there is no competing interest.
Compliance with ethics requirements
Manuscript is under compliance with ethical standard of journal.
Rights and permissions
About this article
Cite this article
Afzal, M., Bhardwaj, D.P., Khan, R. et al. Antineoplastic influence of nimesulide in chemically induced hepatocellular carcinoma by inhibition of DNA synthesis. Inflammopharmacol 27, 89–98 (2019). https://doi.org/10.1007/s10787-018-0481-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0481-1