Abstract
This study was designed to examine the potential antitumor effect of some macrolides: clarithromycin, azithromycin, and erythromycin on chemically induced hepatocellular carcinoma (HCC) in rats and on human hepatoma cells (HepG2) as well. The possible underlying antiapoptotic mechanisms were investigated. Antiproliferative activity was assessed in HepG2 using Sulforhodamine-B staining method. In vivo, HCC was induced in rats by initiation-selection-promotion protocol using diethylnitrosamine (200 mg/kg, single i.p. injection)/2-acetylaminofluorene (0.03% w/w supplemented-diet for 2 weeks)/carbon tetrachloride (2 ml/kg diluted in corn oil 1:1, single intra-gastric dose)/phenobarbitone sodium (0.05% w/w supplemented-diet for 28 weeks). Macrolides were administered once daily starting from the 3rd week until the 17th week at a dose of 100 mg/kg in the current 33-week study period. Clarithromycin showed a higher efficacy in the suppression of HepG2 proliferation with lower IC50 value than doxorubicin. In vivo, chemically-induced HCC rat model proved that clarithromycin suppressed HCC via induction of apoptosis through up-regulation of both extrinsic/intrinsic apoptotic pathways’ proteins (TNFR1, cleaved caspase-3, and Bax with an increased Bax/Bcl-2 ratio) along with MMP-9 normalization. Similarly, azithromycin demonstrated antitumorigenic effect through both apoptotic pathways, however, to a lesser extent compared to clarithromycin. Moreover, azithromycin suppressed the proliferation of HepG2, however, at a higher IC50 than doxorubicin. Surprisingly, erythromycin increased HepG2 proliferation in vitro, along with worsened tumorigenic effect of the carcinogenic agents in the in vivo study with ineffective apoptotic outcome. Some macrolides represent potential antitumor agents; however, this evident anticancer activity is an individual effect rather than a group effect and involves modulation of both intrinsic and extrinsic apoptotic pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a prevalent primary liver malignancy (Llovet et al. 2003) and the fifth cause leading to cancer-related death all over the world (Parkin 2001). Because of poor diagnosis and serious side effects of existing treatment protocols, including surgical resection, radiation, and chemotherapy, the chance for curing from hepatocarcinoma is modest (Al-Rejaie et al. 2009). Liver cirrhosis, the major risk factor of HCC (Okuda 1992), is mainly caused by chronic infection by hepatitis B (HBV) and C (HCV) viruses (Bosch et al. 2004; De Giorgi et al. 2009) and/or heavy alcohol consumption (Marra et al. 2011). Cigarette smoking, race, diabetes mellitus, and obesity (Franceschi et al. 2006; Davila et al. 2005) have also been associated with incidence of HCC.
Both dysregulation of the apoptosis/proliferation balance (Fabregat 2009) and over expression of matrix metalloproteinase-9 (MMP-9) (Arii et al. 1996) contribute to the development of HCC (Dong et al. 1999). Regarding apoptosis, both intrinsic and extrinsic pathways show a significant dysregulation in HCC in such a manner that plays an important role in its prognosis (Fabregat 2009). Recently, matrix metalloproteinases (MMPs), namely MMP-9, was found to promote the development of HCC growth (Elewa et al. 2015) by releasing growth factors from their inactive membrane-bound form (Dong et al. 1999) and destruction of the extracellular matrix leading to the invasion of HCC (Arii et al. 1996; The Liver Cancer Study Group of Japan, T.L.C.S.G.O.J, 1994). Moreover, MMP-9 inhibitors were found to induce apoptosis through a cleavage of caspases-9 and -3, leading to a significant tumor eradication (Bhoopathi et al. 2008; Elewa et al. 2015).
Many antitumor agents are from microbial origin like adriamycin and epoxomicin (Hanada et al. 1992; Ohara et al. 2004). Macrolides, a class of antibiotics found in streptomycetes, are used to treat infections caused mainly by Gram-positive and to less extent by Gram-negative bacteria. Aside from their antibacterial properties, some macrolides demonstrated potent antiinflammatory activities that were evident in reducing mucus production (Kaneko et al. 2003) and preventing neutrophil-induced epithelial cell losses (Mitsuyama et al. 1997). They also showed an apoptotic effect (Ohara et al. 2004; Xu et al. 2006), MMP-9 inhibitory effect (Sassa et al. 1999; Li et al. 2010; Zhou et al. 2012), and antitumor activity (Yatsunami et al. 1999; Yongsheng et al. 2011) in some cancer types other than HCC. Additionally, clarithromycin and erythromycin has the same 14-membered macrolide ring of roxithromycin which proved previously an efficacy in the suppression of angiogenesis and modulation of oxidative stress in human hepatoma cells (Aoki et al. 2005; Ueno et al. 2005).
In spite of the documented antitumor effect of macrolides (i.e., clarithromycin, azithromycin, and erythromycin) in several cancer types (Yatsunami et al. 1999; Yongsheng et al. 2011; Lamb et al. 2015), their therapeutic potential in HCC has never been investigated. Hence, the current study is examining the antiproliferative activity of some macrolide antibiotics in human hepatocellular carcinoma (HepG2) cells as well as examining their apoptotic potential with underlying mechanisms in experimental hepatocarcinogenesis model in rats.
Materials and methods
In vitro cell proliferation assay
HepG2 was obtained from the National Cancer Institute, Cairo, Egypt. Sulforhodamine-B stain (SRB) was purchased from Duchefa-Biochemie (Haarlem, Amsterdam, Netherlands). Tris EDTA buffer and acetic acid were commercially available. Doxorubicin was purchased from Bristol-Myers Squibb Company. Whole study was completed at the National Cancer Institute, Cairo, Egypt. Potential cytotoxicity of macrolides versus the standard anticancer drug (doxorubicin) was examined using the technique of Skehan et al. (1990). HepG2 cells were plated in 96-multiwell plate (104 cells/well) for 24 h before treatment to permit the adherence of the cells to the plate. Then, different concentrations (0, 5, 12.5, 25, and 50 μg/ml) of doxorubicin, erythromycin, clarithromycin, and azithromycin were added to the monolayer cells. Monolayer cells were incubated with the studied compounds for 48 h at 37 °C in 5% CO2. After that, cells were fixed, washed, and stained with SRB stain. Excess stain was washed away using acetic acid, while attached stain was retrieved with Tris EDTA buffer. Color intensity was determined using a microplate reader. The color intensity from vehicle-treated cells was considered as 100% of proliferation. The relations between drugs’ concentration and surviving fraction were plotted. The concentration at which the growth of cells was inhibited to 50% of the control (IC50) was obtained from this dose response curve.
Experimental animals
Male Wister rats (180–200 g) were purchased from Vacsera (Egyptian Organization for Biological Products and Vaccines, Egypt) and retained under controlled temperature (25 ± 1 °C) on the natural dark/light cycle. Animals accessed food and water ad libitum during the study period. The animals were allowed to acclimatize for 2 weeks before experiment initiation. All experimental protocols were executed according to the “Guide for the Care and Use of Laboratory Animals”.
Chemicals and drugs
Diethylnitrosamine (DEN) and 2-acetylaminofluorene (2-AAF) were purchased from Sigma-Aldrich (USA). Phenobarbitone sodium (PB) was obtained from Alexandria Pharmaceutical Co. (Egypt). Clarithromycin, azithromycin, and erythromycin were kindly provided by Sigma Pharmaceutical Co. (Egypt), Amoun Pharmaceutical Co. (Egypt), and Medical Union Pharmaceutical Co. (MUP, Egypt), respectively. Cyclophosphamide (Endoxan 200 mg) was obtained from Baxter oncology (Germany). Carbon tetrachloride (CCl4) and all other chemicals were purchased from Al-Gomhorya Co. (Egypt).
Antibodies and kits
Primary rabbit polyclonal antibodies against TNF receptor type 1 (TNFR1), Bcl-xl, Bcl-2, Bax, cytochrome c, cleaved caspase-3, single-stranded DNA (ssDNA), and protein kinase (Ki-67) were used. All antibodies were purchased from Bioss (USA), except anti-ssDNA was purchased from IBL (Japan). Rat MMP-9 (matrix metalloproteinase-9) ELISA kit was purchased from CUSABIO (Japan). Rat tumor necrosis factor-α (TNF-α) Immunoassay ELISA Kit (Quantikine®) was purchased from “R&D systems” (MI, USA). EnVision™ FLEX immunohistochemistry detection kit was purchased from “Dako cytomation” (USA).
Induction of hepatocellular carcinoma
Hepatocellular carcinoma (HCC) was induced using initiation-selection-promotion model (de Gerlache et al. 1982; Lans et al. 1983) and modified after Solt and Farber (Solt and Farber 1976; Solt et al. 1977). Initiation was induced by single (i.p.) injection of DEN (200 mg/kg) diluted in saline (100 mg/ml). The animals were given no further treatment for 2 weeks as a recovery period. After this, selection was induced by feeding the animals a diet comprising 2-AAF (0.03% W/W) for 2 weeks. This was followed by feeding animals the basal diet (BD) for another week. Promotion was induced by (a) intra-gastric administration of CCl4 as a single necrogenic dose (2 ml/kg diluted in corn oil at a ratio of 1:1) 1 week after starting 2-AAF, (b) feeding the animals a diet comprising PB (0.05%W/W) starting the 6th week post DEN injection for 23 weeks. This was followed by feeding the animals the basal diet till the end of the study (33 weeks) (Fig.1).
Pharmacological treatment
HCC was induced in 75 rats using the previously explained protocol. These rats were divided randomly into five main groups, 15 rats each. First group did not receive any pharmacological treatment till the end of the study and served as HCC control. Second group was treated with five doses of cyclophosphamide (20 mg/kg, i.p.) every 3 days modified after Jang et al. (2011). Third, fourth, and fifth groups received daily 100 mg/kg (i.p. injections) of erythromycin, clarithromycin, and azithromycin, respectively (Zhou et al. 2012). All pharmacological treatments started 2 weeks after DEN injection, and all mortalities were recorded on a daily basis. Rats were sacrificed after 10, 17, and 33 weeks post DEN injection (i.e., 8, 15, and 31 weeks post pharmacological treatment, respectively). Additional 10 rats received daily 100 μl of the saline injection and served as a normal group.
Sample preparation
Blood samples were collected, centrifuged at 4000 rpm for 20 min to separate serum samples that were divided, and kept at −20 °C for measuring MMP-9 and TNF-α levels. Following cervical dislocation, livers were isolated and examined macroscopically, then fixed in 10% neutral buffered formalin for further histopathological evaluation and immunohistochemical staining of apoptotic and antiapoptotic markers, Ki-67, and ssDNA.
Immunohistochemistry
Fixed liver tissues were processed and embedded in paraffin. For analysis, paraffin sections at a thickness of 4 μm were de-paraffinized using xylene, then hydrated in decreasing concentrations of ethyl alcohol (100, 90, 80, and70%). Antigen retrieval was done following Tris/EDTA buffer (pH = 9) retrieval protocol. Liver sections were stained applying “EnVision™ FLEX horseradish peroxidase labeled, High pH” manufacturer protocol (Dako). All primary polyclonal antibodies were diluted in PBS at a ratio of 1:250 (TNF receptor type1, and cleaved caspase-3), 1:200 (Bax, cytochrome c, Bcl-2, and Bcl-xl), and 1:100 (Ki-67 and ssDNA). Sections were incubated with corresponding primary antibody at 4 °C overnight. After conjugation with dextran coupled with peroxidase molecules and goat secondary antibody molecules against rabbit immunoglobulins, coloring was achieved with DAB substrate chromogen then counterstained using Mayer’s hematoxylin. Positive immunoreactions (brown) for all antibodies were captured in 10 serial fields for each section (400×). Moreover, positive apoptotic nuclei for ssDNA were counted in each field. All photomicrographs were analyzed using ImageJ program. The Pearson’s equation was used for the determination of correlation coefficient between cytochrome c expression and the ratios of Bax/Bcl-xl and Bax/Bcl-2.
Determination of serum MMP-9
Serum samples were used for the determination of MMP-9 using quantitative sandwich ELISA protocol (CUSABIO). In brief, to a microplate pre-coated with polyclonal antibody specific for rat MMP-9, 50 μl samples were added. Recombinant rat MMP-9 was used to setup the standard curve. Following incubation for 2 h at 37 °C, the liquids were removed from each well. One hundred microliter of biotin antibody were added to each well without washing; next, they were incubated for further1 h, and plates were washed three time using washing buffer, then 100 μl horseradish peroxidase (HRP)–avidin was added to each well. Incubation at 37 °C was continued for another hour and each well was washed five times. Ninety microliter of TMP substrate were added to each well, and then incubated at 37 °C for15–30 min. The enzyme reaction yielded a blue product that turned yellow when the stop solution was added. The optical density was measured using a microplate reader set to 450 nm. (Metertech, M960).
Determination of serum TNF-α
Serum samples were used for the determination of TNF-α using quantitative solid phase ELISA protocol (R&D). In brief, 50 μl of assay diluent were added to microplate pre-coated with polyclonal antibody specific for rat TNFα, and then 50 μl samples were added. After that, 50 μl of standard, control, or sample were added to each well. Recombinant rat TNF-α was used to setup the standard curve. Following incubation for 2 h, wells were washed, and the liquid was aspirated from each well. Then, 100 μl of conjugate were added to each well. The microplates were incubated for another 2 h. Then the wells were washed five times, and all liquids were aspirated. One hundred microliter of the substrate solution were added to each well and followed by incubation for 30 min. The enzyme reaction yielded a blue product that turned yellow when the stop solution was added. The optical density was measured using a microplate reader set to 450 nm. (Metertech, M960).
Statistical analysis
All data were expressed as mean ± S.E.M. Statistical significance was tested using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc analysis. The Kaplan-Meier test was used to determine any significance in survival data. All treated groups were compared to normal group, HCC control group, and cyclophosphamide group. The confidence limit of P ≤ 0.05 was considered statistically significant.
Results
In vitro antiproliferative activity
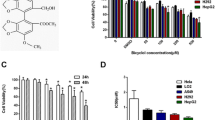
Macrolide antibiotics under study were screened for their ability to reduce cell proliferation in human hepatocellular carcinoma cell line (HepG2) versus doxorubicin as a standard cytotoxic agent. The present data revealed that treatment of HepG2 cells with increasing concentrations (5, 12.5, 25, and 50 μg/ml) of doxorubicin decreased HepG2 cell survival by 51, 44, 35, and 30%, respectively (Table 1 and Fig.2). The half maximal inhibitory concentration (IC50) value for doxorubicin on HepG2 cell line was found to be 5.87 μg/ml at 48 h. Treatment of HepG2 with 5, 12.5, 25, and 50 μg/ml of clarithromycin suppressed surviving of the tumor cells by 24, 23, 28, and 29%, respectively (Table 1 and Fig.2). Interestingly, clarithromycin showed IC50 at a concentration equal to 3.13 μg/ml, which was lower than doxorubicin IC50. On the other hand, the treatment of HepG2 with azithromycin showed 29% inhibition of tumor cell survival only at a concentration equal to 50 μg/ml, while the concentrations 5, 12.5, and 25 did not show any antiproliferative activity. The IC50 of azithromycin was found to be 42.8 μg/ml. Surprisingly, treatment of HepG2 with 5, 12.5, 25, and 50 μg/ml erythromycin increased HepG2 survival and viability by 137, 131, 126, and 125%, respectively (Table 1 and Fig.2).
Survival probability
Survival probability at each individual stage in the present in vivo study was estimated according to Kaplan-Meier equation (number of rats living at the beginning of each stage-number of dead rats at each stage)/number of rats living at the beginning of each stage) (Alman 1992; Goel et al. 2010). Current data showed that all groups displayed no death events at week 10 (Tables 2 and 3). Survival probability of HCC control group decreased gradually with the experiment stage. Interestingly, treatment with cyclophosphamide and clarithromycin showed a higher survival probability than HCC control group with no death events till the 17th week (Tables 2 and 3). In contrast, survival probability decreased to half in the group treated by erythromycin to show less living animals than HCC control group at week 33. It is worthy of note that treatment with azithromycin showed a gradual decrease in survival probability yet, higher than HCC control group (Tables 2 and 3).
Gross liver examination
In the current in vivo study, at week 10 post DEN, HCC control group had abnormal morphology with mild cirrhotic nodules (Fig. 3a). However, treatment with cyclophosphamide showed normal liver morphology at the same time point. Conversely, treatment with erythromycin showed an abnormal appearance of the liver with well differentiated cirrhotic nodules (Fig. 3a). In contrast, treatment with clarithromycin showed nearly normal liver appearance. Similarly, treatment with azithromycin showed almost normal appearance with rough edges.
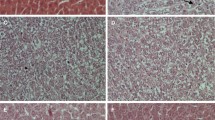
a Macroscopic images of gross liver. b Microscopic images of H&E-stained liver sections that were captured at different stages of initiation-selection-promotion model (10, 17, and 33 weeks). c Time course scoring of dysplasia grades along the study period. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal (data of normal group are not represented on the bar graph), #P ≤ 0.05 with respect to corresponding control (i.e., either stage A or B), •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5)
At week 17 post DEN, HCC control group showed enlarged and swollen liver with early neoplastic changes (Fig. 3a). However, treatment with cyclophosphamide showed mild liver swelling. In contrast, treatment with erythromycin showed abnormal swelling and enlargement of the liver with numerous well-differentiated cirrhotic and early neoplastic nodules. Conversely, treatment with clarithromycin or azithromycin showed almost normal appearance of the liver with mild liver enlargement (Fig. 3a).
At week 33 post DEN, HCC control group showed a liver full of well-differentiated early neoplastic changes accompanied by swelling and enlargement of the liver. However, treatment with cyclophosphamide showed mild liver enlargement with abnormal appearance. On the other hand, treatment with erythromycin showed abnormal enlarged swollen liver with numerous developed early neoplastic and cirrhotic nodules. Conversely, treatment with clarithromycin showed enlarged liver with few early neoplastic changes. In the same way, treatment with azithromycin showed enlarged swollen liver with early neoplastic changes (Fig. 3a).
Histopathological examination
Current in vivo results revealed that, at week 10, HCC control group showed grade I dysplasia (Fig. 3b, c). Hepatocytes showed enlarged nuclei, arranged in thick plates with disturbed architecture, mildly increased nucleocytoplasmic ratio, some showed coarse chromatin, others showed distinct nucleoli. There was moderate hydropic degeneration with minimal lymphocytic infiltrate (Fig. 3b). However, treatment with cyclophosphamide at the same time point showed marked improvement with no dysplasia. The liver showed restored architecture; hepatocytes are arranged in thin plates of one to two cell thickness and smaller nuclei. In contrast, treatment with erythromycin showed grade I dysplasia at week 10 (Fig. 3b, c). On the other hand, treatment with clarithromycin, for 10 weeks, showed almost normal liver tissue which indicated marked improvement. Moderate improvement was observed in azithromycin-treated group at week 10 post DEN (Fig. 3b).
At week 17, grade II dysplasia was observed in HCC control group where hepatocytes showed enlarged nuclei arranged in thick plates with disrupted architecture, along with moderately increased nucleocytoplasmic ratio (Fig. 3b, c). Some hepatocytes showed coarse chromatin, while other showed nucleoli along with bi-nucleated cells. There was a mild hydropic degeneration with mild lymphocytic infiltrate. The treatment with cyclophosphamide showed marked improvement with a focal grade I dysplasia. On the other hand, erythromycin-treated group showed grade II dysplasia. In contrast, moderate improvement was observed in the groups treated with clarithromycin or azithromycin where grade I dysplasia was detected at week 17 (Fig. 3b, c).
At week 33, HCC control group demonstrated grade III dysplasia where hepatocytes showed markedly enlarged nuclei arranged in thick plates, nests, and sheets with disturbed architecture, markedly increased nucleocytoplasmic ratio, some showed coarse chromatin, others showed more than one nucleolus, moderate number of bi-nucleated and multi-nucleated cells, minimal hydropic degeneration with mild lymphocytic infiltrate (Fig. 3b, c). In contrast, treatment with cyclophosphamide showed, at the same time point, marked improvement with focal grade I dysplasia. Surprisingly, grade III dysplasia was observed in erythromycin-treated group at week 33 (Fig. 3b, c). In contrast, treatment with clarithromycin showed a minimal focal improvement where livers had a restored architecture; hepatocytes were arranged in thin plates of one to two cell thicknesses with smaller nuclei. But most fields showed grade II dysplasia with thick plates and nest arrangement with large nuclei along with some bi-nucleated cells, and mild hydropic degeneration. On the other hand, no improvement was observed in azithromycin-treated group at the same time point where hepatocytes showed grade III dysplasia (Fig. 3b, c).
Serum levels of MMP-9
To reveal the influence of MMP-9 on the HCC development, the differences in serum levels of MMP-9 among different groups were statistically compared. All groups showed, after 10 and 17 weeks post DEN insult, significant higher levels of MMP-9 compared to normal group, except for groups treated with clarithromycin (Fig. 4a). Moreover, the group treated with cyclophosphamide did not show any significant change compared to normal group at week 10. Additionally, the individual treatment with the reference anticarcinogenic agent (i.e., cyclophosphamide) or the studied macrolides showed a significant reduction in MMP-9 serum levels after 10 weeks compared to corresponding HCC control group (Fig. 4a). It is worth mentioning that erythromycin group showed a significant increase in MMP-9 serum levels after 10 weeks post DEN compared to corresponding cyclophosphamide group. However, after 17 weeks post DEN, treatment with cyclophosphamide, erythromycin, or azithromycin did not show any significant difference compared to corresponding HCC control. On the other hand, treatment with clarithromycin showed a significant reduction in MMP-9 serum levels compared to both HCC control and cyclophosphamide groups at week 17 post DEN (Fig. 4a).
a and b Differential effect of cyclophosphamide (20 mg/kg, i.p.) and macrolide antibiotics (erythromycin, clarithromycin, and azithromycin; 100 mg/kg, i.p.) on serum levels of MMP-9 and TNF-α, respectively as measured by ELISA. c Photomicrographs represent the expression of TNF receptor type-1 assessed immunohistochemically (400×). d Optical density (O.D) of positive immunohistochemical reaction (brown) determined using ImageJ program. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal, #P ≤ 0.05 with respect to corresponding HCC control (i.e., either stage A or B), •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5)
Serum levels of TNF-α
TNF-α is the agonist of TNF receptor type-1 that play an initiator role in the extrinsic pathway of apoptosis (Locksley et al. 2001). Moreover, TNF-α has a critical role in the inflammation, which is an important key in the pathogenesis of HCC (Martin and Herceg 2012). Hence, serum levels of TNF-α was determined to reveal its role in the current HCC model. A significant difference in serum levels of TNF-α was observed among all groups (Fig. 4b). HCC control group showed a significant increase at both 10 and 17 weeks post DEN insult compared to normal group. Treatment with cyclophosphamide or macrolide antibiotics showed a significant reduction in the serum TNF-α compared to corresponding HCC control group after 10 weeks. In the same way, treatment with cyclophosphamide, clarithromycin, or azithromycin showed, after 17 weeks, a significant reduction at TNF-α serum levels compared to corresponding HCC control (Fig. 4b).
Interestingly, treatment with cyclophosphamide reduced TNF-α serum levels significantly compared to normal group at week 17 post DEN. On the other hand, treatment with erythromycin showed, after 17 weeks, a significant increase compared to normal group. It is worth mentioning that treatment with macrolide showed, after 17 weeks, a significant increase at TNF-α serum levels compared to corresponding cyclophosphamide group (Fig. 4b).
Expression of TNF receptor type-1
TNF receptor type-1 is an initiator of extrinsic apoptotic pathway (Hsu et al. 1995). To reveal the influence of macrolides on extrinsic pathway of apoptosis through TNF receptor type-1, its expression in hepatic tissues was assayed. At week 10 post DEN insult, treatment with azithromycin increased the expression of TNF receptor type-1 significantly (Fig. 4c, d). Moreover, treatment with clarithromycin or azithromycin increased the expression of TNF receptor type-1 significantly at week 17 post DEN (Fig. 4c, d).
Expression of Bcl-xl, Bcl-2, and Bax
Since Bcl-2 family members are essential regulators of the mitochondrial pathway that induce the intrinsic activation of caspases, IHC was carried out to determine the expression of the Bcl-2 family proteins Bcl-xl, Bcl-2, and Bax. Regarding antiapoptotic markers, HCC control group showed a significant increase in the expression of Bcl-xl, at week 10 post DEN insult, compared to normal group (Fig. 5a, b). Similarly, treatment with cyclophosphamide or erythromycin maintained the higher expression of Bcl-xl compared to normal group, at the same time point. However, a significant reduction in the expression of Bcl-xl was observed after treatment with clarithromycin or azithromycin, at week 10 post DEN, compared to the corresponding HCC control group and cyclophosphamide-treated group as well (Fig. 5a, b). On the other hand, at week 17 post DEN insult, both HCC control group and erythromycin-treated group showed a significant increase in the expression of Bcl-xl compared to normal group. In contrast, treatment with cyclophosphamide significantly reduced the expression of Bcl-xl compared to corresponding control group, at the same time point, yet remained higher than normal group (Fig. 5a, b). On the other hand, treatment with clarithromycin or azithromycin showed, at week 17 post DEN, a significantly reduced expression of Bcl-xl compared to normal, corresponding HCC control, and corresponding cyclophosphamide groups (Fig. 5a, b).
Differential effect of cyclophosphamide (20 mg/kg, i.p.) and macrolide antibiotics (erythromycin, clarithromycin, and azithromycin; 100 mg/kg, i.p.) on Bcl-xl and Bcl-2 expression. a and c Photomicrographs represent expression of Bcl-xl and Bcl-2, respectively as assessed immunohistochemically (400×). b and d Optical density (O.D) of positive immunohistochemical reactions (brown) determined using ImageJ program. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal, #P ≤ 0.05 with respect to corresponding HCC control (i.e., either stage A or B), •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5)
In the same manner, a significant increase in Bcl-2 expression was observed in both HCC control and erythromycin-treated groups at weeks 10 and 17 post DEN insult compared to normal group (Fig. 5c, d). Conversely, at week 17 post DEN, treatment with clarithromycin or azithromycin significantly decreased the expression of Bcl-2 compared to corresponding HCC control group. It is worth mentioning that Bcl-2 expression, in both clarithromycin and azithromycin groups, was comparable to normal group at the same former time point (Fig. 5c, d).
Concerning the apoptotic marker Bax, HCC control group showed a significantly lower Bax expression compared to normal group at both weeks 10 and 17 post DEN insult (Fig. 6a, b). On the other hand, significant increase in Bax expression was observed at both time points upon treatment with cyclophosphamide or clarithromycin, compared to either normal group or corresponding HCC control group. In the same way, treatment with erythromycin or azithromycin showed a significantly higher expression of that pro-apoptotic protein compared to corresponding HCC control at both weeks 10 and 17 post DEN, to reach by that the normal level. It is worthy of note that treatment with erythromycin showed a lower Bax expression compared to cyclophosphamide-treated group at both weeks 10 and 17 post DEN. Similarly, treatment with azithromycin showed lower Bax expression in comparison with cyclophosphamide group at week 17 post DEN (Fig. 6a, b).
Differential effect of cyclophosphamide (20 mg/kg, i.p.) and macrolide antibiotics (erythromycin, clarithromycin, and azithromycin; 100 mg/kg, i.p.) on Bax expression, Bax/Bcl-xl ratio, and Bax/Bcl-2 ratio. a Photomicrographs represent Bax expression assessed immunohistochemically (400×). b Optical density (O.D) of positive immunohistochemical reaction (brown) determined using ImageJ program. c and d represent Bax/Bcl-xl ratio and Bax/Bcl-2 ratio, respectively. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal, #P ≤ 0.05 with respect to corresponding HCC control (i.e., either stage A or B), •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5)
Determination of Bax/Bcl-xl and Bax/Bcl-2 ratios
The effect of macrolide antibiotics on Bax/Bcl-xl and Bax/Bcl-2 ratios was calculated. Regarding Bax/Bcl-xl, HCC control group showed a significant reduction compared to normal group after 10 and 17 weeks as well (Fig. 6c). However, treatment with cyclophosphamide, clarithromycin, or azithromycin increased the Bax/Bcl-xl ratio significantly compared to HCC control group at week 10 post DEN. Moreover, treatment with clarithromycin showed a significant higher ratio compared to the normal group at week 10. However, treatment with erythromycin showed a significant lower ratio than corresponding HCC control group at the same time point. On the other hand, at week 17 post DEN, only treatment with clarithromycin or azithromycin showed a significant increase in Bax/Bcl-xl ratio compared to normal, corresponding HCC control, and cyclophosphamide groups (Fig. 6c).
Regarding Bax/Bcl-2 ratio, a significant reduction was observed in HCC control group compared to normal group after 10 and 17 weeks as well (Fig. 6d). Treatment with cyclophosphamide, clarithromycin, or azithromycin significantly increased the ratio, compared to corresponding HCC control group, to reach the normal level at week 10 post DEN insult. However, at the same former time point, treatment with erythromycin showed a significant reduction in Bax/Bcl-2 ratio compared to normal and corresponding cyclophosphamide groups. On the other hand, at week 17 post DEN, treatment with clarithromycin or azithromycin significantly increased the ratio, compared to corresponding HCC control group, to reach the normal level (Fig. 6d).
Expression of cytochrome c
Cytochrome c was measured immunohistochemically to investigate whether the effect of the macrolides on the ratios of Bax/Bcl-xl and Bax/Bcl-2 has affected the expression of cytochrome c. A significant decrease in the expression of cytochrome c was observed in HCC control group compared to normal group, after 10 and 17 weeks as well (Fig. 7a, b). In contrast, treatment with cyclophosphamide or macrolides at week 10 post DEN resulted in a significant increase in the expression of cytochrome c compared to both normal group and corresponding HCC control group. On the other hand, treatment with cyclophosphamide, clarithromycin, or azithromycin showed, at week 17 post DEN, a significant increase in the expression of cytochrome c compared to both normal group and corresponding HCC control group (Fig. 7a, b). Additionally, treatment with erythromycin showed a higher cytochrome c expression than the corresponding HCC control group that was comparable to normal group at week 17 post DEN. It is worth mentioning that at week 17 post DEN, only treatment with clarithromycin or azithromycin showed a significant increase in cytochrome c expression compared to corresponding cyclophosphamide group. In contrast, treatment with erythromycin showed a significant reduction in cytochrome c expression compared to corresponding cyclophosphamide group at the same time point (Fig. 7a, b).
Differential effect of cyclophosphamide (20 mg/kg, i.p.) and macrolide antibiotics (erythromycin, clarithromycin, and azithromycin; 100 mg/kg, i.p.) on cytochrome c and b cleaved caspase-3 expression. a and c: Photomicrographs represent the expression of cytochrome c and cleaved caspase-3 as assessed immunohistochemically (400×). b and d Optical density (O.D) of positive immunohistochemical reactions (brown) determined using ImageJ program. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal, #P ≤ 0.05 with respect to corresponding HCC control (i.e., either stage A or B). •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5). e Correlation between cytochrome c expression and Bax/Bcl-xl or Bax/Bcl-2 expression. r denotes the Pearson’s correlation coefficient obtained from the linear regression line
Correlation between cytochrome c expression and pro-apoptotic/antiapoptotic proteins’ ratios
The Pearson’s correlation coefficient was determined for the relationship between cytochrome c expression and the ratios of Bax/Bcl-xl and Bax/Bcl-2. Correlation studies showed a strong positive association between cytochrome c and the ratios of Bax/Bcl-xl (r = 0.86) and Bax/Bcl-2 (r = 0.85), indicating the influence of macrolides on cytochrome c expression and consequently intrinsic apoptotic pathway through the modulation of pro-apoptotic/antiapoptotic proteins’ ratios. (Fig. 7e).
Expression of cleaved caspase-3
As cleaved caspase-3 plays a critical role in proceeding apoptosis, it was assessed in the present study. The current results revealed that HCC control group had a significantly lower expression of cleaved caspase-3 at both weeks 10 and 17 post DEN insult compared to normal group (Fig. 7c, d). However, treatment with cyclophosphamide, clarithromycin, or azithromycin significantly increased cleaved caspase-3 expression compared to normal group and corresponding HCC control group as well at both weeks 10 and 17 post DEN. Interestingly, treatment with clarithromycin or azithromycin showed a significant increase in cleaved caspase-3 expression at week 10 compared to corresponding cyclophosphamide group (Fig. 7c, d). On the other hand, treatment with erythromycin showed a rise in the expression of cleaved caspase-3 compared to normal and corresponding HCC control groups at week 10 post DEN. It is worth mentioning that in spite of higher expression of cleaved caspase-3 in erythromycin-treated group compared to HCC control group, it remained significantly lower than cyclophosphamide-treated group and comparable to normal group at week 17 post DEN (Fig. 7c, d).
Expression of ssDNA
Since single-stranded DNA (ssDNA) modification in the nucleosomal linker region might constitute a critical early step in apoptosis (Tomei 1991); it was used in the current study to detect the differences in the apoptotic cells among all groups. Statistical analysis showed a lower degree of apoptosis in HCC control group at week 17 compared to normal group (Fig. 8a, b). Treatment with cyclophosphamide or clarithromycin showed a significant increase in apoptotic cells compared to normal group and corresponding control group at both weeks 10 and 17 post DEN. Moreover, treatment with clarithromycin increased the number of apoptotic cells significantly compared to corresponding cyclophosphamide group at week 17 post DEN (Fig. 8a, b). On the other hand, treatment with erythromycin or azithromycin showed, at week 17 post DEN, a significant increase in apoptosis compared to corresponding HCC control group. Treatment with erythromycin or azithromycin showed a significantly lower number of ssDNA positive cells compared to corresponding cyclophosphamide group at week 10 or 17, respectively (Fig. 8a, b).
Differential effect of cyclophosphamide (20 mg/kg, i.p.) and macrolide antibiotics (erythromycin, clarithromycin, and azithromycin; 100 mg/kg, i.p.) on ssDNA and ki-67 expression. a and c Photomicrographs represent expression of ssDNA and ki-67, respectively as assessed immunohistochemically (400×). b and d Optical density (O.D) of positive immunohistochemical reactions (brown) determined using ImageJ program. Values are expressed as mean ± S.E.M. All data were analyzed using ANOVA followed by the Bonferroni post hoc test. *P ≤ 0.05 with respect to normal, #P ≤ 0.05 with respect to corresponding HCC control (i.e., either stage A or B). •P ≤ 0.05 with respect to corresponding cyclophosphamide (i.e., either stage A or B). (n = 4–5)
Expression of Ki-67
To assess the cellular proliferation, Ki-67 was estimated immunohistochemically. A significant increase at Ki-67 expression was observed in all groups both at weeks 10 and 17 post DEN insult compared to normal group (Fig. 8c, d). Interestingly, treatment with cyclophosphamide or clarithromycin showed a lower expression compared to HCC control group at both weeks 10 and 17 post DEN. In contrast, treatment with erythromycin or azithromycin increased Ki-67 expression significantly compared to corresponding cyclophosphamide group at both weeks 10 and 17 post DEN (Fig. 8c, d).
Discussion
The incidence of hepatocellular carcinoma (HCC) is particularly high in developing countries, making it as a major challenge due to a lack of early diagnosis (Bosch et al. 2004). HCC is often diagnosed at advanced stages, resulting in low survival rates. Current treatment protocols including surgical ablation, radiation, and chemotherapy provoke serious side effects with modest chances for recovery (Al-Rejaie et al. 2009).
Unrestrained propagation of immortal cells forms solid cancerous mass (Mukherjee et al. 2012). These excessively proliferating cells along with insufficient apoptosis result in HCC formation (Evan and Vousden 2001). The initiation-selection-promotion protocol selected in the current study has the advantage of distinction of each stimulus (de Gerlache et al. 1984), thus, allowing for a better examination of the apoptotic and antitumorigenic effect of studied drugs. Current treatments started 2 weeks post DEN to investigate their effect on the formation of early neoplastic lesions and to evaluate their secondary preventive effect through their proposed apoptotic potential. According to our findings, HCC control group showed a gradual progression of dysplasia from grade1 to grade 3 along the study period with impaired apoptotic mechanisms.
Indeed, insufficient apoptosis has been associated with development and progression of liver tumors (Guicciardi and Gores 2005; Fabregat et al. 2007). Both intrinsic and extrinsic pathways of apoptosis are significantly dysregulated in HCC in such a manner that plays an important role in its prognosis (Fabregat 2009). Among the most common alterations in the apoptotic mechanisms that were observed in HCC: (a) mutations in p53 tumor suppressor gene (Hussain et al. 2007) (b) imbalance in the pro- and antiapoptotic members of the Bcl-2 family (Mott and Gores 2007) (c) loss of response to Fas in HCC cells as a result of down-regulation of Fas expression (Shin et al. 1998; Lee et al. 2001) (d) over expression of the antiapoptotic protein “Brain and Reproductive organ-Expressed protein (BRE)” that binds to the cytoplasmic domains of TNF receptor type-1 and Fas, thus, attenuating death receptor-initiated apoptosis in HCC tissues (Chan et al. 2008).
Current study showed a dysregulated intrinsic apoptotic pathway in HCC control group as a result of significant lower Bax/Bcl-2 and Bax/Bcl-xl ratios which correlated with the lower release of cytochrome c into cytosol along with the lower expression of cleaved caspase-3 in hepatic tissues. These results were supported by previous studies that reported over expression of the antiapoptotic protein (Bcl-xl) and down-regulation of the pro-apoptotic protein (Bax) in a great percentage of HCCs (Beerheide et al. 2000). On the other hand, the significant high expression of TNF-α, that was detected in the promotion phase with HCC control group, was not accompanied by any significant difference in the expression of TNF receptor type 1 indicating that the extrinsic apoptotic pathway is not the main regulator of apoptosis in the current model. The high TNF-α expression observed in the current study is suggested to be related to inflammation which is known to play a major role in HCC pathogenesis (Martin and Herceg 2012).
Although the antitumor effect of clarithromycin, azithromycin, and erythromycin was not previously elucidated in HepG2 studies, in 2005, Aoki et al. has investigated the antitumor effect of roxithromycin, another 14-member macrolide antibiotic, on HepG2. Roxithromycin showed an inhibition of several newly developed vasculatures in HepG2 cells in a dose dependent manner; however, it did not inhibit the growth of HepG2 cells at concentrations up to 100 μM. In contrary, clarithromycin showed a different pattern in the current study where it decreased the survival of HepG2 by 50% at a concentration lower than doxorubicin. Similarly, treatment with azithromycin decreased 50% of the HepG2 survival yet, at a higher concentration. Surprisingly, treatment with erythromycin increased HepG2 survival and viability.
Recent studies demonstrated the induction of apoptosis as a protective and curative mechanism in the HCC therapy (Wilhelm et al. 2008; Wang et al. 2014). Clarithromycin showed an antiinflammatory effect beside its antibacterial activity in the treatment of chronic lower respiratory tract disease (Takizawa et al. 1995; Mitsuyama et al. 1997). In the current study, antiinflammatory effect of clarithromycin and azithromycin was evident by normalization of serum TNF-α. This normal TNF-α serum level that was detected after treatment with clarithromycin or azithromycin was compensated by an up-regulation of TNF receptor type-1and subsequent activation of extrinsic apoptotic pathway. Moreover, the two drugs showed a significant increase in both Bax/Bcl-xl and Bax/Bcl-2 ratios that correlated with the significant release of cytochrome c along with elevation in the expression of cleaved caspase-3, indicating activation of intrinsic apoptotic pathway. Those previously mentioned changes in the balance of pro-apoptotic versus antiapoptotic markers in treated groups were in favor of the induction of apoptosis in cancerous tissues at the expense of proliferation. Current results are supported by previous models which reported that clarithromycin and azithromycin suppressed Bcl-xl significantly, and this effect was involved in the augmentation of apoptosis (Mizunoe et al. 2004). Similarly, clarithromycin metabolite (N-demethyl-clarithromycin) was found to increase the ratios of Bax/Bcl-2 and Bax/Bcl-xl resulting in high expression of caspase-3 in cervical carcinoma (Qiao et al. 2006). Additionally, clarithromycin and azithromycin were reported to attenuate lymphoma through induction of apoptosis (Ohara et al. 2004).
Although Mitsuyama et al. (1997) reported an antiinflammatory effect of erythromycin in an endothelial cell damage model, the current results showed an opposite effect where the treatment with erythromycin at week 17 significantly increased the serum TNF-α expression, however, without any significant difference in the expression of TNF receptor type-1. On the other hand, erythromycin increased the expression of cytochrome c along with cleaved caspase-3 suggesting that erythromycin induced apoptosis through only intrinsic pathway. The previously mentioned apoptotic effect of erythromycin was supported by Xu et al. (2006) who detected the high expression of Bax in nasal polyps. The currently observed inflammatory effect of erythromycin that overweighed its apoptotic effect besides the previously reported data of causing cholestasis (Ansede et al. 2010) may explain the noticed accelerated tumor growth in this HCC model with erythromycin treatment.
In line with the previous in vitro results and immunohistochemical findings, histopathological results showed that clarithromycin delayed and decreased the appearance of early neoplastic changes. Similarly, azithromycin showed histopathological improvement yet, to a lesser degree compared to clarithromycin. The antitumor effect of clarithromycin and azithromycin was previously reported by Yatsunami et al. (1999) in melanoma growth. Surprisingly, erythromycin accelerated the appearance of early neoplastic changes which is contradictory to a previous finding by Yongsheng et al. (2011) who detected an antitumor effect of erythromycin in vitro using neuroblastoma cell line. This discrepancy may be explained based on different experimental settings and unlike susceptibility of the studied cells. The apoptotic and antitumor effects of the cytotoxic agent cyclophosphamide were comparable to clarithromycin and to less extent to azithromycin.
On the other hand, cyclophosphamide showed a stronger antiinflammatory effect compared to macrolides. In contrast to erythromycin, cyclophosphamide showed antiinflammatory properties in the current model which is consistent with a recent report in an experimental myocardial ischemia-reperfusion model (Jin et al. 2013). Cyclophosphamide has been previously reported to induce activation of intrinsic and extrinsic pathways of apoptosis (Singh et al. 2009). However, current results showed that cyclophosphamide induced apoptosis in hepatocytes mainly via intrinsic pathway. This resulted from, at least in part, the elevated Bax/Bcl-xl and Bax/Bcl-2 ratios that correlated with the high expression of cytochrome c and cleaved caspase-3.
In 2015, Elewa et al. showed an efficient inhibitory effect of HCC invasion cascade by significant decrease of the activity of MMP-9. Similarly, Bhoopathi et al. (2008) have reported MMP-9 inhibition as one of the apoptosis-inducing mechanisms. Its inhibition was associated with cytochrome c release and consequently increased a cleavage of caspases-9 and -3 resulting in significant tumor eradication (Bhoopathi et al. 2008). Our findings showed a higher MMP-9 expression in HCC control group which may have contributed to the malignant hepatocytes growth and progression. Earlier studies supported the current findings where the Liver Cancer Study Group of Japan, T.L.C.S.G.O.J (1994) and Arii et al. (1996) reported an over expression of MMP-9 that lead to infiltration into the fibrous tumor capsule and destruction of the extracellular matrix resulting in HCC invasion. In addition, induction of the gene transcription of MMP-9 was found to be correlated with the promoter region which is extremely responsive to cytokines and growth factors in numerous cells (Van den Steen et al. 2002). Clarithromycin, azithromycin, and erythromycin were reported earlier as inhibitors of MMP-9 expression in rat mammary adenocarcinoma, human corneal epithelial cells, and cigarette-smoke-induced emphysema and inflammation in rats, respectively (Sassa et al. 1999; Li et al. 2010; Zhou et al. 2012). In the same way, clarithromycin and azithromycin showed, in the current study, a lower expression of MMP-9 that might have contributed to their observed apoptotic effect and consequently decreased HCC progression. Although erythromycin showed an inhibitory effect on MMP-9, it did not inhibit the progression of the dysplasia. In contrast to erythromycin, cyclophosphamide suppressed the expression of MMP-9 that was associated with its antitumor activity and might have participated in its apoptotic effect. In line with current results, previous studies reported that cyclophosphamide down-regulated MMP-9 in breast cancer model (Liu et al. 2006) and hepatocellular carcinoma model by metronomic therapy (Jang et al. 2011).
Conclusions
It is concluded that the activation of both intrinsic and extrinsic apoptotic pathways plays an important role in mediating the antitumor effect that was detected for some macrolide antibiotics (clarithromycin and azithromycin) in the current study. Nevertheless, this evident anticancer potential is an individual effect rather than a group effect as some other macrolides, like erythromycin, could enhance the carcinogenic effect of hepatotoxic xenobiotics and ought to be avoided in patients with risk factors for developing HCC. All of all, current study suggests further clinical investigations for the use of clarithromycin or azithromycin as adjuvant to chemotherapy in HCC patients, especially if microbial infections with susceptible bacteria are suspected. On the other hand, erythromycin should be contraindicated as an antibacterial agent for HCC patients.
References
Al-Rejaie SS, Aleisa AM, Al-Yahya AA, Bakheet SA, Alsheikh A, Fatani AG, Al-Shabanah OA, Sayed-Ahmed MM (2009) Progression of diethylnitrosamine-induced hepatic carcinogenesis in carnitine-depleted rats. World J Gastroenterol 15:1373–1380
Ansede JH, Smith WR, Perry CH, St Claire RL 3rd, Brouwer KR (2010) An in vitro assay to assess transporter-based cholestatic hepatotoxicity using sandwich-cultured rat hepatocytes. Drug Metabolism and Disposition: the Biological Fate of Chemicals 38:276–280
Aoki D, Ueno S, Kubo F, Oyama T, Sakuta T, Matsushita K, Maruyama I, Aikou T (2005) Roxithromycin inhibits angiogenesis of human hepatoma cells in vivo by suppressing VEGF production. Anticancer Res 25:133–138
Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, Mizumoto M, Fukumoto M, Imamura M (1996) Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology 24:316–322
Beerheide W, Tan YJ, Teng E, Ting AE, Jedpiyawongse A, Srivatanakul P (2000) Downregulation of proapoptotic proteins Bax and Bcl-X (S) in p53 overexpressing hepatocellular carcinomas. Biochem Biophys Res Commun 273:54–61
Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS (2008) Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem 283:1545–1552
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–s16
Chan BC, Ching AK, To KF, Leung JC, Chen S, Li Q, Lai PB, Tang NL, Shaw PC, Chan JY, James AE, Lai KN, Lim PL, Lee KK, Chui YL (2008) BRE is an antiapoptotic protein in vivo and overexpressed in human hepatocellular carcinoma. Oncogene 27:1208–1217
Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB (2005) Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 54:533–539
de Gerlache J, Lans M, Preat V, Taper H, Roberfroid M (1984) Comparison of different models of rat liver carcinogenesis: conclusions from a systemic analysis. Toxicol Pathol 12:374–382
de Gerlache J, Lans M, Taper H, Preat V, Roberfroid M (1982) Promotion of chemically initiated hepatocarcinogenesis. Prog Clin Biol Res 109:35–46
De Giorgi V, Monaco A, Worchech A, Tornesello M, Izzo F, Buonaguro L, Marincola FM, Wang E, Buonaguro FM (2009) Gene profiling, biomarkers and pathways characterizing HCV-related hepatocellular carcinoma. J Transl Med 7:85
Alman DG (1992) Analysis of survival times. Practical statistics for medical research. Chapman and Hall, London (UK), pp 365–393
Dong J, Opresko LK, Dempsey PJ, Lauffenburger DA, Coffey RJ, Wiley HS (1999) Metalloprotease-mediated ligand release regulates autocrine signaling through the epidermal growth factor receptor. Proc Natl Acad Sci U S A 96:6235–6240
Elewa MA, Al-Gayyar MM, Schaalan MF, Abd El Galil KH, Ebrahim MA, El-Shishtawy MM (2015) Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clinical & experimental metastasis 32:479–493
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Fabregat I (2009) Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol 15:513–520
Fabregat I, Roncero C, Fernandez M (2007) Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int 27:155–162
Franceschi S, Montella M, Polesel J, La Vecchia C, Crispo A, Dal Maso L, Casarin P, Izzo F, Tommasi LG, Chemin I, Trepo C, Crovatto M, Talamini R (2006) Hepatitis viruses, alcohol, and tobacco in the etiology of hepatocellular carcinoma in Italy. Cancer Epidemiol Biomark Prev 15:683–689
Goel MK, Khanna P, Kishore J (2010) Understanding survival analysis: Kaplan-Meier estimate. International journal of Ayurveda research 1:274–278
Guicciardi ME, Gores GJ (2005) Apoptosis: a mechanism of acute and chronic liver injury. Gut 54:1024–1033
Hanada M, Sugawara K, Kaneta K, Toda S, Nishiyama Y, Tomita K, Yamamoto H, Konishi M, Oki T (1992) Epoxomicin, a new antitumor agent of microbial origin. J Antibiot (Tokyo) 45:1746–1752
Hsu H, Xiong J, Goeddel DV (1995) The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495–504
Hussain SP, Schwank J, Staib F, Wang XW, Harris CC (2007) TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene 26:2166–2176
Jang JW, Park ST, Kwon JH, You CR, Choi JY, Jung CK, Bae SH, Yoon SK (2011) Suppression of hepatic tumor growth and metastasis by metronomic therapy in a rat model of hepatocellular carcinoma. Exp Mol Med 43:305–312
Jin J, Chen F, Wang Q, Qiu Y, Zhao L, Guo Z (2013) Inhibition of TNF-alpha by cyclophosphamide reduces myocardial injury after ischemia-reperfusion. Ann Thorac Cardiovasc Surg 19:24–29
Kaneko Y, Yanagihara K, Seki M, Kuroki M, Miyazaki Y, Hirakata Y, Mukae H, Tomono K, Kadota J, Kohno S (2003) Clarithromycin inhibits overproduction of muc5ac core protein in murine model of diffuse panbronchiolitis. Am J Physiol Lung Cell Mol Physiol 285:L847–L853
Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 6:4569–4584
Lans M, de Gerlache J, Taper HS, Preat V, Roberfroid MB (1983) Phenobarbital as a promoter in the initiation/selection process of experimental rat hepatocarcinogenesis. Carcinogenesis 4:141–144
Lee SH, Shin MS, Lee HS, Bae JH, Lee HK, Kim HS, Kim SY, Jang JJ, Joo M, Kang YK, Park WS, Park JY, Oh RR, Han SY, Lee JH, Kim SH, Lee JY, Yoo NJ (2001) Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum Pathol 32:250–256
Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC (2010) Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci 51:5623–5629
Liu S, Xue XH, Yang XW, Lu DM (2006) Effects of Runing Recipe medicated serum on expressions of genes in breast cancer cells. Zhong Xi Yi Jie He Xue Bao 4:490–494
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501
Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R, Accardo M, Agresti M, Caraglia M, Sperlongano P (2011) Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med 9:171
Martin M, Herceg Z (2012) From hepatitis to hepatocellular carcinoma: a proposed model for cross-talk between inflammation and epigenetic mechanisms. Genome Med 4:8
Mitsuyama T, Hidaka K, Furuno T, Hara N (1997) Neutrophil-induced endothelial cell damage: inhibition by a 14-membered ring macrolide through the action of nitric oxide. Int Arch Allergy Immunol 114:111–115
Mizunoe S, Kadota J, Tokimatsu I, Kishi K, Nagai H, Nasu M (2004) Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. Int Immunopharmacol 4:1201–1207
Mott JL, Gores GJ (2007) Piercing the armor of hepatobiliary cancer: Bcl-2 homology domain 3 (BH3) mimetics and cell death. Hepatology 46:906–911
Mukherjee B, Ghosh MK, Hossain CM (2012) Chemically induced hepatocellular carcinoma and stages of development with biochemical and genetic modulation: a special reference to insulin-like-growth factor II and Raf gene signaling. In: Lau DJWY (ed.) Hepatocellular Carcinoma - Basic Research. InTech, pp. 201–218
Ohara T, Morishita T, Suzuki H, Masaoka T, Ishii H, Hibi T (2004) Antibiotics directly induce apoptosis in B cell lymphoma cells derived from BALB/c mice. Anticancer Res 24:3723–3730
Okuda K (1992) Hepatocellular carcinoma: recent progress. Hepatology 15:948–963
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2:533–543
Qiao AM, Ikejima T, Tashiro S, Onodera S, Zhang WG, Wu YL (2006) Involvement of mitochondria and caspase pathways in N-demethyl-clarithromycin-induced apoptosis in human cervical cancer HeLa cell. Acta Pharmacol Sin 27:1622–1629
Sassa K, Mizushima Y, Kobayashi M (1999) Differential modulatory effects of clarithromycin on the production of cytokines by a tumor. Antimicrob Agents Chemother 43:2787–2789
Shin EC, Shin JS, Park JH, Kim JJ, Kim H, Kim SJ (1998) Expression of Fas-related genes in human hepatocellular carcinomas. Cancer Lett 134:155–162
Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK (2009) Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J Pharmacol Sci 109:473–485
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Solt D, Farber E (1976) New principle for the analysis of chemical carcinogenesis. Nature:701–703
Solt DB, Medline A, Farber E (1977) Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol 88:595–618
Takizawa H, Desaki M, Ohtoshi T, Kikutani T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K (1995) Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun 210:781–786
The Liver Cancer Study Group of Japan, T.L.C.S.G.O.J (1994) Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer 74:2772–2780
Tomei LD (1991) Apoptosis: a program for death or survival. In: Tomei LD, Cope FD (eds) Apoptosis, the molecular basis of cell death. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 279–316
Ueno S, Aoki D, Kubo F, Hiwatashi K, Matsushita K, Oyama T, Maruyama I, Aikou T (2005) Roxithromycin inhibits constitutive activation of nuclear factor {kappa}B by diminishing oxidative stress in a rat model of hepatocellular carcinoma. Clin Cancer Res 11:5645–5650
Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G (2002) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol 37:375–536
Wang L, Wei D, Han X, Zhang W, Fan C, Zhang J, Mo C, Yang M, Li J, Wang Z, Zhou Q, Xiao H (2014) The combinational effect of vincristine and berberine on growth inhibition and apoptosis induction in hepatoma cells. J Cell Biochem 115:721–730
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M (2008) Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 7:3129–3140
Xu SN, Feng Y, Zhang Y, Zhou YF, Yang L, Jin YG (2006) Effect of erythromycin on apoptosis of epithelial cell in nasal polyps. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 41:510–513
Yatsunami J, Fukuno Y, Nagata M, Tominaga M, Aoki S, Tsuruta N, Kawashima M, Taniguchi S, Hayashi S (1999) Antiangiogenic and antitumor effects of 14-membered ring macrolides on mouse B16 melanoma cells. Clin Exp Metastasis 17:361–367
Yongsheng J, Xiaoyun M, Xiaoli W, Xin L, Haitao Y, Xiaoyan L, Jianquan Z (2011) Antitumor activity of erythromycin on human neuroblastoma cell line (SH-SY5Y). J Huazhong Univ Sci Technolog Med Sci 31:33–38
Zhou Y, Tan X, Kuang W, Liu L, Wan L (2012) Erythromycin ameliorates cigarette-smoke-induced emphysema and inflammation in rats. Transl Res 159:464–472
Acknowledgements
The authors thank Dr. Mohamed Kamal Abdel-Monim El-Kherbetawy, Assistant Lecturer of Pathology, Faculty of Medicine, Suez Canal University, for his valuable assistance with the evaluation of histopathology photomicrographs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 62 kb.)
Rights and permissions
About this article
Cite this article
Abdel-Hamid, N.I., El-Azab, M.F. & Moustafa, Y.M. Macrolide antibiotics differentially influence human HepG2 cytotoxicity and modulate intrinsic/extrinsic apoptotic pathways in rat hepatocellular carcinoma model. Naunyn-Schmiedeberg's Arch Pharmacol 390, 379–395 (2017). https://doi.org/10.1007/s00210-016-1337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1337-0