Abstract
Neuropathic pain is a debilitating disease which affects central as well as peripheral nervous system. Transient receptor potential (TRP) channels are ligand-gated ion channels that detect physical and chemical stimuli and promote painful sensations via nociceptor activation. TRP channels have physiological role in the mechanisms controlling several physiological responses like temperature and mechanical sensations, response to painful stimuli, taste, and pheromones. TRP channel family involves six different TRPs (TRPV1, TRPV2, TRPV3, TRPV4, TRPM8, and TRPA1) which are expressed in pain sensing neurons and primary afferent nociceptors. They function as transducers for mechanical, chemical, and thermal stimuli into inward currents, an essential first step for provoking pain sensations. TRP ion channels activated by temperature (thermo TRPs) are important molecular players in acute, inflammatory, and chronic pain states. Different degree of heat activates four TRP channels (TRPV1–4), while cold temperature ranging from affable to painful activate two indistinctly related thermo TRP channels (TRPM8 and TRPA1). Targeting primary afferent nociceptive neurons containing TRP channels that play pivotal role in revealing physical stimuli may be an effective target for the development of successful pharmacotherapeutics for clinical pain syndromes. In this review, we highlighted the potential role of various TRP channels in different types of neuropathic pain. We also discussed the pharmacological activity of naturally and synthetically originated TRP channel modulators for pharmacotherapeutics of nociception and neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain (NP), according to the International Association for the Study of Pain (IASP), is “pain initiated or caused by a primary lesion or dysfunction in the nervous system”. Neuropathic pain is debilitating heterogeneous consequence caused by destruction of nerves (in the central or peripheral or somatosensory nervous system) (Treede et al. 2008). Neuropathic pain can be inherent and usually depicted as shooting, burning, or stabbing. Neuropathic pain involves positive and negative sensory symptoms concomitant in neuropathic pain (Baron 2006). Negative symptoms comprise different somatosensory functional deficits, such as tactile hypoesthesia or anesthesia, thermal hypoesthesia, pinprick hypoalgesia, and loss of vibratory sensation, are uncomfortable but not painful. Instinctive positive symptoms are paroxysmal and ongoing superficial pain, paresthesia, and dysesthesia, and stimulus evoked positive symptoms include allodynia and hyperalgesia (Baron 2006).
TRP channels are ion channel family members involved in different physiological and pathological conditions, such as neuropathic pain, pulmonary hypertension, asthma, parkinsonism, and prostate cancer (Nilius et al. 2007). These are of six different types, such as TRPV1, TRPV2, TRPV3, TRPV4, TRPM8, and TRPA, which have been expressed in primary afferent nociceptors and pain sensing neurons, act as transducers for chemical, thermal, and mechanical stimuli (Clapham 2003; Corey 2003; Montell et al. 2002). In this article, we discuss recent developments associated with different types of TRP channels as potential targets for pharmatherapeutics of neuropathic pain.
TRP channels family
TRP channels were discovered in 1969 as mutant Drosophila photoreceptors (Cosens and Manning 1969). Process of phototransduction in the fruit fly, Drosophila melanogaster, comprises membrane cation channels activation leading to a depolarizing current. Activation of Drosophila photoreceptors, i.e., light-sensitive G protein-coupled receptor rhodopsin, results in the stimulation of phospholipase C-β (PLC-β). The light-induced current resolving components escorted to identification of a Drosophila mutant exhibiting a transient LIC in response to light, in comparison to the sustained LIC in wild-type flies, and mutant strain was termed trp, for transient receptor potential. Trp gene mutations headed to a disruption of a Ca2+ entry channel in the photoreceptors, representing that TRP, the protein encoded by the trp gene, forms whole or part of a Ca2+ influx channel (Nilius et al. 2007). TRP channels are Ca2+-permeable non-selective cation channels.
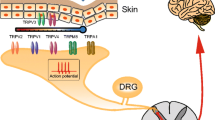
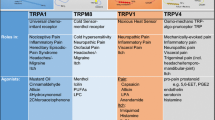
Mammalian TRP channels are classified into different subfamilies (as shown in Table 1): TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), and TRPA (ankyrin) (Caterina 2007). All TRP subunits have six transmembrane domains (TMD), a pore-forming loop (between 5th and 6th transmembrane segments) and having widely varying intracellularly located amino (N) and carboxyl (C) terminal in length (Clapham 2003; Vriens et al. 2004a). Mammalian TRP channels are with low-sequence homology, and have different modes of activation (mechanical stimulation, temperature, chemical compounds, osmolarity, lipids, light, oxidative stress, acid, and pheromones), regulation (glycosylation, transcription, phosphorylation, and alternative splicing), broad tissue distribution (at least one member of the family present virtually in all cells), ion selectivity, and physiological functions (Levine and Alessandri-Haber 2007). After TRPV1 cloning, some other TRPs have been depicted in dorsal root ganglia (DRG), such as TRPV2, TRPV3, TRPV4, TRPA1, and TRPM8, which act as sensory transducers and play an important role in the generation of pain sensations evoked by thermal, mechanical, and chemical stimuli. In Table 1, TRPV1, TRPV2, TRPV3, and TRPM8 are thermoreceptors, and TRPV4 and TRPA1 referred as mechanoreceptors, while TRPV1, TRPV3, TRPM8, and TRPA1 are known as chemoreceptors, respectively, receptive to capsaicin and endocannabinoids, camphor, menthol, mustard, and cinnamon oil (Bandell et al. 2004; Jordt et al. 2004).
TRPV1
TRPV1 is a polymodal receptor named as Vanilloid receptor 1(VR1) whose invertebrate families are necessary to sensory transduction (mechanosensation, osmosensation, phototransduction, and thermosensation) and in mammals, it is activated by heat and protons, and leads to the influx of cations which depolarize the cell for action potential generation (Vriens et al. 2004a). TRPV1 was predominantly found in a subpopulation of small-to-medium-diameter neurons in dorsal root and trigeminal ganglia. TRPV1 plays an amenable role in thermal and chemical hyperalgesia in a model of diabetic neuropathy (Hong and Wiley 2005; Kamei et al. 2001). They play role by altered cell-specific expression like by decrease of TRPV1 protein expression in C-fibers paralleled by an increase in A-fibers which coupled to an increase in its function (reallocation of channels to cell-surface plasma membrane and/or increase of TRPV1 phosphorylation coupled to oligomerization and impaired desensitization) (Levine and Alessandri-Haber 2007).
TRPV1 is activated by capsaicin, thermal heat (≥43 °C), low pH (<5.9) (Caterina et al. 2000; Tominaga et al. 1998), camphor (Xu et al. 2005a), allicin (Macpherson et al. 2005), nitric oxide (Yoshida et al. 2006), spider toxins (Siemens et al. 2006), vanilloids (Caterina and Julius 2001), protons (Caterina and Julius 2001), and proalgesic substances (Julius and Basbaum 2001), and modulated and potentiated by extracellular cations and ethanol, respectively. TRPV1 can be sensitized and up-regulated during inflammation and injury. In several conditions, TRPV1 activation demonstrated as different models of pain like in inflammatory conditions and temperature threshold of activation is reduced causing the channel to be active at normal body temperatures. TRPV1 sensitization depends on phosphorylation of TRPV1 by protein kinase A (PKA) and protein kinase C (PKC). TRPV1 activation-associated pain conditions are inflammatory thermal hypersensitivity, acute thermal pain, post-herpetic neuralgia, constriction-type nerve injury, trigeminal neuralgia, diabetic peripheral neuropathy, headache and cardiac pain, pain arising from GI diseases, cluster, lung diseases, cancer pain, and migraine (Cortright and Szallasi 2004; Premkumar 2010; Premkumar and Sikand 2008; Szallasi 2006; Szallasi and Appendino 2004; Szallasi et al. 2007). TRPV1 channel is incriminated in a variety of human diseases, including gastrointestinal reflex disease, osteoarthritis, inflammatory disorders of the airways, and urinary bladder (Groneberg et al. 2004; Matthews et al. 2004; Nilius et al. 2005).
The contribution of TRPV1 in inflammatory, nociceptive, and neuropathic pain created an interest in discovery of specific vanilloid receptor antagonists. TRPV1 is involved in heat evoked pain, thermal hyperalgesia, and deep tissue pain in CNS and primary terminals of primary afferents. TRPV1 can be activated by noxious heat and its threshold temperature (≥43 °C) lies close to sense as painful for humans. TRPV1-null mice exhibited reduced nocifensive responses to acute thermal stimuli arguing for the role of TRPV1 in transducing thermal pain in vivo (Caterina et al. 2000; Davis et al. 2000; Levine and Alessandri-Haber 2007). TRPV1 is involved in the hyperalgesia after the injection of inflammatory mediators, such as bradykinin, adenosine triphosphate (ATP), nerve growth factor (NGF), and protease (Caterina et al. 2000; Chuang et al. 2001; Davis et al. 2000). TRPV1 acts as a crucial molecular site of nociceptor sensitization where activity of both a noxious stimuli (heat) and inflammatory mediators is required for nociceptor activation. TRPV1 involves in nociception in deep tissues, such as musculoskeletal and visceral tissues. Deep tissues pathological conditions mainly produce mechanical hyperalgesia rather than thermal hyperalgesia, and a number of deep tissue pain models have been shown the involvement of TRPV1 (Chung et al. 2011). TRPV1 is necessary for sensitization of afferent fibers of mouse colon by inflammatory mediators (Jones et al. 2005; Miranda et al. 2007; Ravnefjord et al. 2009).
TRPV1 plays a critical role in joint pain in an arthritis model and increased number of sensory neurons expressing TRPV1 has been found in rats after induction of arthritis (Fernihough et al. 2005). TRPV1 is implicated in pain associated with bone cancer and the movement-induced nocifensive behaviour in bone cancer model is ameliorated by specific TRPV1 antagonists (Niiyama et al. 2007). The number of TRPV1-expressing neurons in DRG was found to be increased in experimental bone cancer. TRPV1 acts as a transducer of thermal stimuli at the peripheral terminal of primary afferents and also expression of TRPV1 has been demonstrated in the spinal cord, mainly at the lamina I and II of the superficial dorsal horn area (Guo et al. 1999; Valtschanoff et al. 2001). TRPV1 is activated by central branches of primary afferents to release excitatory amino-acid glutamate and produces excitatory synaptic transmission in superficial dorsal horn (Pan and Pan 2004; Sikand and Premkumar 2007; Yang et al. 1999, 1998). Strong correlation between therapeutic efficacy and CNS penetrability of TRPV1 antagonists tells that centrally located TRPV1 blockade is also involved in the anti-nociceptive effects of TRPV1 antagonists.
TRPV2
TRPV2 was discovered as structural homologue of TRPV1 with 50 % amino-acid identity and originally named as vanilloid receptor-like protein 1 (VR-L1) (Caterina et al. 1999). Higher temperature (∼52 °C), 2-aminoethoxyphenyl borate (2-APB) at higher dose, inflammation, osmotic stimuli, and mechanical stretch are activators of TRPV2, but it is insensitive to capsaicin (Caterina et al. 1999; Jordt et al. 2004; Muraki et al. 2003). The growth factor (insulin-like growth factor-I) and PI3-kinase signalling pathways enhance TRPV2 activity. TRPV2 is usually expressed in neuronal and non-neuronal cells, Aδ and Aβ fibers of DRG, trigeminal ganglia (TG), and nodose ganglion (NG) (Lewinter et al. 2004; Stokes et al. 2005; Wainwright et al. 2004). The expression of TRPV2 in neurons innervating the larynx, bladder, and intestine suggests its role in sensory functions of internal organs (Kashiba et al. 2004) and is activated by 2-aminoethoxydiphenylborate (2-APB) at higher concentration. TRPV2 is expressed throughout the spinal cord, including laminae III and IV, suggesting a role other than nociception (Caterina and Julius 1999; Lewinter et al. 2004). TRPV2 has been distributed in neurotropin-3-dependent subpopulation of DRG neurons and its protein level release beyond the normal level following inflammation and has ability to hetromultimerize and ability to be activated by 2-APB indicates its role in pain associated with inflammation and neuropathy (Tamura et al. 2005). Fewer studies related to pain that focuses on TRPV2 because of its very high heat threshold as well as differential distribution.
TRPV3
TRPV3 shows 40–50 % homology with TRPV1 and is activated by warm temperature (≥34 °C). They are having ability to show augmented responses to higher noxious thermal stimuli and increased current following repetitive heat stimulation (Peier et al. 2002b; Smith et al. 2002; Xu et al. 2002). These are strongly activated and sensitized by cloves, camphor, oregano, and irritants extracted from thyme (Xu et al. 2006). Initially, TRPV3 exhibited to be expressed only in keratinocytes, while some other studies have also shown to be expressed in sensory neurons (Facer et al. 2007). TRPV3 is found in TG, DRG, and NG neurons, keratinocytes, and certain regions of the brain and have a role in thermoregulation (Moqrich et al. 2005). It is suggested that TRPV1 and TRPV3 receptors act as a potential therapeutic target for the treatment of pain and inflammation.
TRPV4
TRPV4 is mechano/osmosensitive channel expressed in many cell types, including sensory neurons and a polymodal receptor involved in nociception and activated by low pH, shear stress, hypotonicity, diacylglycerol (DAG), innocuous heat with threshold >27 °C, citrate, endocannabinoids, and nitric oxide (Guler et al. 2002; Watanabe et al. 2002). These are mainly present in cochlear hair cells, sensory ganglia (Guler et al. 2002), as well as in cutaneous A and C-fiber terminals and free nerve endings, and suggested a role in mechano-transduction, beyond osmosensation. OSM-9, a homologue of the C. elegans osmosensory channel, expressed in cochlear hair cells, sensory neurons, vascular smooth muscle cells, hypothalamus, trachea, kidney, keratinocytes, and endothelial cells (Strotmann et al. 2000). Mice lacking functional TRPV4 show normal response to low-threshold mechanical stimuli and noxious heat (Vriens et al. 2004b). Agonists of TRPV4 promote the liberation of the neuropeptides like substance P and calcitonin gene-related peptide (CGRP) from the central projections of primary afferents in the spinal cord. These studies suggest a role of TRPV4 in detection of warm temperature, nociception, and chemically induced hyperalgesia (Grant et al. 2007; Todaka et al. 2004). In TRPV4 knockout mice, the sensitivity of tail to pressure and acidic nociception is diminished as compared with wild-type mice (Suzuki et al. 2003). TRPV4 channels can also act as target for treatment of nociceptive and neuropathic pain.
TRPA1
TRPVA1 is new TRP subfamily member, characterized by the presence of a large number of ankyrin repeat motifs located on the cytosolic amino terminal domain (TRPAnkyrin) (Story et al. 2003), was identified as a protein overexpressed in liposarcoma cell lines (ANKTM1) (Jaquemar et al. 1999). TRPA1 is expressed in the inner ear, lung fibroblast, trigeminal and DRG neurons, motor neurons, postganglionic sympathetic neurons, and neurons of the intestinal myenteric plexus (Corey et al. 2004; Munns et al. 2007; Poole et al. 2011; Smith et al. 2004). TRPVA1 activation by physical stimuli, such as noxious cold (<18 °C) temperatures, mechanical force (Story et al. 2003), by garlic, mustard oil, wintergreen oil, ginger, clove oil, and cinnamon oil leads to induction of acute painful burning or pricking sensation (Bandell et al. 2004; Jordt et al. 2004; Macpherson et al. 2005). It acts as sensor for mechanical stimuli and plays a role in mechanical nociception because of its Drosophila homologue (Xu et al. 2005a). Its involvement in cold allodynia and mechanical hyperalgesia is reported in different behavioural models (Baron 2006; Katsura et al. 2006; Obata et al. 2005) that suggest TRPVA1 as good target for neuropathic pain treatment.
TRPC3 and TRPC6
TRP channel expression in human monocytes is affected by high glucose-induced oxidative stress. TRPC3 and TRPC6 protein expression was enhanced by increased 1-oleoyl-2-acetyl-sn-glycerol induced Ca2+ influx, which was blocked by the TRPC channel inhibitor, i.e., 2-aminoethoxydiphenylborane (2-APB) (Wuensch et al. 2010). These may also be act as potential targets for treatment of diabetic neuropathy.
TRPM5, TRPM6, and TRPM7
TRPM5 are present in taste bud tissues and papillae. TRPM6 and TRPM7 supposed to involve in type-2 diabetes mellitus because of their gene variation (Romero et al. 2010). TRPM7 gene variation could play a role in the risk of ischemic stroke.
TRPM8
TRPM8, a cold-sensitive receptor, is known as cold and methanol-activated channel with voltage-dependent gating properties (McKemy et al. 2002; Peier et al. 2002a). It is thermally regulated channel activated in vitro by neurons originating from both TG and DRG (Dhaka et al. 2008). It may be involved in cold-evoked nocifensive responses under temperatures ranging from innocuous cold (26–15 °C) to noxious cold (<15 °C) (McKemy et al. 2002) and by various other chemicals, including eucalyptol, menthone, spearmint, and icilin (Peier et al. 2002a; Tominaga and Caterina 2004). TRPM8 is expressed in a subpopulation of primary afferent sensory pathological conditions. In a chronic constriction injury (CCI) model, the percentage of sensory neurons expressing TRPM8-like immunoreactivity is increased (Xing et al. 2007).TRPM8 is a good target for treatment of cold allodynia, a common feature of neuropathic pain.
TRP channels in neuropathic pain
TRPV1
TRPV1 channels are prominently associated with neuropathic pain as shown by experimental evidences. Desensitization or amputations of TRPV1-positive sensory nerve endings exhibit analgesic effect and make it potential therapeutic target in treatment of neuropathic pain (Haanpaa and Treede 2012; Moran et al. 2011). TRPV1 exhibits Ca2+-dependent desensitization mediated by calmodulin (CaM) which directly binds with calmodulin-binding sites present on several TRP channels (Lambers et al. 2004). TRPV1 shows its expression and function in sensory ganglia in neuropathic pain. Spinal nerve ligation (SNL)-induced nerve injury increases the proportion of TRPV1-expressing IB4-positive DRG neurons and improves TRPV1 function, resulting in persistent thermal hyperalgesia (Vilceanu et al. 2010). After sciatic nerve transection in rats, TRPV1 at the central terminals of primary afferent neurons in the spinal cord is up-regulated, and augment release of inflammatory neuropeptides like CGRP (calcitonin gene-related peptide), substance P from the presynaptic central terminals along with enhanced glutamatergic neurotransmission, is involved in the neuropathic pain (Kanai et al. 2005; Lappin et al. 2006; Lee and Kim 2007; Spicarova et al. 2011). Activity of TRPV1 enhanced in neuropathic pain, and administration of selective TRPV1 inhibitors allays SNL-induced hyperalgesia and mechanical allodynia (Jhaveri et al. 2005; Urano et al. 2012; Vilceanu et al. 2010; Watabiki et al. 2011).
TRPA1 and TRPM8
TRPA1 and TRPM8 proposed to act as a cold transducer and deliberated as a major candidate for mediating cold allodynia, common feature of neuropathic pain (del Camino et al. 2010; Ji et al. 2008; Knowlton et al. 2011; Obata et al. 2005). TRPA1 function inhibition peculiarly diminishes cold allodynia induced in chronic constriction injury (CCI)-induced neuropathy model of neuropathic pain Chen et al. 2011). Both TRPV1 and TRPA1 are involved in chemotherapy-induced peripheral neuropathy and neuropathic pain. Inhibition of TRPA1 function eradicates both mechanical and cold allodynia induced by cisplatin and oxaliplatin, most commonly used chemotherapeutic agents (Baron 2009; Brederson et al. 2013; Nassini et al. 2011; Zhao et al. 2012). Neuropathy induced by paclitaxel chemotherapy is reported to elicit the release of mast cell tryptase to activate protease-activated receptor 2 (PAR2), which sensitizes TRPV1, TRPV4, and TRPA1 through PLC, PKC, and PKA signalling to initiate neuropathic pain behaviours (Chen et al. 2011) and also enhances the TRPV1 mRNA transcripts and amount of TRPV1 protein in small-to-medium diameter DRG neurons that contribute to neuropathic pain (Hara et al. 2013). Therefore, different TRP channels play a crucial role in the management of neuropathic pain.
Mechanistic involvement of TRP channels in neuropathic pain
Neuropathic pain can be evoked by raising local Ca2+ ion concentration at the site of injury or in the spinal cord (Fernyhough and Calcutt 2010) by influx of calcium ions through voltage-dependent Ca2+ channels like high-voltage activated or low-voltage activated or transient (T-type) Ca2+ channels. When pain impulse transmitted from the periphery to the central nervous system, the nociceptive transmitters like substance P released via exocytosis from the primary sensory terminals present in the spinal dorsal horn, which is regulated by high-threshold voltage-dependent Ca2+ channels (Verkhratsky and Fernyhough 2008). Increased responsiveness of the spinal pain transmission is probably due to the increased awareness of the primary afferent neurons, which can results in enhanced neurotransmitter exocytosis through the opening of voltage-dependent Ca2+ channels or due to the postsynaptic hyper-excitability in dorsal horn projection neurons, which is possibly induced by enhanced Ca2+-influx through voltage-dependent Ca2+ channels (TRP Channels). Oxidative stress-dependent Ca2+ over influxes through the TRP channel also has important role in diabetic neuropathic pain (Umeda et al. 2006) and other types of neuropathy (Fig. 1).
Schematic representation of Mechanistic pathway involved in neuropathic pain. a Role of TRPV1 ant TRPA1: different TRPV1 agonist like capsaicin, camphor, high temperature, low pH, etc., activates the transient type Ca2+-channel to increase the intracellular Ca2+ influx in cytosol. This intracellular Ca2+ activates TRPA1 channels as well as increase Ca2+ concentration in EPR which leads to neurotransmitter release like substance P, activation of PKA/PKC and cGMP/NO pathway which results in progression of neuropathic pain. b Role of Arachidonic acid (AA) metabolites: AA metabolites like 5-HETPE also activates TRPV1. Leukotrienes produced by LOX pathway and PGs from COX pathway also involved in neuropathic pain. (Ca ++ /Ca 2+ calcium ion, LTs leukotrienes, AA arachidonic acid, COX cyclooxygenase, LOX lypooxygenase, PGs prostaglandins, PLA 2 phospholipase A2, 5-HPETE 5-hydroperoxyeicosatetraenoic acid, EPR endoplasmic reticulum)
TRP channel modulators
TRP channel modulators possess strong pharmacotherapeutic potential for management of neuropathic pain. Natural compounds which act as agonists to modulate TRPV1 channels are capsaicinoids, triprenyl phenols, unsaturated dialdehydesterpenes (Thapsigargin), gingerols, and gingenosides (Calixto et al. 2005) (Table 2).
Capsaicin
It is a TRPV1 modulator that leads to degeneration of a large portion of C-fibers as well as a small portion of Aδ-fibers, resulting in a prolonged analgesic period (in adult and neonatal experimental animals) (Holzer 1991). TRP channel agonists directly gate the channel by reduction of the heat threshold of activation. Persistent exposure of receptor to the agonist in the presence of Ca2+ induces channel closure by desensitisation and tachyphylaxis (Szallasi and Blumberg 1999) and also act by phosphorylation of a key residue at the C-terminus of the protein (Bhave and Gereau 2004). Capsaicin is used to diminish pain, due to its ability to desensitize TRPV1. Capsaicin (as 0.025–0.075 % cream preparations) is used for treating pain produced by peripheral neuropathy, osteoarthritis, and rheumatoid arthritis (Brito et al. 2014). Capsaicin desensitises TRP channels and selectively depletes TRPV1-expressing nociceptors due to Ca2+ overload (Karai et al. 2004). High-affinity agonists that promote receptor tachyphylaxia and/or nociceptor ablation could be used as efficacious pain relievers. Intrathecal administration of oligodeoxynucleotide antisense for TRPA1 completely repressed the cold hyperalgesia induced by nerve damage in neuropathic pain (Katsura et al. 2006).
Camphor
Camphor is a well-known TRPA1 antagonist. It activates TRPV1, TRPV3, and TRPA1 channels at low concentration, but inhibits TRPA1 currents at high concentrations (Xu et al. 2005a).
Mecamylamine
Mecamylamine is a TRPA1 antagonist as well as a non-selective and non-competitive antagonist of the nicotinic acetylcholine receptors (nAChRs) (Bacher et al. 2009) used to treat hypertension and measures cigarette smoke extract (CSE)-evoked vascular endothelial growth factor (VEGF) release.
Capsazepine
It blocks painful sensation of heat caused by capsaicin. TRPV1 expression is elevated in uninjured ganglia in nerve injury model and capsazepine allays nerve injury induced hyperalgesia and mechanical allodynia. It acts as TRPM8 antagonist to treat cold allodynia (Behrendt et al. 2004) and inhibits voltage gated Ca2+ channels (Docherty et al. 1997) along with nicotinic acetylcholine receptors (Liu and Simon 1997). TRPV1 levels are elevated in visceral sensory afferents in inflammatory bowel disease in humans (Holzer 2004). Analgesic effect of TRPV1 antagonists due to dual (both peripheral and central) action is vital for full analgesic action (Cui et al. 2006). Inflammatory mediators, such as glutamate (acting on metabotropic receptors 5), bradykinin (acting on B2 receptors), prostaglandins E2 (acting on EP receptors), or NGF (acting on trkA receptors), extracellular ATP (acting on P2Y2 receptors), indirectly trigger and stimulate TRPV1 (Chuang et al. 2001; Ferreira et al. 2004; Hu et al. 2002; Moriyama et al. 2003; Premkumar 2010; Shin et al. 2002; Tominaga et al. 2001).
Thapsigargin
Thapsigargin is a sesquiterpine containing tricyclic diterpene ring, isolated from Thapsiagarganica (Apiaceae). It acts as selective inhibitor of Ca2+-ATPases (SERCAs) (Luo et al. 2000) in the endoplasmic and sarcoplasmic reticulum of animal cells. It is used traditionally in treatment of rheumatic pain in European and Arabian medicine system and seems to be a prototype for TRPV1 inhibitor.
Yohimbine
An indole alkaloid, isolated from the root of Rauwolfia serpentine (Aponcynaceae) and bark of the tree Pausinystalia yohimbe (Rubiaceae), blocks Na+ channels and TRPV1 receptors which revealed to hinder the firing activities of DRG neurons of rat (Dessaint et al. 2004).
Resiniferatoxin (RTX)
RTX is a naturally occurring ultrapotent analog of capsaicin found in resin spurge Euphorbia resinifera and Euphorbia poissonii having important anti-nociceptive properties (Walpole et al. 1996) mainly related to the dysfunction of various specific classes of pain receptors, but due to the stinging, burning pain and erythema primarily produced by these agonists make them difficult to use clinically.
2-aminoethoxydiphenylborate (2-APB)
2-APB is a synthetic diphenylborinic acid derivative that inhibits IP3 receptors (Diver et al. 2001) and TRP channels. It activates TRPV1, TRPV2, and TRPV3 at higher concentrations (Bootman et al. 2002; Xu et al. 2005b). It manipulates intracellular Ca2+ release that modify TRP channel activity. Patients with pathological condition accompanied with persistent or recurrent severe pain, such as neuropathic, herpes zoster, arthritis, cancer, and postoperative pain, are treated with innervations that are inadequate and encompassing devastating side effects. Experimental evidences showed that the TRPV1 is involved in these different pathologies. TRPV1 ligands and modulators are emerging as a new pharmacotherapeutic approach for various painful conditions.
Non-pungent agonists of TRPV1 receptors may be an interesting alternative and are devoid of undesirable effect. Specific antagonists of TRP channels could be used clinically and expected to have more prompt effects, different from the affected sensory fiber destruction caused by agonists.
TRPM8 channels are involved in oxaliplatin, and chronic constriction nerve injury (CCI)-induced neuropathic pain and its antagonist have ability to treat cold-induced allodynia (Descoeur et al. 2011; Su et al. 2011; Xing et al. 2007). TRPM8 plays a role in core body temperature regulation and detection of TRPM8 antagonist (PF-05105679) shows its competence in treatment of pain in humans (Andrews et al. 2015).
Conclusions
TRPs are transmembrane ligand-gated Ca2+ channels that play pivotal role in cellular functioning. TRP channels mainly include TRPV1, TRPA1, and TRPM8 gates for Ca2+ ion exclusively and tend to increase the intracellular Ca2+ concentration. Increased Ca2+ may lead to numerous cellular consequences like muscular contraction, neurotransmitter release, release of Substance P, and action potential generation. Increasing intracellular Ca2+ activates other TRP channels and modulates cellular signalling that leads to generation and propagation of neuropathic pain. 5-HPETE, the metabolite of LOX pathway, and other arachidonic acid metabolites, and also activates the TRVP1 channels and precipitates neuropathic pain. Furthermore, modulation of TRP channels either by synthetic/natural agents or by inhibition of COX/LOX pathway relieves neuropathic pain.
TRP channels can be explored as potential therapeutic target for treatment of neuropathic pain. TRP channel modulators can be picked up Pharmaceutical Industries and developed as a new class of highly efficacious pharmacotherapeutic agents for the clinical management of neuropathic pain.
References
Andrews MD et al (2015) Discovery of a selective TRPM8 antagonist with clinical efficacy in cold-related pain. ACS Med Chem Lett 6:419–424. doi:10.1021/ml500479v
Bacher I, Wu B, Shytle DR, George TP (2009) Mecamylamine––a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother 10:2709–2721. doi:10.1517/14656560903329102
Bandell M et al (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Baron R (2006) Mechanisms of disease: neuropathic pain––a clinical perspective. Nat Clin Pract Neurol 2:95–106. doi:10.1038/ncpneuro0113
Baron R (2009) Neuropathic pain: a clinical perspective. Handb Exp Pharmacol 194:3–30 doi:10.1007/978-3-540-79090-7_1
Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141:737–745. doi:10.1038/sj.bjp.0705652
Bhave G, Gereau RWt (2004) Posttranslational mechanisms of peripheral sensitization. J Neurobiol 61:88–106. doi:10.1002/neu.20083
Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM (2002) 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP(3)-induced Ca2+ release. Faseb J 16:1145–1150. doi:10.1096/Fj.02-0037rev
Brederson JD, Kym PR, Szallasi A (2013) Targeting TRP channels for pain relief. Eur J Pharmacol 716:61–76. doi:10.1016/j.ejphar.2013.03.003
Brito R, Sheth S, Mukherjea D, Rybak LP, Ramkumar V (2014) TRPV1: a potential drug target for treating various diseases. Cells 3:517–545. doi:10.3390/cells3020517
Calixto JB, Kassuya CA, André E, Ferreira J (2005) Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther 106:179–208
Caterina MJ (2007) Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol 292:64–76. doi:10.1152/ajpregu.00446.2006
Caterina MJ, Julius D (1999) Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol 9:525–530. doi:10.1016/S0959-4388(99)00009-4
Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24:487–517. doi:10.1146/annurev.neuro.24.1.487
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441. doi:10.1038/18906
Caterina MJ et al (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Chen J et al (2011) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152:1165–1172. doi:10.1016/j.pain.2011.01.049
Chuang HH et al (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411:957–962. doi:10.1038/35082088
Chung MK, Jung SJ, Oh SB (2011) Role of TRP channels in pain sensation. Adv Exp Med Biol 704:615–636. doi:10.1007/978-94-007-0265-3_33
Clapham DE (2003) TRP channels as cellular sensors. Nature 426:517–524. doi:10.1038/nature02196
Corey DP (2003) New TRP channels in hearing and mechanosensation. Neuron 39:585–588
Corey DP et al (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730. doi:10.1038/nature03066
Cortright DN, Szallasi A (2004) Biochemical pharmacology of the vanilloid receptor TRPV1. Update Eur J Biochem/FEBS 271:1814–1819. doi:10.1111/j.1432-1033.2004.04082.x
Cosens DJ, Manning A (1969) Abnormal electroretinogram from a Drosophila mutant. Nature 224:285–287
Cui M et al (2006) TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci 26:9385–9393. doi:10.1523/JNEUROSCI.1246-06.2006
Davis JB et al (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187. doi:10.1038/35012076
del Camino D et al (2010) TRPA1 contributes to cold hypersensitivity. J Neurosci 30:15165–15174. doi:10.1523/JNEUROSCI.2580-10.2010
Descoeur J et al (2011) Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med 3:266–278. doi:10.1002/emmm.201100134
Dessaint J, Yu W, Krause JE, Yue L (2004) Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur J Pharmacol 485:11–20
Dhaka A, Earley TJ, Watson J, Patapoutian A (2008) Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J Neurosci 28:566–575. doi:10.1523/JNEUROSCI.3976-07.2008
Diver JM, Sage SO, Rosado JA (2001) The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry channels in human platelets: cautions for its use in studying Ca2+ influx. Cell Calcium 30:323–329. doi:10.1054/ceca.2001.0239
Docherty RJ, Yeats JC, Piper AS (1997) Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol 121:1461–1467. doi:10.1038/sj.bjp.0701272
Facer P et al (2007) Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 7:11. doi:10.1186/1471-2377-7-11
Fernihough J, Gentry C, Bevan S, Winter J (2005) Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett 388:75–80. doi:10.1016/j.neulet.2005.06.044
Fernyhough P, Calcutt NA (2010) Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 47:130–139
Ferreira J, da Silva GL, Calixto JB (2004) Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol 141:787–794. doi:10.1038/sj.bjp.0705546
Grant AD et al (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578:715–733. doi:10.1113/jphysiol.2006.121111
Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF (2004) Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170:1276–1280. doi:10.1164/rccm.200402-174OC
Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414 doi:20026679
Guo A, Vulchanova L, Wang J, Li X, Elde R (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11:946–958
Haanpaa M, Treede RD (2012) Capsaicin for neuropathic pain: linking traditional medicine and molecular biology. Eur Neurol 68:264–275. doi:10.1159/000339944
Hara T et al (2013) Effect of paclitaxel on transient receptor potential vanilloid 1 in rat dorsal root ganglion. PAIN® 154:882–889
Holzer P (1991) Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol 298:3–16
Holzer P (2004) Vanilloid receptor TRPV1: hot on the tongue and inflaming the colon. Neurogastroenterol Motil 16:697–699. doi:10.1111/j.1365-2982.2004.00598.x
Hong S, Wiley JW (2005) Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem 280:618–627. doi:10.1074/jbc.M408500200
Hu HJ, Bhave G, Gereau RWt (2002) Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci 22:7444–7452
Jaquemar D, Schenker T, Trueb B (1999) An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 274:7325–7333
Jhaveri MD, Elmes SJ, Kendall DA, Chapman V (2005) Inhibition of peripheral vanilloid TRPV1 receptors reduces noxious heat-evoked responses of dorsal horn neurons in naive, carrageenan-inflamed and neuropathic rats. Eur J Neurosci 22:361–370. doi:10.1111/j.1460-9568.2005.04227.x
Ji G, Zhou S, Carlton SM (2008) Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience 154:1054–1066. doi:10.1016/j.neuroscience.2008.04.039
Jones RCW, Xu LJ, Gebhart GF (2005) The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25:10981–10989. doi:10.1523/Jneurosci.0703-05.2005
Jordt SE et al (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265. doi:10.1038/nature02282
Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413:203–210. doi:10.1038/35093019
Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S (2001) Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol 422:83–86
Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A (2005) Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology 49:977–984. doi:10.1016/j.neuropharm.2005.05.003
Karai L et al (2004) Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Investig 113:1344–1352. doi:10.1172/JCI20449
Kashiba H, Uchida Y, Takeda D, Nishigori A, Ueda Y, Kuribayashi K, Ohshima M (2004) TRPV2-immunoreactive intrinsic neurons in the rat intestine. Neurosci Lett 366:193–196. doi:10.1016/j.neulet.2004.05.069
Katsura H, Tsuzuki K, Noguchi K, Sakagami M (2006) Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem Senses 31:681–688. doi:10.1093/chemse/bjl009
Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD (2011) Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 6:e25894. doi:10.1371/journal.pone.0025894
Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ (2004) Regulation of the mouse epithelial Ca2(+) channel TRPV6 by the Ca(2+)-sensor calmodulin. J Biol Chem 279:28855–28861. doi:10.1074/jbc.M313637200
Lappin SC, Randall AD, Gunthorpe MJ, Morisset V (2006) TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol 540:73–81. doi:10.1016/j.ejphar.2006.04.046
Lee SE, Kim JH (2007) Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res 58:245–249. doi:10.1016/j.neures.2007.03.004
Levine JD, Alessandri-Haber N (2007) TRP channels: targets for the relief of pain. Biochim Biophys Acta 1772:989–1003. doi:10.1016/j.bbadis.2007.01.008
Lewinter RD, Skinner K, Julius D, Basbaum AI (2004) Immunoreactive TRPV-2 (VRL-1), a capsaicin receptor homolog, in the spinal cord of the rat. J Comp Neurol 470:400–408. doi:10.1002/cne.20024
Liu L, Simon SA (1997) Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett 228:29–32. doi:10.1016/S0304-3940(97)00358-3
Luo D, Nakazawa M, Yoshida Y, Cai J, Imai S (2000) Effects of three different Ca2+ pump ATPase inhibitors on evoked contractions in rabbit aorta and activities of Ca2+ pump ATPases in porcine aorta. Gen Pharmacol Vasc Syst 34:211–220
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol CB 15:929–934. doi:10.1016/j.cub.2005.04.018
Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P (2004) Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol 16:897–902
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58. doi:10.1038/nature719
Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN (2007) The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 148:1021–1032. doi:10.1016/j.neuroscience.2007.05.034
Montell C, Birnbaumer L, Flockerzi V (2002) The TRP channels, a remarkably functional family. Cell 108:595–598
Moqrich A et al (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472. doi:10.1126/science.1108609
Moran MM, McAlexander MA, Biro T, Szallasi A (2011) Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 10:601–620. doi:10.1038/nrd3456
Moriyama T et al (2003) Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci 23:6058–6062
Munns C, AlQatari M, Koltzenburg M (2007) Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium 41:331–342. doi:10.1016/j.ceca.2006.07.008
Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829–838. doi:10.1161/01.RES.0000097263.10220.0C
Nassini R et al (2011) Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152:1621–1631. doi:10.1016/j.pain.2011.02.051
Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A (2007) Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 148:560–572. doi:10.1016/j.neuroscience.2007.05.049
Nilius B, Voets T, Peters J (2005) TRP channels in disease Science’s STKE: signal transduction knowledge environment 2005:re8 doi:10.1126/stke.2952005re8
Nilius B, Owsianik G, Voets T, Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217. doi:10.1152/physrev.00021.2006
Obata K et al (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Investig 115:2393–2401. doi:10.1172/JCI25437
Pan YZ, Pan HL (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol 91:2413–2421. doi:10.1152/jn.01242.2003
Peier AM et al (2002a) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Peier AM et al (2002b) A heat-sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049. doi:10.1126/science.1073140
Poole DP et al (2011) Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology 141:565–575 e561–564. doi:10.1053/j.gastro.2011.04.049
Premkumar LS (2010) Targeting TRPV1 as an alternative approach to narcotic analgesics to treat chronic pain conditions. AAPS J 12:361–370. doi:10.1208/s12248-010-9196-y
Premkumar LS, Sikand P (2008) TRPV1: a target for next generation analgesics. Curr Neuropharmacol 6:151–163. doi:10.2174/157015908784533888
Ravnefjord A, Brusberg M, Kang D, Bauer U, Larsson H, Lindstrom E, Martinez V (2009) Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur J Pharmacol 611:85–91. doi:10.1016/j.ejphar.2009.03.058
Romero JR, Castonguay AJ, Barton NS, Germer S, Martin M, Zee RY (2010) Gene variation of the transient receptor potential cation channel, subfamily M, members 6 (TRPM6) and 7 (TRPM7), and type 2 diabetes mellitus: a case-control study. Transl Res J Lab Clin Med 156:235–241. doi:10.1016/j.trsl.2010.07.001
Shin J et al (2002) Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99:10150–10155. doi:10.1073/pnas.152002699
Siemens J et al (2006) Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444:208–212. doi:10.1038/nature05285
Sikand P, Premkumar LS (2007) Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol 581:631–647. doi:10.1113/jphysiol.2006.118620
Smith GD et al (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418:186–190. doi:10.1038/nature00894
Smith MP, Beacham D, Ensor E, Koltzenburg M (2004) Cold-sensitive, menthol-insensitive neurons in the murine sympathetic nervous system. Neuroreport 15:1399–1403
Spicarova D, Nerandzic V, Palecek J (2011) Modulation of spinal cord synaptic activity by tumor necrosis factor alpha in a model of peripheral neuropathy. J Neuroinflamm 8:177. doi:10.1186/1742-2094-8-177
Stokes AJ, Wakano C, Del Carmen KA, Koblan-Huberson M, Turner H (2005) Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J Cell Biochem 94:669–683. doi:10.1002/jcb.20331
Story GM et al (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702. doi:10.1038/35036318
Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL (2011) Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 12:120. doi:10.1186/1471-2202-12-120
Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664–22668. doi:10.1074/jbc.M302561200
Szallasi A (2006) Small molecule vanilloid TRPV1 receptor antagonists approaching drug status: can they live up to the expectations? Naunyn-Schmiedeberg’s Arch Pharmacol 373:273–286. doi:10.1007/s00210-006-0072-3
Szallasi A, Appendino G (2004) Vanilloid receptor TRPV1 antagonists as the next generation of painkillers. Are we putting the cart before the horse? J Med Chem 47:2717–2723. doi:10.1021/jm030560j
Szallasi A, Blumberg PM (1999) Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51:159–212
Szallasi A, Cortright DN, Blum CA, Eid SR (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6:357–372. doi:10.1038/nrd2280
Tamura S, Morikawa Y, Senba E (2005) TRPV2, a capsaicin receptor homologue, is expressed predominantly in the neurotrophin-3-dependent subpopulation of primary sensory neurons. Neuroscience 130:223–228. doi:10.1016/j.neuroscience.2004.09.021
Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M (2004) Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem 279:35133–35138. doi:10.1074/jbc.M406260200
Tominaga M, Caterina MJ (2004) Thermosensation and pain. J Neurobiol 61:3–12. doi:10.1002/neu.20079
Tominaga M et al (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543
Tominaga M, Wada M, Masu M (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA 98:6951–6956. doi:10.1073/pnas.111025298
Treede RD et al (2008) Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70:1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
Umeda M, Ohkubo T, Ono J, Fukuizumi T, Kitamura K (2006) Molecular and immunohistochemical studies in expression of voltage-dependent Ca 2 + channels in dorsal root ganglia from streptozotocin-induced diabetic mice. Life Sci 79:1995–2000
Urano H, Ara T, Fujinami Y, Hiraoka BY (2012) Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int J Med Sci 9:690–697. doi:10.7150/ijms.4706
Valtschanoff JG, Rustioni A, Guo A, Hwang SJ (2001) Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol 436:225–235
Verkhratsky A, Fernyhough P (2008) Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium 44:112–122
Vilceanu D, Honore P, Hogan QH, Stucky CL (2010) Spinal nerve ligation in mouse upregulates TRPV1 heat function in injured IB4-positive nociceptors. J Pain 11:588–599. doi:10.1016/j.jpain.2009.09.018
Vriens J, Owsianik G, Voets T, Droogmans G, Nilius B (2004a) Invertebrate TRP proteins as functional models for mammalian channels. Pflugers Arch 449:213–226. doi:10.1007/s00424-004-1314-1
Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B (2004b) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101:396–401. doi:10.1073/pnas.0303329101
Wainwright A, Rutter AR, Seabrook GR, Reilly K, Oliver KR (2004) Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J Comp Neurol 474:24–42. doi:10.1002/cne.20100
Walpole CSJ et al (1996) Similarities and differences in the structure-activity relationships of capsaicin and resiniferatoxin analogues. J Med Chem 39:2939–2952. doi:10.1021/Jm960139d
Watabiki T et al (2011) Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther 336:743–750. doi:10.1124/jpet.110.175570
Watanabe H et al (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577. doi:10.1074/jbc.M200062200
Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M (2010) High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes 59:844–849. doi:10.2337/db09-1100
Xing H, Chen M, Ling J, Tan W, Gu JG (2007) TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci 27:13680–13690. doi:10.1523/JNEUROSCI.2203-07.2007
Xu H et al (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418:181–186. doi:10.1038/nature00882
Xu H, Blair NT, Clapham DE (2005a) Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci 25:8924–8937. doi:10.1523/JNEUROSCI.2574-05.2005
Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ (2005b) Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145:405–414. doi:10.1038/sj.bjp.0706197
Xu H, Delling M, Jun JC, Clapham DE (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9:628–635. doi:10.1038/nn1692
Yang K, Kumamoto E, Furue H, Yoshimura M (1998) Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett 255:135–138
Yang K, Kumamoto E, Furue H, Li YQ, Yoshimura M (1999) Action of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Res 830:268–273
Yoshida T et al (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2:596–607. doi:10.1038/nchembio821
Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S (2012) Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain 8:55. doi:10.1186/1744-8069-8-55
Acknowledgments
Research grants sanctioned by SERB, Department of Science & Technology (DST), All India Council of Technical Education (F. No. 11-25/RIFD/CAYT/POL-II/2013-14), and University Grants Commission (UGC), New Delhi to Dr. Anurag Kuhad are gratefully acknowledged. Junior Research Fellowship sanctioned by All India Council of Technical Education (AICTE), New Delhi to Mr. Lovish Marwaha is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marwaha, L., Bansal, Y., Singh, R. et al. TRP channels: potential drug target for neuropathic pain. Inflammopharmacol 24, 305–317 (2016). https://doi.org/10.1007/s10787-016-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-016-0288-x