Abstract
Thermal lens spectrometry is a very sensitive non-evasive technique that offers a reliable alternative for measurement of materials thermal diffusivity. In this work, silver nanoparticles from leaf extract of Azadirachta Indica (neem) and silver nitrate were synthesized using reverse micelle microemulsion. The thermal lens technique was used to obtain the thermal diffusivity of silver nanoparticles emulsions contained in grapefruit centrifuged oil. Thermal diffusivity of silver nanoparticles was measured as a function of concentration. The results showed an increase in the thermal diffusivity of the nanoemulsion with the increase in nanoparticle concentration. Also, the nanoemulsion exhibited improved thermal diffusivity in comparison with the base fluid. Transmission electron microscopy was used to determine the morphology of the nanoparticles. Results showed that they were spherical in shape with an average size of 35 nm. UV–Vis spectrometry was used to observe the absorption spectra of nanoparticles plasmon. This study has future applications in dermatological therapies against allergies, in tissue regeneration and in the cosmetic area, due to silver nanoparticles antibacterial properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Grapefruit oil extracted from the Citrus Paradisi (also known as Citrus Racemosa or C. Maxima Var. Racemosa) belongs to the family Rutaceae or Pomelo. Other fruits of the genus are: oranges, lemons, limes, citrons, and tangerines. Citrus fruits are remarkable berries with internal parts divided into segments. The amount of natural species is not clear, since many of the species mentioned are hybrids [1].Grapefruit is native from Asia, but cultivated in the US, Brazil, Israel and Mexico, among other countries. Grapefruit is collected from a tree of about 10 m high, with white flowers and large pale yellow fruit. The glands penetrate deep into the shell and produce a small amount of essential oil [2]. For the growth of the nanoparticles, neem (a tree native from India) is used, taking advantage of its natural reducing agents and its low toxicity. Green chemistry is a process that involves the use of natural products as an alternative to conventional chemical processes for the synthesis of nanoparticles [1]. Metal nanoparticles can be obtained using reducing agents contained in the Azadirachta Indica leaves or neem [3]. Different productive sectors seek to apply the benefits of metallic nanoparticles such as silver nanoparticles to enhance a harmless environment or to eliminate pathogens from their products (coatings for food products, cosmetics and medicines). Neem extracts are a viable option for these applications, guaranteeing a low environmental impact and sustainable processes [4]. The development of technologies to produce silver nanoparticles has had a boom in the last decade due to the wide range of applications and its effectiveness as bactericide, fungus growth inhibitor, anti-inflammatory and antiangiogenesis [5].
The study of the thermal behavior of oils for corporal use and in general for human use is important. The change of the thermal diffusivity of the compounds could be affected when substances of different nature are incorporated, and more importantly, because of the presence of metallic particles, even in the nanoscale, could induce significant changes in the diffusivity. The value obtained for this coefficient is contrasted according to the concentration, size and dimension of the silver nanostructures obtained. The use of nano-sized particles with large surface area cannot only improve heat transfer. Also, can have a higher stabilization effect against particle sedimentation and act as a heat dissipator. Various types of nanoparticles such as metallic and non-metallic, with different shapes and sizes, can be suspended in different fluids forming the so-called nanofluids.
A series of experimental studies on the properties of nanofluids show improvements in their thermal conductivity. The results were obtained for different carbon allotropes. For example, a great improvement in thermal conductivity of 160 % and of 70 % for a suspension containing 1 % MWCNTs (multiwalled carbon nanotubes) in oil was achieved [6, 7]. There is a promising way to characterize the thermal properties of oils, and this is the thermal lens (TL). The thermal lens (TL) effect is an optical phenomenon that arises when a proportion of the energy of the light beam through the medium where it propagates is absorbed. This results in an increase in temperature, causing its refractive index to change with temperature. Using a probing laser in this region, it is possible that a focalization or defocusing is formed, thus producing the effect of the thermal lens. With the signal obtained as a function of the time of the thermal lens, the thermal diffusivity of the sample under study is obtained. To perform the thermal lens effect, medium-power lasers and optical instruments of relatively large dimensions are used. Also, electronic equipment is used for the detection and observation of the photothermal signal and cells as solutions containers [8]. It is important that the sample being analyzed is at a wavelength of excitation laser beam so that the thermal lens signal can be detected. Thermal lens system calibration is done with water and rhodamine (natural dye). In this work, the thermal diffusivity of grapefruit clarified oil emulsions, containing silver nanoparticles (AgNPs), was studied as a function of AgNPs concentration.
2 Theory
The TL technique of dual-beam mode mismatched was used to carry out experiments and detect the thermal properties of a sample. Two Gaussian laser beams are guided to the sample. One was used for sample excitation to produce a local temperature increase, creating a lens-like element in the heated region, and the other was used to probe the thermal effect. When the probe beam passes through the created lens, its optical path length undergoes a temporal change that can be observed by measuring the beam center intensity in the far field. To carry out the thermal lens technique and to detect the thermal properties of a sample, the Fresnell diffraction theory is used, which explains the intensity of the probe beam captured in the detector. It is represented by the following analytical expression (Shen) [9]:
I (0) is the signal intensity when t = 0. θ is proportional to the difference of phase change induced in the probe beam and when it passes through the sample area. θ is represented by the following equation:
where Pe (40 mW, 532 nm) is the incident power, A (cm−1) is sample of the absorption coefficient, λp (632 nm) is the wavelength of probe beam, L (1 cm) is the cuvette thickness, dn/dT (is the change of refractive index temperature coefficient). The so-called characteristic time constant of the TL effect’s formation is defined as tc = w 2e /4D, with we = 3.98 ± 0.02 × 103 cm which is the spot size of the excitation laser beam at the sample; D is the thermal diffusivity for D = k/ρc where k (W K−1⋅cm−1) is the thermal conductivity, ρ is the density and c is the specific heat of the nanofluid; m = (w1p/we)2, with w1p (cm) being the probe beam’s radius in the sample position and V = Z1/Zc with Z1 (cm) being the distance between the minimum beam waist and the sample position; Zc is the confocal distance with Zc = π w 2op /λp where wop (cm) being the minimum probe beam radius, the values of m and V are constants. From Eq. 1, the following constant parameters: m = 13.7, V = 1.22, Zc = 6.56 cm and we = 49 µm, w1p = 181 µm [10] were used to obtain the experimental fitting values of tc and θ.
2.1 Experimental Setup
Schematic diagram of the thermal lens spectroscopy (TLS) experimental is shown in Fig. 1 [11]. The measurement was made using an argon laser (Melles Griot) as an excitation source and a He–Ne laser (JDS Uniphase) as a probe beam. The excitation light beam was pulsed by a shutter (Uniblitz electronic), and a lens of 10 cm focal length is focused onto a liquid sample contained in a 1-cm-length cuvette positioned at its focal plane. The probe beam also was focused by a lens of 25 cm onto the cell and aligned at an angle smaller than 1.5° at its respect to the excitation beam. The transmitted beam was detected by a photodiode. A band-pass filter is placed in front of a pinhole to remove the stray light from excitation beam entering photodiode. Photodiode output was coupled to a digital storage to record the time evolution of TL signal.
3 Experimental
3.1 Synthesis
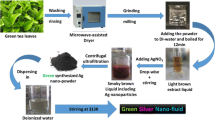
The neem extract was obtained from neem leaf extract prepared with deionized water from crushed neem leaves, heated at 70° C for 10 min, and subsequently centrifuged. To the previously elaborated neem extract, 5 mM of silver nitrate (AgNO3, 99.99 %) was added and it was kept under stirring for 15 min. The mixture was allowed to stand for 4 h. Then, the mixture was added to a 0.1 M solution of docusate sodium (AOT, 95.0 %) in isooctane drop by drop and stirring again for 15 min. After 1 h, 60 μl of dodecanethiol was added to the solution and stirred for 5 min. After 24 h, the supernatant was decanted. Nanoparticles (NPs) were added to grapefruit citrus oil in concentrations (NPs/grapefruit) of: 0.1/1 mg · mL−1; 0.2/1 mg · mL−1; 0.4/1 mg · mL−1; 0.5/1 mg · mL−1; 0.7/1 mg · mL−1; and 1/1 mg · mL−1 for the nanofluids. Samples were vortexed before each measurement (Fig. 2).
3.2 Characterization
The morphology of the metal nanoparticles was characterized using a transmission electron microscope JEOL (JEM-1010). The size distribution and the average number of particle diameters were obtained using the AMT Image Captures software. A UV–Vis Genesys 10 S spectrophotometer was used to obtain the absorbance of the samples.
4 Results and Discussion
In Fig. 3, TEM images and particle size distribution of the nanoparticles is seen. Figure 3a shows a typical TEM image of AgNPs between 30 nm and 40 nm, with an average size of 35 nm. Figure 3b shows the size distribution of the nanoparticles.
UV–Vis spectrophotometer was used to obtain the absorption spectra of grapefruit oil containing AgNPs with an average size of 35 nm for concentrations ranging from 0.1/1 mg · mL−1 to 1/1 mg · mL−1 (Fig. 4). In the spectra, a peak around 450 nm is observed, assigned to the surface plasmon resonance of the AgNPs [12, 13]. It can be seen that there is an increase in the absorption peak with an increase in the concentration.
First, the initial calibration using the experimental arrangement for TL described in the previous section was carried out using known samples such as water and olive oil as a base sample or control. The thermal diffusivity of grapefruit oil was measured obtaining a value of 7.3 ± 0.04 × 10−4 cm2 · s−1 [14,15,16]. Measurements of grapefruit oil with AgNPs were made at different concentrations. The TL signal of the aromatic grapefruit oil as a function of time is shown in Fig. 5. The signal intensity decreased with time.
The TL curve is characteristic of a defocalization of the probe beam as it passes through the sample analyzed. It is possible to see in the detector an image where the center of the beam is scattered, so it is characteristic of a diverging lens. In Fig. 5, the open circles represent the obtained experimental data and the solid line represents the best fitting of Eq. 1 to the experimental data. The obtained fitting parameters were θ = 1.9 ± 0.003 and tc = 82.1 ± 0.4 s for grapefruit essential oil, using tc = w 2e /4 D and Eq. 1 with D = 7.3 ± 0.04 × 10−4 cm2 · s−1. For the nanofluids, the thermal diffusivity was calculated for the sample with a concentration of 0.1 mg/1 mL and the diffusivity was D = 7.6 ± 0.11 × 10−4 cm2 · s−1 (Figure 6a). In a similar way, the other diffusivities of samples were obtained, for different concentrations, at room temperature. Table 1 summarizes the obtained fitting parameters and the calculated thermal diffusivities for each AgNPs concentration. Also, in Fig. 6a–f, the TL signal for the different concentrations and D value is observed.
It was observed that there was an improvement of 7.6 × 10−4 cm2 · s−1 to 13.3 × 10−4 cm2 · s−1 of thermal diffusivity. All the results of concentration, adjustment parameters and diffusivities are summarized in Table 1.
In the literature, several authors have mentioned several causes of the thermal increase in nanofluids. It is possible to name some physical reasons to explain an improvement in heat transfer of nanofluids: The suspended nanoparticles increase the surface area and the heat capacity of the fluid and increase the effective thermal conductivity of the fluid; the interaction of the particle, fluid and flow are intensified; the dispersion of the nanoparticles slightly modifies the transverse temperature gradient of the fluid. Also, stabilizers, pH, concentrations, type of nanoparticles, synthesis times and sizes of nanoparticles and solvents caused thermal increase in nanofluids. All these parameters influence the change in the diffusivity and conductivity of the medium [16].
For the reasons mentioned above, a linear increase in thermal diffusivity with the increase in concentration of nanoparticles was observed as function of concentration of the metal nanoparticles contained in grapefruit oil as shown in Fig. 7. This enhancement in thermal diffusivity can be due to the phonon scattering between the particle–liquid interfaces. Then, the energy deposited into the phonon mode is subsequently transferred to the surrounding medium. When the particle concentration is increased, the optical absorption intensity is increased, as well as the thermal diffusivity of the surrounding medium [17, 18].
Due to the fact that there is not an exact model for the determination of thermal diffusivity in nanofluids, it was not possible to compare our experimental results and the theoretical predictions using the traditional models where thermal conductivity is an important parameter.
5 Conclusions
It was observed that the formation of silver nanoparticles was successfully obtained by the microemulsion process, and also their incorporation into an aromatic oil. In general, this synthesis is a good alternative to the development of new methods of making nanoparticles, in addition to being friendly to the environment. It was also observed that there was an increase in the thermal diffusivity of the nanofluid when the concentration of nanoparticles increases with a diffusivity improvement from 7.6 × 10−4 cm2 · s−1 to 13.3 × 10−4 cm2 · s−1. This increase in thermal diffusivity is attributed to the phonons scattering in the particle–liquid interface of the largest contact surface between the nanoparticle and the liquid that surrounds it. From these results, it is possible to predict the behavior of this type of nanoparticles when incorporated to different systems in relation to the heat transport that it can lead. And perhaps, it can be applied to cosmetic to combat health problems in medicine.
References
Sallamander Concepts. Grapefruit essential oil information. Esoteric oils http://essentialoils.co.za/essential-oils/grapefruit.htm. Accessed 13 March 2018
B. Uysal, F. Sozmen, O. Birsen, S. Oksal, E. Kose, Int. J. Food Sci. Technol. 46, 1455 (2011)
M. Khalil, E. Ismail, K. El-Baghdady, D. Mohamed, Arab. J. Chem. 7, 1131 (2013)
S. Shankar, A. Rai, A. Ahmad, M. Sastry, J. Colloid Interface Sci. 275, 496 (2004)
S. Ahmed, M. Ahmad, B.L. Swami, S. Ikram, J. Radiat. Res. Appl. Sci. 9, 2 (2016)
S. Choi, G. Zhang, W. Yu, E. Lockwood, A. Grolke, Appl. Phys. Lett. 79, 2252 (2001)
S. Shaikh, K. Lafdi, R. Ponnappan, J. Appl. Phys. 101, 064302 (2007)
L. Rodríguez, J. Ramírez, O. Marcano, Rev. Mex. Fis. 51, 1 (2005)
J. Shen, R.D. Lowe, R.D. Snook, Chem. Phys. 165, 385 (1992)
R. Carbajal-Valdez, J.L. Jiménez-Pérez, A. Cruz-Orea, Z.N. Correa-Pacheco, M.L. Alvarado-Noguez, I.C. Romero-Ibarra, J.G. Mendoza-Alvarez, Thermochim. Acta 65, 66 (2017)
R.C. Valdez, J.L.J. Pérez, A. Cruz-Orea, E.S. Martín-Martínez, Int. J. Thermophys. 27, 1890 (2006)
A. Verma, M. Singh, J. Radiat. Res. Appl. Sci. 9, 109 (2016)
S. Rojas, V. Guerrero, Propiedad Bactericida de Telas de Algodón Impregnadas con Nanopartículas de Plata, X Congreso de Ciencia y Tecnología ESPE Conference (Ecuador, 2015)
G. López Muñoz, R. López González, J. Balderas López, L. Martínez Pérez, Int. J. Thermophys. 32, 1006 (2011)
W. Zhang, X. Qiao, J. Chen, H. Wang, J. Colloid Interface Sci. 302, 4370 (2016)
J.L. Jiménez-Pérez, G. López-Gamboa, Z.N. Correa-Pacheco, J.F. Sánchez-Ramírez, M. Sánchez-Rivera, M. Salazar-Villanueva, Int. J. Eng. Tech. Res. 3, 161 (2015)
S. Rotkin, V. Perebeinos, A. Petrov, P. Avouris, Nano Lett. 9, 1850 (2009)
E. Shahriari, M. Maradi, M. Raeisi, J. Theor. Appl. Phys. 10, 259 (2016)
Acknowledgements
Authors would like to thank CONACYT, COFAA, Red Temática de Biofotónica, and CGPI-IPN, Mexico, for their partial financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera-Aquino, R., Jiménez-Pérez, J.L., Altamirano-Juárez, D.C. et al. Green Synthesis of Silver Nanoparticles Contained in Centrifuged Citrus Oil and Their Thermal Diffusivity Study by Using Thermal Lens Technique. Int J Thermophys 40, 3 (2019). https://doi.org/10.1007/s10765-018-2466-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-018-2466-0