Abstract
Green synthesis was successfully used for silver nanoparticles (Ag NPs) preparation using Berberis vulgaris aqueous extracts. The ultraviolet and visible (UV–Vis) spectroscopy, photon cross-correlation spectroscopy (PCCS), transmission electron microscopy (TEM) and selected area electron diffraction (SAED) techniques were used for characterization of the Ag NPs and confirmed the presence of Ag(0) in nanoparticles. The bimodal morphology was discovered, namely, the larger particles had elongated shape and size around 200 nm, while the smaller ones were spherical with a size of up to ten microns. All the nanoparticles (NPs) showed antioxidant activity against radical DPPH and ferric-reducing antioxidant power. The Ag NPs possess antibacterial effects against Escherichia coli and Staphylococcus aureus comparable to silver nitrate solution. In addition, also irritation potential of the produced Ag NPs has been investigated ex Ovo and no irritation of vessels and their surroundings was found, which shows the harmless character of the products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles are defined as objects with a size of 1–100 nm [1]. Based on their small size and relatively broad surface, they have different properties compared to the same material with larger diameters. Nanoparticles have extensive application possibilities in various industries from agriculture to medicine. In the field of medicine, NPs are constantly being improved, for drug transport, screening for various diseases, and cancer therapy [2,3,4]. Due to increasing resistance to antibiotics, AgNPs are also studied as antimicrobial agents [5,6,7,8,9]. These facts lead to the study of Ag NPs as agents inhibiting resistant bacterial strains by multiple mechanisms of action, involving induction of oxidative stress, inhibition of DNA replication, or interaction with proteins and enzymes [10, 11].
Various ways of NPs synthesis (chemical, physical, biological) are studied. Green methods narrow down the reducing abilities of chemical substances obtained from natural sources. The advantages of green synthesis are economic simplicity and environmental aspects, due to the reduction of using toxic and harmful substances [12, 13]. In general, the plant extract consists of several secondary metabolites and biomolecules responsible for the reduction of metal ions. These are terpenoids, flavones, ketones, aldehydes, flavonoids, polyphenols, carboxylic acids, carbohydrates, proteins and vitamins [13].
Berberis vulgaris L. (barberry, family Berberidaceae) has been used in herbal medicine for more than 2500 years [14]. More than 20 alkaloids (mainly isoquinoline alkaloids) with pharmacological effects were identified in different parts (also in fruit) of this plant, for example, berberine, berbamine and palmatine [15]. In traditional medicine in Asia (mainly Iran), the different part from Berberis vulgaris L. is used for its antibacterial, anti-fever and anti-pruritic properties [16].
Berberis vulgaris fruit methanolic extract was successfully used for Ag NPs preparation [17]. The authors used methanolic extract as a prerequisite to potentially extract phenolic substances and investigated the impact of experimental conditions [17]. There are two more studies dealing with Ag NPs biosynthesis using extracts of B. vulgaris [16, 18]; however, leaves were used in this case.
Antibacterial agents may be used for topical or systemic administration. In the case of Ag NPs, the local application is preferred [19]. They can be used for the treatment of diseases on the skin, ocular or mucosal membrane [20]. For optical, nasal, vaginal or oral application, the test for irritancy must be realized. The Hen´s Egg Test on chorioalantoic membrane HET-CAM is such method recommended by ICCVAM (Recommended Test Methods (NIH Publication No. 10–7553 – 2010) [21].
In comparison with the mentioned works, our study is focused on the greener synthesis of Ag NPs preparation using an aqueous medium for extraction. We also studied the biological activities of prepared Ag NPs using a selected set of aqueous extracts of B. vulgaris L. fruit. Namely, antioxidant and antimicrobial properties were investigated. Moreover, we report the results of ex Ovo irritation potential of vessels by HET-CAM for the first time for Ag NPs prepared using berberis.
2 Materials and Methods
2.1 Green Synthesis of Silver Nanoparticles
2.1.1 Extract Preparation
A total of 200 mg of dried and milled fruit of commercial B. vulgaris L. (Sanny Tea, Prague, Czech Republic) was suspended in 4 mL of freshly distilled water, and the suspension was mixed at room temperature for 2 h, or sonicated for 15 or 30 min without temperature control (Bandelin Sonorex Digitec, Berlin, Germany). The solids were filtered out and the filtrates were used for Ag NPs biosynthesis.
2.1.2 Silver Nanoparticles Synthesis
Ag NPs were synthesized according to our previous study [18]. A total of 2700 µL of 5.5 mM silver nitrate solution (Mikrochem, Pezinok, Slovakia) was inserted into a quartz cuvette and heated at the required temperature (80 °C or room temperature) using the Peltier heating tool. Then, 300 µL of B. vulgaris fruit extract was added. The reaction process of Ag NPs preparation was detected visually by the change of colour and by UV–Vis spectra monitoring. The reaction process was stopped when the SPR absorbance did not increase in the monitored region. Ag NPs were also synthesized at room temperature, when 27 mL of 5.5 mM silver nitrate and 3 mL of berberis extract were stirred for 16 h, and monitored by UV–Vis spectroscopy using 10% dilution. The UV–Vis spectra were measured by UV–Vis spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA) equipped with a Peltier heating system.
2.1.3 Size Distribution Measurement
Size distribution measurements of the obtained nanosuspensions were performed using the PCCS methodology by Nanophox particle size analyzer (Sympatec, Clausthal-Zellerfeld, Germany). The refractive index of water was 1.33 and measurements were repeated three times for all the samples.
2.1.4 TEM Analysis
The morphology of Ag NPs in the extract prepared by sonication for 30 min was studied by transmission electron microscopy (TEM). The nanosuspension was homogenized in an ultrasonic bath Emmi-30HC (EMAG, Mörfelden-Walldorf, Germany) operating at 40 kHz at room temperature. A droplet of the suspension was applied onto a lacey carbon 200 mesh nickel grid (SPI Supplies, West Chester, PA, USA) and dried. Prior to TEM analyses, the grid was coated with a thin layer of amorphous carbon to improve the surface electron conductivity and to prevent charging under the high-energy electron beam. TEM analyses were performed using a 200 kV microscope JEM 2100 (JEOL, Tokyo, Japan) with a LaB6 electron source.
2.2 Antioxidant Activity
2.2.1 DPPH Assay
The free radical scavenging activity was determined by the methodology developed by Brand-Williams [22] with some changes. To 2 mL of 0.1 mM methanolic solution of free radical DPPH (2,2-diphenyl-1-picrylhydrazyl; Sigma Aldrich, St. Louis, MO, USA), 250 μL of extract or nanosuspension was added. The mixtures were then incubated in the dark at room temperature for 30 min. The negative control was a DPPH solution with 250 µL of methanol (Mikrochem, Slovakia). The decrease of absorbance was measured at the wavelength of 517 nm using UV–Vis spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA) and the percentage of antioxidant effect was calculated using Eq. (1),
where A0 is the absorbance of the sample with DPPH, and Ax is the absorbance of the negative control. The data are expressed as a triplicate average ± standard deviation (SD).
2.2.2 Total Phenolic Content
The content of total phenolic compounds was determined using Folin–Ciocalteau’s reagent (Sigma Aldrich, St. Louis, MO, USA) by a spectrophotometric method by Waterhouse with some modifications [23]. A total of 20 μL of extract standard solutions of gallic acid (Sigma Aldrich, St. Louis, MO, USA) with the concentration of 0.5–1.5 mg/mL or Ag NPs were added to 1.6 mL of distilled water and 100 µL of Folin–Ciocalteau’s reagent. The reaction mixtures were stirred and after 5 min; 300 µL of sodium bicarbonate (Mikrochem, Slovakia) was added. The samples were left in the dark at room temperature for 2 h. The absorbance at 765 nm was measured spectrophotometrically using UV–Vis spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA) against blanc (distilled water). The equation obtained from the gallic acid calibration line (y = 0.3007x − 0.0237; R2 = 0.9985) gave total phenolic content expressed as mg gallic acid equivalents (mg GAE/mL). All measurements were in triplicate and standard deviations were also provided.
2.2.3 Ferric Reducing Antioxidant Power
The FRAP (ferric reducing antioxidant power) method is based on the reduction of Fe(3 +) ions into Fe(2 +). The reaction mixture was prepared by mixing the acetate buffer with pH 3.6, 0.01 M solution of 2,4,6-tripyridyl-S-triazine (Sigma Aldrich, Steinheim, Germany) in 0.04 M HCl (Mikrochem, Pezinok, Slovakia) and 0.02 M ferric chloride (Mikrochem, Pezinok, Slovakia) in a ratio 3:1:1 (FRAP solution) [24].
To 2.25 mL of FRAP solution, 0.225 mL of freshly distilled water was added, and the mixture was incubated in the dark at room temperature for 5 min. Then, 75 μL of the sample of extract, nanosuspension or standard solution of ferric sulphate (Mikrochem, Slovakia) with concentrations 0.1–2 mM was added. After 5 min, the absorbance at 593 nm was measured spectrophotometrically using UV–Vis spectrophotometer Cary 60 (Agilent Technologies, Santa Clara, CA, USA) against blank (distilled water). The reduction ability was calculated from the calibration line equation (y = 0.0768x + 0.0728; R2 = 0.9929). All the measurements were in triplicate and standard deviations were also provided.
2.3 Irritation Potential Ex Ovo
Freshly laid fertilized quail eggs (Coturnix coturnix japonica,120 pcs) were purchased from the local certified hatchery (Malá Ida, Košice, Slovakia). The entire exterior of the eggs was cleaned and disinfected by ethanol (70%). They were incubated in a blunt end position in a forced-draft incubator (River ET549/A, River Systems, Italy) with automatic continuous rocking mechanisms (rotating of the eggs every 3 h) and with standard ambient conditions of 38.2 ± 0.5 °C temperature and 60 ± 2% relative humidity. After 56 h, the embryos were transferred from eggshell to a sterile 6-well tissue culture plate (Sigma Aldrich) under sterile conditions. They were then transferred back to the incubator and stored under the same conditions as before, however, without rotating this time. Embryos were controlled every day and dead embryos were discarded humanely. At embryonic day 7, the vasoactivity was tested by Luepke method [25]. The changes on vascularized CAM were photographed without samples and 30 s, 2 min and 5 min after application of 20 μL of samples by stereomicroscope Olympus SZ61 (Olympus Corporation, Japan), digital camera ARTCAM-300MI (ARTRAY, Japan) and software Quick Photo 2.3 (PROMICRA, Czech Republic). To minimalise subjective observations, two independent scientists evaluated irritant effects (a total of 5 eggs per solution). The numerical time-dependent scores for irritant effect including haemorrhage, hyperaemia and coagulation were summed to give a single numerical value which indicates the irritation assessment [25]. The chick embryos are considered experimental models as they are exempt from the horizontal legislation on the protection of animals used for scientific purposes in Europe (2010/63/EU), as well as in the USA, which means that no animal protocol approval for the chick embryo is requested.
2.4 Antimicrobial Properties
The tested microorganisms (Escherichia coli CCM 3988 and Staphylococcus aureus CCM 4223) were obtained from a Czech collection of microorganisms (CCM, Brno, Czech Republic). Bacteria were cultured overnight, aerobically at 37 °C in LB medium (Sigma-Aldrich, Saint-Louis, MO, USA) with agitation. The inoculum from these overnight cultures was prepared by adjusting the density of the culture to equal that of the 0.5 McFarland standard (1–2 × 108 CFU/mL) by adding a sterile saline solution. These bacterial suspensions were diluted 1:300 in liquid plate count agar (HIMEDIA, Mumbai, India) resulting in a final concentration of bacteria approximately 5 × 105 CFU/mL, and 20 mL of this inoculated agar was poured into a Petri dish (diameter 90 mm). Once the agar was solidified, 5-mm-diameter wells were punched in the agar and filled with 50 µL of samples. Gentamicin sulphate (Biosera, Nuaille, France) with a concentration of 50 µg/mL was used as a positive control and the plant extract used for the preparation of Ag NPs was used as a negative control. The plates were incubated for 20 h at 37 °C. The plates were incubated for 24 h at 37 °C. Afterwards, plates were photographed and the inhibition zone diameter (IZD) was measured using the software ImageJ 1.53e (U.S. National Institutes of Health, Bethesda, MD, USA). All the tests were applied in triplicate and the diameter of the clear zone was calculated by Eq. (2) [26]:
where % RIZD is the relative inhibition zone diameter (%) and IZD is the inhibition zone diameter (mm).
2.5 Statistical Analysis
All analyses were performed in triplicate. The results were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). The level of p < 0.001 was used.
3 Results and Discussion
3.1 Green Synthesis of Silver Nanoparticles
3.1.1 Silver Nanoparticles Synthesis
The reduction of Ag(+) ions to neutral form Ag(0) was achieved by using aqueous plant extracts (prepared at room temperature, sonically 15 or 30 min) of B. vulgaris (BV, fruit extract) serving as the reducing, stabilizing and capping agent [27,28,29]. The observed visual colour change was the first detection of the reduction of silver ions into Ag NPs. The colour of the produced nanosuspensions was red–brown.
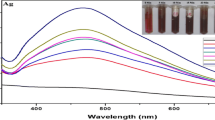
To monitor the reaction process, the UV–Vis spectra were measured in the region of 200–500 nm each minute directly in the cuvette at the temperature of 80 °C. For better visualization, we showed the spectra only for every fifth, respectively, tenth minutes (Fig. 1). It should be noticed that between 200 and 220 nm was observed n→σ* transitions of water. During the reaction, an increase of absorbance maximum was detected due to the surface plasmon resonance (SPR) phenomenon. The maximum of SPR absorption bands was between 425 and 438 nm (Table 1) with a slight red shift with the time of reaction, pointing to the possible increase of particle size. The heating was stopped after reaching the maximum of absorbance.
UV–Vis spectra monitoring of a sonical extraction Ag NPs synthesis by different extracts of B. vulgaris fruit at room temperature. b Extract prepared without USG for 2 h. c Extract prepared using USG for 15 min. d Extract prepared using USG for 30 min, and at 80 °C. e Extract prepared without USG for 2 h. f Extract prepared using USG for 15 min. g Extract prepared using USG for 30 min
The effect of the precursor concentration on the synthesis of Ag NPs using B. vulgaris fruit extracts is the object of our study in preparation, and the preliminary results have shown that increasing temperature and AgNO3 concentration decrease the reaction time, so we used the 5.5 mM silver nitrate as precursor and temperature of reaction was 80 °C. In concentration of precursor point of view, we also synthesized nanoparticles with precursor concentration of 2.2 mM; the reaction time was 120 min for extract prepared without sonification (no USG). In comparison with 5.5 mM precursor, there is significant difference in the reaction rate. Similarly, it was observed for sonically prepared extracts, when the reaction rate with 2.2 mM silver nitrate was 180 min for both extract with only slight decrease of absorption bands representing surface plasmon resonance. As the temperature change, we synthesized Ag NPs at 70 and 80 °C, and all the extracts reduced silver ions four times slowly than at 80 °C. These facts were confirmed also by our previous study when higher temperature and concentration of silver nitrate accelerated the reaction process [18].
In the present case, the rate of bio-reduction was the highest using extract prepared at room temperature (no USG) (26 min), followed by sonication for 30 min (32 min), and finally, the slowest reduction of Ag( +) was observed for extract prepared ultrasonically during 15 min (36 min). In the study of reaction process within temperature impact, we prepared Ag NPs at room temperature. The time of reaction increased from a few minutes into 16 h. The SPR band positions were between 428 and 434 nm, that is no significant difference, but there are interesting observations in polyphenols region (Fig. 1). The bands at 291 and 323 nm can be assigned to polyphenols, such as quercetin, gallic acid, caffeic acid, and coumaric acid. The band at 323 nm was slowly dissapeared with the red shift (from 323 nm to 304–344 nm) in all the spectra at room temperature; on the other hand, absorption band at 291 nm did not. This leads us to suggest that not all the polyphenols are responsible for bioreduction and/or are not able to follow this proccess due to structural properties. Similar situation was observed for Ag NPS prepared at 80 °C from extract without sonication, but with decreasing of absorption band was also detected red shift from 323 to 345 nm. This observation can be explicated by change in polyphonol structure, for example forming a new double bond that may correspond to oxidation of hydroxyl group (C–OH) into oxo group (C = O). For the Ag NPs prepared from sonicated extracts at room temperature, the red shift for 30 min sonicated extract was detected from 323 to 344 nm and 15 min sonicated extract from 323 to 343 nm. Also, Ag NPs prepared at 80 °C from sonicated extract (30 min) was observed red shift of absorption maximum from 323 to 342 nm. These observations may lead us to predict that polyphenols are engaged in bioreduction of silver during the Ag NPs synthesis. At the temperature of 80 °C for sonicated extract (15 min), the situation was different. The two absorption bands disappeared and the new one was detected between them at absorption maximum of 310 nm and was slightly blue shifted within the reaction progress. It may be the result of disappearance of the band at 323 nm and red shift of band at 291 nm, or, on the other side, the convolution of absorption bands affected by the environment of reaction process. This fact can also support the opinion that polyphenols are serving as reducing agents, but it needs more detailed study in the future.
Berberis vulgaris fruit extract was also successfully used for Ag NPs preparation in another study published in 2022 [17]. The authors used methanolic extract as a prerequisite to potentially extract phenolic substances and investigated the impact of experimental conditions such as pH, temperature, time and precursor concentration. The authors observed the SPR bands around 420 nm and the reaction time was from 5 to 180 min, depending on the used conditions [17]. The authors also proposed the mechanism of Ag NPs synthesis by B. vulgaris extract. The hydroxyl groups of polyhydroxy(phenyl)carboxylic acids are oxidized by Ag( +) ions into oxo groups and the carboxylate anions are capping the individual nanoparticles [17]. There are two more studies dealing with the Ag NPs biosynthesis using extracts of B. vulgaris [16, 18]; however, in this case, leaves were used.
3.1.2 Characterization of prepared Ag NPs
In order to obtain information about the prepared Ag NPs’ size, shape and monodispersity in colloidal solutions, the prepared nanosuspensions were characterized by various methods. In getting information about the size of grains, the samples were subjected to photon cross-correlation (PCCS) measurements. By grain size, we mean the size of nanoparticle aggregations (individual nanoparticles joined together via the organic matrix), not the diameter of individual NPs [30]. The grain size distribution data are shown in Fig. 2 for all three prepared colloidal solutions.
Ag NPs prepared by various extracts of B. vulgaris fruit at 80 °C showed the average grain size distribution between 97 and 127 nm (Fig. 2).
The results for all three nanosuspensions were quite similar, with the mean (d50) value being in the range of 97 to 127 nm. However, for the sample prepared without USG (black curve) and for the one subjected to the treatment to USG for 30 min (red curve), also coarse particles with sizes larger than 5 µm can be seen. Nevertheless, the content of these coarse particles does not seem to be substantial, and therefore, the sample with the lowest d50 was futher analyzed by TEM.
Nanosuspension of the extract prepared by sonication for 30 min was analyzed with TEM (Fig. 3). The low magnification image shows that the sample is composed of silver NPs with two distinctly different particle sizes (Fig. 3a). Selected area electron diffraction (SAED) was used to confirm that the particles are crystalline silver with face-centred cubic structure (s.g. Fm‒3 m). The large silver NPs show irregular morphologies; many of these particles are elongated and reach more than 200 nm in length. In addition to the large NPs, a fraction of smaller Ag NPs with sizes up to a few tens of nanometres and more isometric morphology and developed facets is observed in the sample. The observed nanoparticle morphology is in accordance with the other studies using plant extracts as reducing agents [18, 31,32,33].
The analysis of SAED confirmed the formation of silver in its elemental form, as the interplanar distances were in accordance with the following crystallographic planes of cubic Ag(0) (from the centre of the SAED pattern to the outside: 111, 200, 220, 113). This assignment is in accordance with the majority of studies describing the presence of elemental Ag(0), e.g., in [32, 34,35,36].
3.2 Antioxidant Activity Study
Antioxidant activity studies were determined for aqueous extracts and Ag NPs using a DPPH assay, TPC and FRAP method. The results are summarized in Table 2, where all the extract types and Ag NPs synthesized by them are expressed as an average of three independent measurements ± standard deviation (SD).
The antioxidant capacity of the biological sample, pure chemicals or isolated compounds is broadly studied these days. The most frequently used method for antioxidant capacity determination is the DPPH assay. The purple stable radical DPPH• (2,2-diphenyl-1-picrylhydrazyl) is a free radical which reacts with the donor of hydrogen. The presence of delocalized spare electrons on the molecule prevents dimerization and gives the colour to the molecule of DPPH with an absorption maximum in UV–Vis spectra at around the value of 520 nm. The reaction DPPH• radical leads to its reduced hydrazine form DPPH, which results in the colour change to pale yellow. The level of the disappearance of the purple colour depends on the concentration of the antioxidant. The scavenging capacity is usually determined in organic solvents, not in aqueous media [37,38,39]. The scavenging activity of plant extracts is in the range of 85.17–85.64%; for Ag NPs, we observed antioxidant activity between 88.4 and 90.24% (Table 2, Fig. 4a).
As we can see, all the NPs showed higher antioxidant activity against DPPH radical in comparison with the extract. The highest antioxidant activity was observed for extract prepared at room temperature (85.64%) as well as Ag NPs synthesized using it (90.24%). The lowest antioxidant properties were detected for ultrasonically (15 min) extract (85.17%) and silver NPs prepared by it (88.45%). The differences between the methods of extract preparation and the antioxidant capacity of Ag NPs are not significant. The antioxidant capacity of extracts of B. vulgaris fruit has not been studied yet; some studies were published on extract of root [18, 40].
On the other hand, an uncountable number of studies dealing with the antioxidant activity of Ag NPs using the DPPH method have been released. For example, Ag NPs prepared by Khorrami et al. [41] determined the higher scavenging activity of DPPH free radicals than the walnut (Juglans regia) extract they applied. Authors speculated that the improved antioxidant effect of Ag NPs is due to the simultaneous activity of polyphenols as antioxidant agents and Ag NPs serving as a catalyst [41]. Similar results were obtained by Elemike et al. [42], who prepared Ag NPs by Costus afer. Nanoparticles showed higher capacity against DPPH than the leaf extract, and their activity was comparable to that of ascorbic acid as a standard. The phytochemicals (mainly flavonoids) presented in the extract and silver ions are both able to serve as antioxidants through the single electron and hydrogen atom transfer [42]. A different theory was presented by Vijayan et al. [31], who indicate that the increased antioxidant properties of nanoparticles compared to the extract can be attributed to the adsorption of bioactive compounds of leaf extract over spherically shaped nanoparticles. Another theory suggested that the antioxidant ability of Ag NPs is caused by the presence of phenolic compounds, terpenoids and flavonoids in plants which allow nanoparticles to act as singlet oxygen quenchers, hydrogen donors and reducing agents [43].
The method of determining total phenolic content is based on the oxidation of phenolic compounds. Folin–Ciocalteau’s phenol reagent consists of a mixture of phosphomolybdic acid and phosphotungstic acid, in which the molybdenum and tungsten are both in the oxidation state 6 + and are reduced to the oxidation state of 5 + during the reaction. This is accompanied by a colour change of the solution of Na2WO4/Na2MoO4 from yellow to blue because of the formation of complexes with phenols (Phenol-MoW11O404−). The absorbance is usually measured in the range of 750–765 nm [44, 45]. The known fact is that the content of total phenolics (TPC) in the aqueous extracts is correlated with antioxidant activity due to the dependence of antioxidant activity on phenolic compounds amount [46, 47]. Our results are in agreement with this theory. The highest amount of TPC was observed for RT extract and Ag NPs prepared by this extract (0.634 and 0.552 mg GAE/mL), on the other hand, the lowest, as well as DPPH, for the USG 15 min extract and Ag NPs fabricated by it (0.479 and 0.439 mg GAE/mL) (Table 2, Fig. 4b).
Our results are in line with the literature [33, 48] as phenolic compounds are reducing agents for Ag( +) ions and thus determine the bioreduction potential of extract in silver NPs production.
The FRAP (ferric reducing antioxidant power) assay is based on the reduction of colourless Fe(3 +)-2,4,6-tripyridyl-S-triazine complex to the intensively blue Fe(2 +)-2,4,6-tripyridyl-s-triazine complex in acidic medium. FRAP values are calculated from increasing absorbances measured at 593 nm [39, 44]. The results of our observation lead to similarity to other used techniques in our study (Table 2, Fig. 4c). Similarly, to DPPH, the FRAP method also showed higher reducing power of Ag NPs than extracts. The highest reducing capacity was determined for extract prepared at room temperature and for Ag NPs prepared from the extract (14.28 and 15.86 mg/mL, respectively), so the ability to reduce Fe(3 +) ions to Fe(2 +) of Ag NPs is better than that of the extracts.
As a conclusion of the antioxidant properties of silver nanoparticles, we observed the correlation between total phenolic content and antioxidant activities (DPPH, FRAP) of extracts. The Ag NPs showed in general higher antioxidant properties, with no significant differences between extracts used. We expect that the increased antioxidant activities of Ag NPs are caused by bioactive compounds present in the organic matrix that is adsorbed on the surface of nanoparticles. This leads to the reduction of DPPH free radicals and Fe3 + ions to a better extent in comparison to the extract.
3.3 Irritation Potential Ex Ovo
To determine the safety of the application of Ag NPs obtained using berberis extract prepared sonically for 30 min, the irritation potential to vessels by CAM method ex Ovo was evaluated. According to Luepke, the vasogenic effect is determined by observation of changes in vessels, in particular hyperaemia, haemorrhage and coagulation [25].
The irritation potential of the Ag NPs-containing nanosuspensions prepared in this study was tested by an environmentally, economically and animal-friendly CAM model. This assay belongs to Recommended Test Methods by the National Institutes of Health (NIH) and its division Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) (NIH Publication No. 10–7553 – 2010) [21]. We found that the biosynthesized Ag NPs have antibacterial activity. It means that they can be used topically not only on artificial surfaces but also on human or animal skin and mucous. For application to different mucous, the irritation potential of Ag NPs must be analyzed. Based on their potential future mucous application, we tested the safety of Ag NPs by Hen’s Egg Test on chorioalantoic membrane (HET-CAM). The extract from berberis and the solution of AgNO3 were also tested. The results were compared to evaluate the effect of Ag NPs in comparison with input substances for their synthesis. Extract from berberis, 5.5 mM AgNO3 and Ag NPs did not show any of the three phenomena that determine the irritating capacity, namely hyperaemia, haemorrhage and coagulation (Fig. 5). No other effects of AgNO3 and berberis were observed. In the case of Ag NPs, the small vasoconstriction after 2 min were visible. However, after 5 min, the vessels returned to the same state as before the application. Vasoconstriction is not considered an irritating effect according to Luepke [25]. In general, the results show, that application of berberis extract, 5.5 mM AgNO3 and Ag NPs prepared from them do not cause any irritation of vessels and their surroundings. Ag NPs with antibacterial activity can be used topically not only on artificial surfaces but also on human and animal skin and mucous. For application to oral, vaginal, nasal mucous or ophthalmological applications, irritation tests are necessary. According to our best knowledge, this is the first report of the non-irritable potential of Ag NPs ex Ovo tested by HET CAM. Based on our results, the Ag NPs may apply to oral mucosa for the treatment of dental [49] or mouth [50] diseases connected with bacterial infections. The positive effect of Ag NPs was tested in the oral mucosa of rats [51]. Moreover, the results of the HET CAM assay predicted its nonirritable usage on the vaginal mucosa, which is in accordance with the ex vivo test of tannic acid/silver nanoparticle-based mucoadhesive hydrogel on porcine vaginal mucosa [52]. The HET-CAM replaces the Draize rabbit irritancy assay [53, 54], so it can used for therapy of ophthalmological infections. Biosynthesized Ag NPs or different Ag NPs were tested by in ovo methods for their antiangiogenic potential [55] or effect on embryogenesis [56]. However, up to now, no Ag NPs have been tested by the HET-CAM method recommended by NIH.
3.4 Antimicrobial Properties
Silver and its compounds are known as antibacterial agents against a broad spectrum of microorganisms. The antibacterial effect of the biogenic Ag NPs against pathogenic bacteria was studied by the agar well-diffusion method on two types of bacteria, namely S. aureus (as a Gram-positive bacteria) and E. coli (as a Gram-negative bacteria). Both bacteria species were resistant to plant extracts. Silver nitrate (5.5 mM) as well as Ag NPs showed significant antimicrobial activity (Table 3, Fig. 6). The values of antibacterial activities of 10 mM gentamicin sulphate as a positive control represent 100% of RIZD.
The antibacterial study showed that Ag NPs fabricated by various plant extracts of B. vulgaris and AgNO3 exhibit an antibacterial effect. All the studied samples of Ag NPs showed antibacterial activity similar to a positive control (gentamicin sulphate). The most effective were Ag NPs from extract prepared sonically (30 min) against both bacterial strains (S. aureus and E. coli) and showed better antibacterial effect than silver nitrate. The size and morphology of NPs play a significant role in their antibacterial efficiency. Smaller particles can easily penetrate through the cell membrane and are able to rapidly release Ag( +) ions during the oxidation process leading to the apoptosis of the cell [17].
There were observed no significant differences between gram-positive and gram-negative bacteria, but slightly better antimicrobial effect indicated for gram-positive S. aureus strain. The synthesized nanoparticles were slightly more effective against the gram-positive strain of S. aureus in comparison to E. coli, but the difference between both strains was not significant (Table 3). Similar results were published in 2015 [57].
The results of other studies mentioned various effects on gram-positive and gram-negative bacteria [58]. These contrasts in sensitivity may be explained by differentiation in thickness and/or biomolecule composition of bacterial cell membranes. Gram-positive bacterial membranes are composed mainly of peptidoglycans and are more resistant than the cell walls of gram-negative bacteria [30]. B. vulgaris Ag NPs prepared from extracts of root and leaves exhibited better antimicrobial activity against gram-negative bacteria E. coli. The authors assumed that Ag NPs react with proteins containing the thiol group inside or outside of the cell membrane. Ag NPs inhibit the absorption and release of phosphates and so lead to cell apoptosis [16]. In our results, it was observed better efficiency against gram-negative bacteria only for extract prepared at room temperature, so extraction procedure affect the antimicrobial properties of prepared Ag NPs and may be a key step in selectivity of relationship between antimicrobial activity and extraction procedure resulted to better control of antimicrobial resistance.
In general, the biosynthesized Ag NPs using all three extracts exhibited antibacterial properties. The antibacterial activity of the prepared Ag NPs is much higher than the activity of the corresponding plant extracts. On the other side, the antibacterial effect may be affected by the silver ion activity of unreacted silver nitrate or bioactive compounds present in the organic matrix.
4 Conclusions
We successfully prepared Ag NPs using B. vulgaris fruit aqueous extracts prepared by different extraction procedures and investigated the role of three different extract preparation conditions. All the extraction conditions, as well as sonication for 15, or 30 min, as maceration at room temperature (without sonification) led to extract with good antioxidant properties, which finally resulted in the reduction of silver ions into nanosized particles. The presence of elemental silver was confirmed by the SAED pattern. TEM analysis was applied to obtain information about the size and shape of prepared Ag NPs.
As the antioxidant studies showed, the Ag NPs exhibited higher antioxidant capacity against radical DPPH as a reduction ability of ferric (3 +) ions due to bioactive compounds presented in an organic matrix. We expect that the silver ion reduction is mainly responsible for phenolic compounds due to the decrease of total phenolic content in nanoparticles compared to the extracts.
Our first-ever study of vessel irritation by Ag NPs showed that silver nanoparticles prepared by B. vulgaris extract (USG 30) did not affect the vessels and their surroundings, so there is a potential for using them for an application on animal or human skin, not only on synthetic surfaces. From an antimicrobial point of view, prepared NPs showed antibacterial activities comparable with silver nitrate.
Data Availability
Not applicable.
References
Vert, M., Doi, Y., Hellwich, K., et al. (2012). Terminology for biorelated polymers and applications IUPAC Recommendations 2012. Pure and Applied Chemistry, 84, 377–410.
Davis, M. E., Chen, Z., & Shin, D. M. (2008). Nanoparticle therapeutics: An emerging treatment modality for cancer. Nature Review Drug Discovery, 7, 771–782. https://doi.org/10.1038/nrd2614
Justin Packia Jacob, S., Finub, J. S., & Narayanan, A. (2012). Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surfaces B Biointerfaces, 91, 212–214. https://doi.org/10.1016/j.colsurfb.2011.11.001
Nazem, A., & Mansoori, G. A. (2008). Diagnostic methods and therapeutic agents. Journal of Alzheimer’s Disease, 13, 199–223.
Abbasi, E., Milani, M., Fekri Aval, S., et al. (2014). Silver nanoparticles: Synthesis methods, bio-applications and properties. Critical Reviews in Microbiology, 7828, 1–8. https://doi.org/10.3109/1040841X.2014.912200
Jiang, H., Manolache, S., Wong, A. C. L., & Denes, F. S. (2004). Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. Journal of Applied Polymer Science, 93, 1411–1422. https://doi.org/10.1002/app.20561
Kováčová, M., Daneu, N., Tkáčiková, L., et al. (2020). Sustainable one-step solid-state synthesis of antibacterially active silver nanoparticles using mechanochemistry. Nanomaterials, 10, 1–17. https://doi.org/10.3390/nano10112119
Sintubin, L., Verstraete, W., & Boon, N. (2012). Biologically produced nanosilver: Current state and future perspectives. Biotechnology and Bioengineering, 109, 2422–2436. https://doi.org/10.1002/bit.24570
Sharma, V. K., Yngard, R. A., & Lin, Y. (2009). Silver nanoparticles: Green synthesis and their antimicrobial activities. Advances in Colloid and Interface Science, 145, 83–96. https://doi.org/10.1016/j.cis.2008.09.002
Tian, X., Jiang, X., Welch, C., et al. (2018). Bactericidal effects of silver nanoparticles on Lactobacilli and the underlying mechanism. ACS Applied Materials & Interfaces, 10, 8443–8450. https://doi.org/10.1021/acsami.7b17274
Hwang, I., Hwang, J. H., Choi, H., et al. (2012). Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. Journal of Medical Microbiology, 61, 1719–1726. https://doi.org/10.1099/jmm.0.047100-0
Siavash, I. (2011). Green synthesis of metal nanoparticles using plants. Green Chemistry, 13, 2638–2650. https://doi.org/10.1039/c1gc15386b
Jha, A. K., Prasad, K., Prasad, K., & Kulkarni, A. R. (2009). Plant system: Nature’s nanofactory. Colloids and Surfaces. B Biointerfaces, 73, 219–223. https://doi.org/10.1016/j.colsurfb.2009.05.018
Arayne, M. S., Sultana, N., & Bahadur, S. S. (2007). The berberis story: Berberis vulgaris in therapeutics. Pakistan Journal of Pharmaceutical Sciences, 20, 83–92.
Imanshahidi, M., & Hosseinzadeh, H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy Research, 22, 999–1012. https://doi.org/10.1002/ptr.2399
Behravan, M., Hossein Panahi, A., Naghizadeh, A., et al. (2019). Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. International Journal of Biological Macromolecules, 124, 148–154. https://doi.org/10.1016/j.ijbiomac.2018.11.101
Hashemi, Z., Shirzadi-Ahodashti, M., Mortazavi-Derazkola, S., & Ebrahimzadeh, M. A. (2022). Sustainable biosynthesis of metallic silver nanoparticles using barberry phenolic extract: Optimization and evaluation of photocatalytic, in vitro cytotoxicity, and antibacterial activities against multidrug-resistant bacteria. Inorganic Chemistry Communications, 139, 109320. https://doi.org/10.1016/j.inoche.2022.109320
Salayová, A., Bedlovičová, Z., Daneu, N., et al. (2021). Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials, 11: https://doi.org/10.3390/nano11041005
Gao, W., Chen, Y., Zhang, Y., et al. (2018). Nanoparticle-based local antimicrobial drug delivery. Advanced Drug Delivery Reviews, 127, 46–57. https://doi.org/10.1016/j.addr.2017.09.015
Gaslin, M. T., Rubin, C., & Pribitkin, E. A. (2008). Silver nasal sprays: Misleading Internet marketing. Ear, Nose, and Throat Journal, 87, 217–220. https://doi.org/10.1177/014556130808700414
Luepke, N. P., & Kemper, F. H. (1986). The HET-CAM test: An alternative to the draize eye test. Food Chem Toxicol, 24, 495–496. https://doi.org/10.1016/0278-6915(86)90099-2
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science Technology, 28, 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Waterhouse, A. L. (2002). Determination of total phenolics. Current Protocols Food Analytical Chemistry 6:I1.1.1-I1.1.8. https://doi.org/10.1002/0471142913.fai0101s06
Huang, D., Boxin, O. U., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agriculture and Food Chemistry, 53, 1841–1856. https://doi.org/10.1021/jf030723c
Luepke, N. P. (1985). Hen’s egg chorioallantoic membrane test for irritation potential. Food and Chemical Toxicology, 23, 287–291. https://doi.org/10.1016/0278-6915(85)90030-4
Rojas, J. J., Ochoa, V. J., Ocampo, S. A., & Muñoz, J. F. (2006). Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complementary and Alternative Medicine, 6, 1–6. https://doi.org/10.1186/1472-6882-6-2
Jayaprakash, N., Vijaya, J. J., Kaviyarasu, K., et al. (2017). Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. Journal of Photochemistry and Photobiology B: Biology, 169, 178–185. https://doi.org/10.1016/j.jphotobiol.2017.03.013
Hemmati, S., Rashtiani, A., Zangeneh, M. M., et al. (2019). Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron, 158, 8–14. https://doi.org/10.1016/j.poly.2018.10.049
Baláž, M., Balážová, Ľ., Daneu, N., et al. (2017). Plant-mediated synthesis of silver nanoparticles and their stabilization by wet stirred media milling. 12: https://doi.org/10.1186/s11671-017-1860-z
Baláž, M., Bedlovičová, Z., Kováčová, M., et al. (2020). Green and bio-mechanochemical approach to silver nanoparticles synthesis, characterization and antibacterial potential. In R. Prasad, B. Siddhardha, & M. Dyavaiah (Eds.), Nanostructures for antimicrobial and antibiofilm applications, Nanotechno (pp. 145–183). Springer Cham.
Vijayan, R., Joseph, S., & Mathew, B. (2018). Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artificial Cells Nanomedicine Biotechnology, 46, 861–871. https://doi.org/10.1080/21691401.2017.1345930
Baláž, M., Bedlovičová, Z., Daneu, N., et al. (2021). Mechanochemistry as an alternative method of green synthesis of silver nanoparticles with antibacterial activity: A comparative study. Nanomaterials, 11, 1–26. https://doi.org/10.3390/nano11051139
Siakavella, I. K., Lamari, F., Papoulis, D., et al. (2020). Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics, 12, 1–17. https://doi.org/10.3390/pharmaceutics12121244
Cai, Y., Piao, X., Gao, W., et al. (2017). Large-scale and facile synthesis of silver nanoparticles via a microwave method for a conductive pen. RSC Advances, 7, 34041–34048. https://doi.org/10.1039/C7RA05125E
Haritha, V. S., Maya Balan, J., de Hosson, J. T. M., & Gopi, K. (2020). Vapour confinement as a strategy to fabricate metal and bimetallic nanostructures. Nanoscale Advances, 2, 4251–4260. https://doi.org/10.1039/d0na00467g
Lengke, M. F., Fleet, M. E., & Southam, G. (2007). Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir, 23, 2694–2699. https://doi.org/10.1021/la0613124
Apak, R., Özyürek, M., Güçlü, K., & Çapanoʇlu, E. (2016). Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agriculture and Food Chemistry, 64, 997–1027. https://doi.org/10.1021/acs.jafc.5b04739
Floegel, A., Kim, D. O., Chung, S. J., et al. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, 24, 1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008
Pisoschi, A. M., & Negulescu, G. P. (2012). Methods for total antioxidant activity determination: A review. Biochemistry and Analytical Biochemistry, 01, 1–10. https://doi.org/10.4172/2161-1009.1000106
ZovkoKončić, M., Kremer, D., Karlović, K., & Kosalec, I. (2010). Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food and Chemical Toxicology, 48, 2176–2180. https://doi.org/10.1016/j.fct.2010.05.025
Khorrami, S., Zarrabi, A., Khaleghi, M., et al. (2018). Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. International Journal of Nanomedicine, 13, 8013–8024.
Elemike, E. E., Onwudiwe, D. C., Ekennia, A. C., & Katata-Seru, L. (2017). Biosynthesis, characterization, and antimicrobial effect of silver nanoparticles obtained using Lavandula × intermedia. Research on Chemical Intermediates, 43, 1383–1394. https://doi.org/10.1007/s11164-016-2704-7
Chinnasamy, G., Chandrasekharan, S., & Bhatnagar, S. (2019). Biosynthesis of silver nanoparticles from Melia azedarach: Enhancement of antibacterial, wound healing, antidiabetic and antioxidant activities. International Journal of Nanomedicine, 14, 9823–9836. https://doi.org/10.2147/IJN.S231340
Magalhães, L. M., Segundo, M. A., Reis, S., & Lima, J. L. F. C. (2008). Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta, 613, 1–19. https://doi.org/10.1016/j.aca.2008.02.047
A Agbor, G., Vinson, J. A., Donnelly, P. E. (2014). Folin-Ciocalteau reagent for polyphenolic assay. International Journal of Food Science Nutrition and Dietetics, 147–156. https://doi.org/10.19070/2326-3350-1400028
Bhutto, A. A., Kalay, Ş, Sherazi, S. T. H., & Culha, M. (2018). Quantitative structure–activity relationship between antioxidant capacity of phenolic compounds and the plasmonic properties of silver nanoparticles. Talanta, 189, 174–181. https://doi.org/10.1016/j.talanta.2018.06.080
Liu, Y. S., Chang, Y. C., & Chen, H. H. (2018). Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. Journal of Food and Drug Analysis, 26, 649–656. https://doi.org/10.1016/j.jfda.2017.07.005
Sharifi-Rad, M., Pohl, P., Epifano, F. (2021). Phytofabrication of silver nanoparticles (AgNPs) with pharmaceutical capabilities using Otostegia persica (burm.) boiss. leaf extract. Nanomaterials, 11: https://doi.org/10.3390/nano11041045
Dahl, J. E. (2007). Potential of dental adhesives to induce mucosal irritation evaluated by the HET–CAM method. Acta Odontologica Scandinavica, 65, 275–283. https://doi.org/10.1080/00016350701589286
Ortega, A., da Silva, A. B., da Costa, L. M., et al. (2023). Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Delivery and Translational Research, 13, 642–657. https://doi.org/10.1007/s13346-022-01227-1
Curtolo, G., de Araújo, J. P., Lima, J. A., et al. (2021). Silver nanoparticles formulations for healing traumatic injuries in oral mucosa of rats. Archives of Oral Biology, 129, 105202. https://doi.org/10.1016/j.archoralbio.2021.105202
Szymańska, E., Orłowski P., Winnicka K., et al. (2018). Multifunctional tannic acid/silver nanoparticle-based mucoadhesive hydrogel for improved local treatment of HSV infection: In vitro and in vivo studies. International Journal of Molecular Sciences, 19, 387. https://doi.org/10.3390/ijms19020387
Draize, J. H., Woodard, G., & Calvery, H. O. (1944). Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. Journal of Pharmacology and Experimental Therapeutics, 82, 377–390.
Scheel, J., Kleber, M., Kreutz, J., et al. (2011). Eye irritation potential: Usefulness of the HET-CAM under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Regulatory Toxicology and Pharmacology, 59, 471–492. https://doi.org/10.1016/j.yrtph.2011.02.003
Craciunescu, O., Seciu, A.-M., & Zarnescu, O. (2021). In vitro and in vivo evaluation of a biomimetic scaffold embedding silver nanoparticles for improved treatment of oral lesions. Materials Science and Engineering C, 123, 112015. https://doi.org/10.1016/j.msec.2021.112015
Subramaniyan, S. A., Kang, D. R., Park, J. R., et al. (2019). Effect of in ovo injection of L-arginine in different chicken embryonic development stages on post-hatchability, immune response, and myo-D and myogenin proteins. Animals, 9(6), 357. https://doi.org/10.3390/ani9060357
Nikbakht, M., Yahyaei, B., & Pourali, P. (2015). Green synthesis, characterization and antibacterial activity of silver nanoparticles using fruit aqueous and methanolic extracts of Berberis vulgaris and Ziziphus vulgaris. Journal Pure Applied Microbiology, 9, 349–355.
Kim, J. S., Kuk, E., Yu, K. N., et al. (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnology Biology and Medicine, 3, 95–101. https://doi.org/10.1016/j.nano.2006.12.001
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic This study was supported by the Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (projects 2/0112/22 and 1/0071/21) and by the Slovak Research and Development Agency (project 18–0357).
Author information
Authors and Affiliations
Contributions
Conceptualization: Z.B., Ľ.B., and M.B.; methodology: Z.B., Ľ.B., M.B., Z.L.B., and Ľ.T.; investigation: Z.B., Ľ.B., N.D., Z.L.B., A.J., and Ľ.T.; resources: M.B.; data curation: Z.B.; formal analysis: M.T.; writing—original draft preparation: Z.B. and Ľ.B.; writing—review and editing: Z.B., Ľ.B., N.D., M.B., and Ľ.T.; visualization: Z.B., Ľ.B., and N.D.; funding acquisition: M.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
None.
Research Involving Humans and Animals Statement and Informed Consent
The authors declare that ethical review and approval were waived for this study due to the chick embryo is considered as an experimental model, as they are exempt from the horizontal legislation on the protection of animals used for scientific purposes (2010/63/EU), as well as applicable laws in the USA, it means that no animal protocol approval for the chick embryo is requested.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balážová, Ľ., Bedlovičová, Z., Daneu, N. et al. Silver Nanoparticles Produced In Vitro by Berberis vulgaris Fruit and Their Antioxidant, Antimicrobial and Ex Ovo Irritation Potential Study. BioNanoSci. 14, 867–879 (2024). https://doi.org/10.1007/s12668-024-01400-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-024-01400-5