Abstract
Photothermal techniques were used for the thermal characterization of linear alcohols. This was carried out by measuring the thermal diffusivity (by means of a photopyroelectric technique) and thermal effusivity (by means of a photoacoustic technique) of ten linear alcohols, from methanol to 1-decanol. The thermal conductivity and specific heat for these substances were obtained by means of their relations with the two previous thermal properties, by using the values reported for the densities of the alcohols. The values of thermal effusivity showed a decreasing behavior with the increase in length of the linear molecule, and the values of thermal diffusivity, on the other hand, showed a similar behavior but only up to 1-pentanol, from which these values began to increase; this latter behavior was also observed, although to a lesser extent, for thermal conductivities. This peculiar behavior for thermal diffusivity and thermal conductivities is attributed to the influence of the hydroxyl group, which is strong for low molecular weight alcohols, but it diminishes as the size of alcohol’s molecule increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Alcohols occupy a prime place among all organic substances. Their applications range from solvents, in various industries, to antifreezes; even more, they are starting point for the synthesis of many substances [1,2,3]. Its wide use in science and engineering makes the knowledge of its physical properties relevant, especially the thermal ones. Linear alcohols have, among all alcohols, the simplest molecular structure that consists in a carbon–hydrogen chain with a hydroxyl group in one end. This particular structure makes them ideal substances to figure out the manner in which the molecule’s size and/or the functional group influence its physical properties, particularly their thermal properties.
Photothermal techniques have been largely used for thermal and optical characterization of substances in condensed phase [4,5,6,7,8]; among these methodologies, photoacoustic [5, 6] and photopyroelectric [7, 8] techniques have been used, respectively, for the measurement of thermal effusivity and thermal diffusivity of liquid substances. In this work, these photothermal techniques were used for thermal characterization of linear alcohols; this was done by measuring the thermal effusivity and diffusivity of the first ten linear alcohols (from methanol to 1-decanol). Thermal conductivity and specific heat for these substances were obtained taking into account their relationship with thermal diffusivity and thermal effusivity, by using reported densities for calculations of specific heat. Empirical dependence of these four thermal properties on the molecular size was established by plotting them as a function of the alcohol´s molecular weight.
2 Theoretical Considerations
Photothermal methods, involving modulated radiation as heat source, are experimental techniques very convenient for the measurement of thermal and optical properties of liquids. Two important thermal properties can be measured with these methodologies, thermal effusivity and thermal diffusivity. The corresponding photothermal techniques are already reported elsewhere [5,6,7,8], so only some relevant aspects of them are provided here.
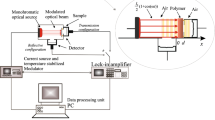
Thermal Effusivity Figure 1 shows a cross section of the experimental setup for thermal effusivity measurements for liquids by means of a photoacoustic (PA) technique in which modulated radiation, at a modulation frequency f, is taken as variable [5, 6]. The amplitude of the self-normalized PA signal, in the thermally thin regime for the absorbing material, m, can be written as [5]
where bsm = es/em is the ratio of thermal effusivities for the liquid sample (s) and absorbing material (m), and x = (πf/αm)1/2L (αm and L are, respectively, the thermal diffusivity and thickness of the light’s absorber).
The analytical procedure to obtain the sample’s thermal effusivity, es, involves the PA signal as a function of the modulation frequency in two different cases: one of them with the sample of interest in the sampler´s container and the other one with air. Ratio of PA amplitudes is taken for each modulation frequency, f, and these values are plotted as a function of f. Once the modulation frequency range for the analysis is determined [5], a fitting procedure is carried out to obtain parameters \( P_{S} \left( { = \frac{{\sqrt {\alpha_{m} } }}{2\sqrt \pi L}\left[ {\frac{{e_{s} }}{{e_{m} }}} \right]} \right) \) and \( P_{R} \left( { = \frac{{\sqrt {\alpha_{m} } }}{2\sqrt \pi L}\left[ {\frac{{e_{R} }}{{e_{m} }}} \right]} \right) \), from which the sample’s thermal effusivity can be obtained as es = (PS/PR)eR, where eR is the thermal effusivity of the reference sample.
Thermal Diffusivity Figure 2 shows a cross section of the photopyroelectric (PPE) experimental setup (the so-called thermal wave resonator cavity), at fix modulation frequency, f, for thermal diffusivity measurements [7, 8]. The PPE signal, as a function of the sample’s thickness l, in the thermally thick regime for the sample can be written as
where σm= (1 + i)(πf/αm)1/2, αm is the thermal diffusivity for the liquid sample, and H is a complex expression which is independent of the sample’s thickness. Linear fits of the PPE amplitude (in semi-log scale) and phase, as a function of the sample’s thickness, provide the slope M, from which sample’s thermal diffusivity can be obtained as αm = πf/M2.
3 Experimental Procedures
Thermal effusivity and diffusivity were measured for ten linear alcohols, from methanol to 1-decanol (Table 1, column 1). Thermal effusivity measurements were taken taking photoacoustic signals in a modulation frequency range of 1 Hz to 91 Hz, in steps of 2 Hz. A red diode laser (Thorlabs model L785P090, 785 nm wave-length, 90 mW CW power) was used as a light source and a thin steel foil (40 microns) as a highly opaque material. Distilled water was used as reference substance to obtain parameter PR, taking its thermal effusivity as 0.1588 Ws1/2 ·cm−2·K−1 [5].
Thermal diffusivity measurements, on the other hand, were taken at a fix modulation frequency of 1 Hz, setting the lock-in’s time constant as 3 s, using the same light source as the one for thermal effusivity measurements and a PVDF film (52 μm thickness) as pyroelectric sensor. Fifteen experimental data were taken for the analysis by means of the photopyroelectric phase (which has a linear behavior and it is independent of the light source fluctuations) starting at a sample’s thickness of around 300 μm, with increments of 50 μm. (The actual sample’s thickness is not required for the analysis.)
In both cases, lock-in amplifier (Stanford research systems, model SR830) and a current source (Thorlabs, model LDC202C) were used, respectively, to obtain the photothermal signal. Laser-intensity modulation was obtained by means of the TTL lock-in’s output that drives the current source. The signal was first pre-amplified (Stanford research systems, model SR540) before it was sent to the lock-in, for the case of PPE technique. All measurements were taken at room temperature of 23.0 ± 0.05 °C (Thermocouple monitor, Stanford Research Systems, model SR630) and at Mexico City’s pressure (around 77.9936 kPa).
4 Results and Analysis
Figure 3 shows typical PA amplitude ratios for two of the alcohols samples (methanol and 1-pentanol) and for distilled water. Solid lines in this figure are the best fits to Eq. 1 to obtain the PR and PS parameters. Thermal effusivity values for all samples are summarized in Table 2, column 3.
Ratio of photoacoustic (PA) amplitudes for the liquid sample and air (AmpS: PA amplitude for the liquid sample; AmpA: PA amplitude for air), as a function of the modulation frequency. Empty circles (ο), empty squares (□) and empty triangles (Δ) stand for distilled water, methanol and 1-heptanol, respectively. Continuous curves are the best fits to obtain the experimental parameters for sample’s thermal effusivity measurements
Figure 4 shows, on the other hand, the linear behavior of the photopyroelectric phase, as a function of the sample´s thickness, for three of the alcohol samples (methanol, 1-pentanol and 1-heptanol). The solid lines in this figure are the best linear fits to obtain the slope M, from which sample’s thermal diffusivity can be calculated. Thermal diffusivities for all samples are summarized in Table 2, column 4.
Photopyroelectric (PPE) phase, as a function of the sample’s thickness, for three of the alcohols samples studied in this work. Empty circles (ο), empty squares (□) and empty triangles (Δ) stand for methanol, 1-pentanol and 1-heptanol, respectively. Continuous curves are the best linear fits to obtain the slope for sample’s thermal diffusivity measurements
Thermal conductivities (\( k = e\sqrt \alpha \)) and specific heats (\( c = e/(\rho \sqrt \alpha ) \)) were calculated from these two set of experimental values, taking reported alcohols’ densities (Table 2, column 2) for the last case [1]. The corresponding values are summarized in Table 2, columns 5 and 6, respectively.
The thermal diffusivity and effusivity values summarized in Table 2 were obtained by carrying out the corresponding photothermal technique in a representative sample of each liquid. All analytical procedures were carried out using commercial software (Origin 6.1™), and the corresponding standard errors of the fitted parameters (obtained from the software) were taken to estimate standard uncertainties in the measurements of thermal diffusivity and effusivity. The usual formulas for error propagation [15, 16] were used to calculate the standard uncertainties for the measurements of thermal conductivity and specific heat.
Thermal effusivity values (Table 2, column 3), as a function of the alcohol’s molecular weight, are shown in Fig. 5. This thermal property shows a decreasing monotonous behavior as a function of the molecular weight of the alcohol.
The corresponding thermal diffusivity values (Table 2, column 4), as a function of the alcohol´s molecular weight, are shown in Fig. 6. The remarkable fact is that this thermal property behaves in a different way as thermal effusivity does; in fact, it decreases monotonically up to 1-pentanol, but from this alcohol it increases in a very clear way. This same behavior is evident, but at minor extent, for thermal conductivity, which experimental behavior, as a function of the alcohol’s molecular weight, is shown in Fig. 7. Finally, the specific heat for the alcohols, shown as a function of the molecular weight in Fig. 8, behaves in similar way as thermal effusivity does.
Concerning thermal conductivity, it is worth to discuss discrepancies between reported values and the ones consigned in this work. Figure 9 shows graphically, for this goal, sets of some reported values of this thermal property for linear alcohols.
Relative high deviations in the values reported for this thermal property have been observed for liquids (alcohols are not the exception), and this has been attributed to the different methodologies and/or improvements in the experimental setups. It is worth to point out, at this respect, that thermal conductivity values (at 20 °C) for toluene were recorded over 44 years (from 1923 to 1967) and very large variations were observed [12].
The influence of the experimental methodology on the measured thermal conductivity values can be observed for alcohols when comparing the values in references [9, 11, 12] (squares, triangles up and triangles down, respectively, in Fig. 9). Thermal conductivities for references [9, 12] were obtained by using two related calorimetric techniques (cylindrical configuration); this explains in part the “good” correlation between the corresponding values in Fig. 9 (assuming similar sample’s purity since no information about this aspect is given in Ref. [9]). However, larger variation is observed when comparing these values with the ones reported in Ref. [11,12,13,], which were obtained by using a slightly different methodology (flat plate configuration). But, even for similar experimental techniques, quite different thermal conductivity values can be obtained; this is evident when comparing values in Refs. [13, 14] (diamonds and left pointed triangles) with the ones in Ref. [9] (squares). Essentially the same experimental technique was involved in these three cases (transient hot wire technique in cylindrical configuration). Similar values of thermal conductivity are reported in Refs. [13, 14], but some of these values are quite different to the ones reported in Ref. [9] (some values in Ref. [13] are, by the way, closer to the ones reported in this work (circles in Fig. 9).
Major discrepancies in thermal conductivity values for methanol are expected due to its low boiling point for which convection effects largely affect the measurements; the extent of this effect depends also on the experimental methodology.
It is important to point out, as a final remark on this topic, that the thermal conductivity values reported in Refs. [9, 11,12,13,14] were measured directly by using experimental setups designed specifically for this goal. This contrasts with the thermal conductivity values consigned in this work, which were estimated by using two related thermal properties (thermal diffusivity and thermal effusivity) which were measured directly. Different systematic errors are then involved, and they could explain the large deviation of our reported values with some of the ones reported in the literature. This situation, however, does not affect the interesting behavior on thermal conductivity as a function of the molecular size, as observed in Fig. 9 (circles). Actually, Wang and Yang [12] consigned graphically this “peculiar influence” of the molecular size on thermal conductivity (triangles down in Fig. 9), but they limited to establish that “the smooth change of λ with the number of carbon atoms merits further study.” (In their article, λ is the thermal conductivity.) One of the most important aspects of this work is to point out that the influence of the number of carbons for alcohols on their thermal diffusivity (measured by means of a very precise photopyroelectric technique) is even larger than the one observed for thermal conductivity. A qualitative explanation of this fact is given as follows.
It is interesting to realize that just for kinetic thermal properties, like thermal diffusivity and conductivity, two different regimes for thermal behavior are clearly presented: Both of these thermal properties decrease for linear alcohols of low molecular weight, and they increase for those of high molecular weights. These two different thermal regimes can be qualitatively explained as the result of the influence of the hydroxyl group. It can be concluded that this functional group “drives” the thermal transport properties for linear alcohols of short hydrocarbon chain, but it gradually “loses” its influence as molecular size increases, taking then “control” the hydrocarbon chain for long-chain linear alcohols.
This “competition” between hydroxyl group and hydrocarbon chain affects other physical properties of linear alcohols, such as water solubility. Short-chain linear alcohols (from methanol to 1-pentanol) are water-soluble, whereas long-chain linear alcohols (from 1-hexanol to 1-decanol) are not [3]. According with these facts, it could be expected that, for linear hydrocarbons like alkanes, which consist only of C–H chains, these two thermal transport properties will increase with the molecular size.
5 Conclusions
Photothermal techniques were used for thermal characterization of linear alcohols. These techniques have advantages over other photothermal techniques (like thermal lens) which are also used for thermal characterization of this kind of liquids, the photothermal signals in the present case do not depend on the optical properties of the liquid sample, and in addition, other mechanisms of heat transport do not influence the photothermal signal at large extent [10]. The experimental results suggest that the hydroxyl group has a strong influence on thermal kinetic properties for alcohols, such as thermal diffusivity and conductivity, which results in a monotonous decrease in these two thermal properties at low molecular weights and a monotonous increase in high molecular weights, when the molecular size “hides” the functional group. These two aspects of the kinetic thermal properties behavior (the influence of functional group and/or molecular size) deserve deep study theoretically and experimentally, and this could be carried out taking, for instance, other molecular models in which only the functional group changes.
References
R.T. Morrison, R.N. Boyd, Organic Chemistry (Allyn and Bacon Inc., Boston, 1973)
H. William, Brown, Introduction to Organic Chemistry, 2nd edn. (Willard Grant Press, Boston, 1979)
J. Stuart, Baun, Introduction to Organic and Biological Chemistry, 2nd edn. (Macmillan Publishing Co. Inc, New York, 1978)
R. Fuente, A. Mendioroz, E. Apiñaniz and A. Salazar, Int. J. Thermophys., ICPPP-16 Special Issue 1, 33(10–11), 1876 (2012)
J.A. Balderas-López, Rev. Sci. Instrum. 78, 064901 (2007)
O. Delgado-Vasallo, E. Marín, J. Phys. D Appl. Phys. 32, 593 (1999)
J.A. Balderas-López, A. Mandelis, J.A. García, Rev. Sci. Instrum. 71, 2933 (2000)
J.A. Balderas-López, M.R. Jaime-Fonseca, G. Gálvez-Coyt, A. Muñoz-Diosdado, J. Díaz-Reyes, Int. J. Thermophys. 36, 857 (2015)
J.D. Raal, R.L. Rijsdijk, J. Chem. Eng. Data 26, 351 (1981)
P. Kumar, S. Dinda, A. Chakraborty, D. Goswami, Phys. Chem. Chem. Phys. 16, 12291 (2014)
K. Ogiwara, Y. Arai, S. Saito, J. Chem. Eng. Jpn. 15, 335 (1982)
C.Y. Wang, M.L. Yang, Thermochim. Acta 255, 365 (1995)
M.J. Assael, E. Charitidou, C.A. Nieto de Castro, Int. J. Thermophys. 9, 813 (1988)
G. Cai, H. Zong, Y. Qingsen, R. Lin, J. Chem. Eng. Data 38, 332 (1993)
J. Mandel, The Statistical Analysis of Experimental Data (Dover Publications Inc., New York, 1964)
See http://www.bipm.org/en/publications/guides/gum.html for a detailed discussion about standardization of uncertainties of experimental data
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balderas-López, J.A., Jaime-Fonseca, M.R., Gálvez Coyt, G. et al. Photothermal Techniques for Thermal Characterization of Linear Alcohols. Int J Thermophys 39, 111 (2018). https://doi.org/10.1007/s10765-018-2432-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-018-2432-x