Abstract
Biofuels are an alternative to fossil fuels and can be made from many different raw materials. The use of distinct catalyst and production processes, feedstocks, and types of alcohol results in biofuels with different physical and chemical properties. Even though these diverse options for biodiesel production are considered advantageous, they may pose a setback when quality specifications are considered, since different properties are subject to different reactions during usage, storage and handling. In this work, we present a systematic characterization of biodiesels to investigate how accelerated thermal degradation affects fuel properties. Two different types of biodiesel, commercially obtained from distinct feedstocks, were tested. The thermal degradation process was performed by maintaining the temperature of the sample at \(140 \,^{\circ }\hbox {C}\) under constant air flux for different times: 0 h, 3 h, 6 h, 9 h, 12 h, 24 h and 36 h. Properties such as density, viscosity, activation energy, volumetric thermal expansion coefficient, gross caloric value, acid value, infrared absorption, and temperature coefficient of the refractive index were used to study the thermal degradation of the biodiesel samples. The results show a significant difference in fuel properties before and after the thermal degradation process suggesting the formation of undesirable compounds. All the properties mentioned above were found to be useful to determine whether a biodiesel sample underwent thermal degradation. Moreover, viscosity and acid value were found to be the most sensitive characteristics to detect the thermal degradation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In face of many current environmental problems caused by the extraction of fossil fuels, the threat it poses to climate changes, and the growing demand to reduce carbon footprint, the development of alternative sources of fuel has become a worldwide priority. Fuels derived from renewable energy sources such as biofuels are indeed in line with the demanding actions toward reducing greenhouse gas emissions. Biofuels can be used alongside petroleum-based fuels and presents many suitable characteristics such as: compatibility with the existing infrastructure, renewability, biodegradability, domestic origin, lubricity and competitiveness with petroleum-diesel in terms of fuel properties [1,2,3,4,5,6].
Biodiesel can be produced through distinct feedstocks, types of alcohol, catalyst and production processes. This results in biofuels with different compositions and physical and chemical properties, which are influential in quality specifications. The most widely used feedstocks are soybean, rapeseed, and animal fats. The alcohol of choice is usually methanol, and the preferred production process is transesterification. Through the transesterification of oils and fats, the primarily advantage is the reduction of fuel viscosity. Since the fatty acid profile of biodiesel corresponds to that of the parent oil or fat obtained from [1,2,3], the proper choice of feedstock is crucial for the final biodiesel characteristics. The fatty acid profile or the presence of significant amount of fatty acids with double bonds indeed interfere in the oxidative and thermal stability of biodiesel as it is stored for an extended period of time. Time-degradation problems can be caused by storage conditions such as: exposure to air, light, temperature, and presence of metals with catalyst effect on oxidation [4,5,6].
Oxidation stability is a major concern in the study of biodiesel. Degradation by oxidation generates products that may compromise fuel properties, and impair fuel quality and engine performance [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Therefore, the study of biodiesel’s properties through numerous test procedures can associate such properties with the oxidative, thermal or storage stability of biodiesel.
A number of researchers have investigated the effect of temperature on stability of biodiesel. Dunn [7, 8] examined the effects of oxidation under controlled accelerated conditions on fuel properties of methyl soyate. Conceio et al. [9] studied the degradation process of castor oil biodiesel in different temperatures and exposure times. Lacoste et al. [10] examined the evolution of physical and chemical properties during the oxidation of fatty acid methyl ester using the Rancimat test. Li et al. [11] investigated thermal degradation of biodiesel derived from peanut oil, palm oil and waste cooking oil methyl ester. Ferrari et al. [12] determined the oxidative stability of biodiesel produced by ethanolysis of neutralized, refined, soybean frying oil waste, and partially hydrogenated soybean frying oil waste. Knothe et al. [13] used oil stability index (OSI) to assess oxidation of monoalkyl ester in the presence of metals. Monyem et al. [14,15,16] evaluated the impact of oxidized biodiesel on engine performance and exhaust emissions. Silva et al. [17] studied the thermal degradation of soybean biodiesel obtained by ethanolic route. Lin et al. [18] evaluated the influence of thermal decomposition on biodiesel viscosity and cold flow properties. Ashraful et al. [19] measured fuel properties of biodiesel over storage time to study oxidative stability. Zhou et al. [20] explored the oxidative degradation of biodiesel blends using FTIR, UV/VIS, TGA and two-channel and differential dielectric spectroscopy (TD-DES). Savi et al. [21] used FTIR in association with principal components analysis (PCA) and hierarchical cluster analysis (HCA) as a method for monitoring the oxidation degree of biodiesel before the end of the induced period.
In this report, we present a systematic characterization of physical and chemical properties of two types of biodiesel (derived from soybean oil and animal fat) most commonly used in Brazil. The effect of the thermal oxidation process is investigated by analyzing the accelerated thermal degradation on fuel quality and physical and chemical properties. Different properties were studied to evaluate which ones are more susceptible to the thermal degradation process and the reasons for such behavior.
2 Experimental Methods
2.1 Materials
Two different types of biodiesel were obtained commercially. The first sample of biodiesel is derived strictly from soybean oil (BSO), while the second one is derived from soybean oil and animal fat (BSG) (1:1 ratio). The conventional catalysts in natural oil transesterification processes used are alkaline, and the short-chain alcohol used is methanol. The companies provided certificate of quality for the fuels, indicating that both biodiesel samples met the national quality specifications—ANP 45 / 2014 [22].
2.2 Preparation of Biodiesel Samples
The thermal degradation process was performed by varying the time exposure to heating: 0 h, 3 h, 6 h, 9 h, 12 h, 24 h and 36 h. The temperature of the samples remained constant at \(140\,^{\circ }\hbox {C}\) under constant airflow. This value was established due to previous studies [7, 9] related to oxidative stability and thermal degradation of biodiesel. In this temperature range, the processes of molecular changes are accelerated in the samples. The procedure was as follows: \(250\,\hbox {ml}\) of biodiesel was added to a Becker and then heated using an electronic hot plate with digital temperature control. To verify the sample’s temperature a thermometer was put in direct contact with the sample through one of the vent holes of the Becker’s bin. Both BSO and BSG samples were thermally degraded for posterior analysis of the changes in thermophysical properties.
2.3 Methods
All samples were subjected to physical and chemical analysis using the procedures described below. Each experiment was run in triplicate to rule out experimental bias.
2.3.1 Density
Density analysis was performed based on the ASTM D4052 standard using a digital Anton Paar density meter, model DMA 5000, from \(20\,^{\circ }\hbox {C}\) to \(90\,^{\circ }\hbox {C}\) with an increase rate of \(2\,^{\circ }\hbox {C}\). The volumetric thermal expansion coefficient (\(\beta \)) was obtained from the density values (\(\rho \)) using the following equation
2.3.2 Viscosity
A Brookfield digital rheometer, model DV-III Ultra, linked to a Brookfield bath, model TC-602P, fed the software program data from the chosen temperature range from \(15\,^{\circ }\hbox {C}\) to \(90\,^{\circ }\hbox {C}\). The temperature range was chosen considering the sensibility of biodiesel usage in low temperatures. Therefore, \(15\,^{\circ }\hbox {C}\) in viscosity readings was the lowest temperature in which the biodiesel samples could be analyzed without the crystallization phase of the sample beginning. The apparatus determined the dynamic viscosity as a function of temperature. The kinematic viscosity was obtained by the ratio of the dynamic viscosity to the mass density. Using the linear form of Grunberg and Nissan adapted equation [23]
the activation energy (\(E_\mathrm{A}\)) of shear viscosity variation with temperature was obtained. Here, \(\eta \) is the dynamic viscosity (m Pas), \(\eta _0\) is the dynamic viscosity at high temperatures, T is the temperature, \(E_\mathrm{A}\) is the activation energy of shear viscosity variation with temperature, and R is the universal gas constant (\(8.31\,\hbox {J} \cdot \hbox {mol}^{-1} \cdot \hbox {K}^{-1}\)).
2.3.3 Gross Calorific Value
To analyze the gross calorific value of these biodiesel samples, an IKA Works calorimeter, model C-2000 Basic, was used. The experiments were performed in dynamic mode at \(25\,^{\circ }\hbox {C}\) and \(1500\,\hbox {k}\cdot \hbox {Pa}\) pressure oxygen.
2.3.4 Temperature Coefficient of the Refractive Index (dn/dT)
An optical interferometer was used to determine the temperature coefficient of the refractive index (\(\hbox {d}n/\hbox {d}T\)). Basically, the biodiesel samples placed in a \(2\,\hbox {mm}\) quartz cuvette was inserted in the cavity of a resistive furnace. A He–Ne laser beam (\(632.8\,\hbox {nm}\)) irradiated the cuvette uniformly, and the reflections from the two inner surfaces interfered, producing fringe patterns at the sensor. The heating unit slowly heated up the sample, generating a uniform temperature variation in the cuvette, inducing changes in the interference fringes. Measurements were carried out at a heating rate of \(0.5\,^{\circ }\hbox {C}\,\hbox {min}^{-1}\) from \(25\, ^{\circ }\hbox {C}\) to \(35\, ^{\circ }\hbox {C}\). The temperature-dependent optical interferograms were used to calculate the magnitude of \(\hbox {d}n/\hbox {d}T\); since the \(\hbox {d}n/\hbox {d}T\) for a given sample is approximately constant within this range of temperature; averages are reported in this study. References [24,25,26] present details of the theory and experimental setup of this apparatus.
2.3.5 Acid Value
Biodiesel acid value monitoring is an essential study to maintain a high-quality product, since it can indicate the presence of water, especially during long-term storage. With this analysis, it was also possible to verify and quantify the existence of free fatty acids (FFAs) in biodiesel. Besides indicating the presence of FFAs, this study helped to correlate the influence the thermal degradation has on the acidity index. The experimental procedures were executed following EN 14104, which uses an alcoholic solution of potassium hydroxide (KOH) as titrant and phenolphthalein as the indicator.
2.3.6 Fourier Transform Infrared Spectroscopy (FTIR)
The infrared absorption was obtained by a FTIR spectrometer, Perkin Elmer model Frontier, based on a Universal Attenuated Total Reflectance sensor (UATR) in the range from \(4000\,\hbox {cm}^{-1}\) to \(650\,\hbox {cm}^{-1}\) with a resolution of \(4\,\hbox {cm}^{-1}\) with an average of 32 scans. A baseline correction of the spectra was performed and a smoothing procedure performed with FFT filter method.
3 Results and Discussion
Different methods were used to analyze the thermal oxidation of both nondegraded and thermally degraded biodiesel samples. Density analysis indicated a linear decrease of mass density with temperature, as it is shown in Fig. 1a for BSO samples. The same behavior was observed for the BSG samples. To better comprehend the thermal degradation effect on the mass density, the values at \(20\,^{\circ }\hbox {C}\) are plotted against the thermal degradation time. Figure 1b shows these values along with a filled area, which indicates the minimum and maximum values (0.85 to 0.90\(\,\hbox {g}\cdot \hbox {cm}^{-3}\)) established by ANP 45/2014 [22].
Note that, even though BSO and BSG samples thermally degraded for 3 and 6 h have undergone oxidation, the density values are still within the legislated limits. However, the use of these samples as engine fuels should only be considered after analyzing other regulated parameters. In addition, it is observed in Fig. 1b that as the thermal degradation time increases, the density values increases accordingly as a result of generated oxidation products, e.g., insoluble sediment. A major cause for this change is the thermal instability of biodiesel, which generates the formation of insoluble sediments increasing the density [1, 6].
(a) Temperature dependence of density for BSO samples subjected to thermal degradation process for 0 h, 3 h, 6 h, 9 h, 12 h, 24 h and 36 h at \(140\,^{\circ }\hbox {C}\). (b) Density values at \(20\,^{\circ }\hbox {C}\) for BSO and BSG samples as function of the thermal degradation time. The filled area indicates the min and max values of 0.85–\(0.90\,\hbox {g}\cdot \hbox {cm}^{-3}\) specified by Brazilian resolution ANP 45/2014. The error bars for the experimental data are smaller than 0.001 %
Using density analysis to verify the outcome of a thermal degradation process is very useful, given that density and thermal degradation times are proportional; an increase in thermal degradation time generates an increase in density. Density values were higher for the soybean-derived biodiesel compared to the soybean–animal fats sample due to the degree of unsaturation present in each carbohydrate chain. Therefore, the greater the number of double bonds, the higher the density values tend to be [3].
The density of biodiesel also provides information directly relevant to kinematic viscosity, ignition quality and fuel atomization efficiency. Pelegrini et al. [27] noted, in their experiment, a correlation between density and the refractive index at \(15\,^\circ \)C indicating a poorer ignition quality for biodiesel samples whose density values were higher. Since density is implicit in kinematic viscosity, the following analysis took the rheological characteristics of the BSO and BSG samples into consideration.
The rheological analysis, which were useful to determine the thermal degraded behavior of the samples, indicates that, as the temperature increases, there is an apparent exponential decrease in dynamic and kinematic viscosities. However, to better view the results, the data were plotted on a logarithmic scale as a function of the inverse of the temperature (1 / T) as shown in Fig. 2a. To compare the BSO and BSG samples, the kinematic viscosity at \(40\,^{\circ }\hbox {C}\) is showed against the thermal degradation time in Fig. 2b. It shows similar behavior for both types of biodiesel, indicating that viscosity increases as thermal degradation time increases. This behavior might be the result of the formation of free fatty acids, isomerization of cis double bonds to trans, and the formation of products with high molecular weight [1, 3]. The viscosity increase is also associated with isomerization reactions and polymerization [17,18,19]. Therefore, the accelerated degradation of these samples at high temperature leads to the formation of gums and polymerized compounds.
Biodiesel thermal instability is one of the major factors for density and viscosity changes. As it is shown in Fig. 2b, the change in viscosity with the thermal degradation time for BSO is much greater than that for BSG. Unsaturated compounds are significantly more reactive to oxidation than saturated compounds [4, 6, 7, 14]. Thus, it is possible to verify by the thermal degradation process which samples are more unstable with regard to the viscosity analysis.
The activation energy was calculated to verify the sensitivity of viscosity due to the temperature change. The higher the activation energy, the more influence the temperature has on the viscosity. In fact, it means that a high activation energy indicates a more rapid change in viscosity with temperature. Figure 3a shows the activation energy values obtained by linear regression as a function of the thermal degradation time.
(a) Temperature dependence of dynamic viscosity (\(\eta \)) for BSO degraded samples. The solid lines represent the linear fit using Eq. 2. (b) Kinematic viscosity of samples as a function of thermal degradation time at \(40\,^{\circ }\hbox {C}\). The filled area indicates the min and max values specified by Brazilian resolution ANP 45/2014
The activation energy is an important parameter for the characterization of fuels. It shows the energy needed to carry out the breaking of chemical bonds during the different steps of the chain reaction caused by the oxidation process. Typically, thermally degraded samples with lower activation energy require less energy to accomplish breakage of chemical bonds [11].
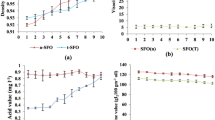
The volumetric thermal expansion coefficient (\(\beta \)) increases with the temperature for all the analyzed samples and decreases with the thermal degradation time, as shown in Fig. 3a. The study related to the calorific value indicated that both biodiesels, BSG and BSO, suffer a loss of heat of combustion as the thermal degradation time increases. As biodiesel undergoes oxidation, there is loss of hydrogen molecules and the addition of oxygen molecules to the fuel chain [5], a fact that contributes to a decrease in the calorific value of these samples. Figure 3b shows the gross calorific value behavior due to the increase of the thermal degradation time (black curves) and the percentage decrease of the gross calorific value (red curves). The maximum heat of combustion percentage loss for these samples were 12.4 % for BSG and 11.7 % for BSO. Even though the heat of combustion is not in any of the standard biodiesel quality specification methods, it can be seen that it is an additional property to verify thermal degradation or oxidation of a given sample.
The acid value or acid number is defined as the mass of potassium hydroxide (KOH) in milligrams required to neutralize one gram of free fatty acid. This qualitative index indicates the fuel’s conservation state during storage. Since biodiesel and its blends are more prone to oxidation than neat vegetable oils and petrodiesel, they can develop a wide variety of alcohols, aldehydes, peroxides, insoluble gums, sediments, etc., causing acidity in the fuel [1, 4, 12, 14, 17, 19]. Thus, the acid value is mostly used to evaluate storage conditions. According to EN, ASTM, and ANP biodiesel testing quality standards, the acid value must not exceed \(0.50\,\hbox {mg}\,\text {KOH}\,\hbox {g}^{-1}\).
BSO and BSG under 0 h, 3 h, and 6 h degradation times are within the standard requirements for acid number. Figure 4 shows the experimental data for the acid values of all samples. It can be observed that the vegetable-oil-based biodiesels have slightly lower acid number than the oil-fat blend biodiesel. A comparison of BSO and BSG samples for 36 h clearly shows that the BSO sample becomes higher in acidity than the BSG one. A high acid number is related to the fact that the oil and/or fat is suffering breakage in its chain, releasing fatty acids. Therefore, the BSO samples suffer greater breakage in their chains and release greater amounts of fatty acids, which can be clearly seen for the 36 h sample.
The optical interferometric measurements as function of temperature were performed for all samples to obtain the temperature coefficient of the refractive index (\(\hbox {d}n/\hbox {d}T\)). As \(\hbox {d}n/\hbox {d}T\) is always negative for these samples, \(-\hbox {d}n/\hbox {d}T\) values are presented to simplify discussion of the magnitude of this parameter. The average \(\hbox {d}n/\hbox {d}T\) obtained in the temperature range is shown in Fig. 4 (red curves).
Note that the behavior of \(\hbox {d}n/\hbox {d}T\) is similar for both samples. For degradation time up to 6h, the absolute value of \(\hbox {d}n/\hbox {d}T\) is approximately constant. However, it decreases (approximately 9 %) for thermally degraded times above 9 h. According to Shen et al. [26], the \(\hbox {d}n/\hbox {d}T\) of samples derived from Canadian tar sands is closely correlated with the cetane number. So, the smaller the magnitude of \(\hbox {d}n/\hbox {d}T\), the lower the cetane number. Apparently, the same correlation is observed for biodiesel samples. The cetane number is also related to chain length and degree of unsaturation [2]. In engine usage, a lower cetane number causes a further delay of ignition, and therefore, a lost in quality of combustion. Further studies are needed to affirm the correlation between cetane number, refractive index, chain length and degree of unsaturation for biodiesel.
The FTIR analysis of both types of biodiesel intended to verify the functional groups present in each one of the samples. The number of unsaturation present in each sample, due to its feedstock, can be estimated observing the wavenumbers \(3009\,\hbox {cm}^{-1}\) and \(722\,\hbox {cm}^{-1}\), which are related with the cis C\(=\)C bond [11]. Figure 5a, b compares the infrared spectra of undegraded BSO and BSG in the above mentioned peaks. It corroborates the information related to the amount of unsaturated acids present in each type of biodiesel according to the literature. As previously mentioned, the feedstock is influential in the composition of the fatty acids. The amount of fatty acids found in each carbohydrate chain is mainly linked to its feedstock. Vegetable-oil-based biodiesels present a larger amount of unsaturation when compared to the animal-fat-produced biodiesel. The greater unsaturation of vegetable-oil-based biodiesel is shown in Fig. 5 by the band broadening and the intensification of the peak compared to the animal-fat-produced biodiesel. Both of these elements indicate the presence of more unsaturations in BSO than in BSG.
The FTIR analysis of the thermal degraded samples showed variations in the infrared spectrum. Figure 5c for BSG and (e) for BSO indicates changes in the vibrational modes. Since the characteristic biodiesel peak is at \(1745\,\hbox {cm}^{-1}\), the wavenumbers from \(1660\,\hbox {cm}^{-1}\) to \(1820\,\hbox {cm}^{-1}\) were analyzed to check the degree of thermal modification of the samples, Fig. 5d for BSG and (f) for BSO. These changes in shape and intensity of the peak indicate a degraded biodiesel quality. There is an apparent widening of the bands as the thermal degradation time increases. It indicates that the sample has suffered some sort of degradation, e.g., thermal or oxidative. The subproduct of the oxidation process maintains the group C\(=\)O, associated with the ester in the sample’s structure and incorporates, as biodiesel is thermally degraded, the C\(=\)O band resultant from oxidation of aldehydes. For this reason, there is an increase in the band area, typically resulting in the widening of the band. Furthermore, the increase absorption in the region between \(1700\,\hbox {cm}^{-1}\) and \(1740\,\hbox {cm}^{-1}\), which corresponds to different C\(=\)O species, is a strong indicator of a fuel’s degradation [20, 28, 29]. Note that, as absorption increases, it causes, within this region, a widening of such band, which is the result of the sum of different bands related to various degradation products containing links to C\(=\)O.
FTIR spectra for undegraded BSG and BSO samples: (a) full spectrum and (b) near wavenumber \(3009\,\hbox {cm}^{-1}\) and \(722\, \hbox {cm}^{-1}\). FTIR spectra of degraded BSG sample: (c) full and (d) near \(1745\,\hbox {cm}^{-1}\). FTIR spectra of degraded BSO sample: (e) full spectrum and (f) near \(1745\,\hbox {cm}^{-1}\)
4 Conclusions
We presented a systematic study of the physical and chemical properties of biodiesels under accelerated thermal degradation. The thermal degradation process in a biodiesel sample can be observed by analyzing its different properties. The results suggest that the date related to the viscosity and acid values presented the most noticeable dependence upon the thermal degradation time. By comparing the undegraded and the thermally degraded (36 h) samples, the kinematic viscosity presented an increase of 970 % for BSG and 2300 % for BSO. On the other hand, the acid number varied 390 % for BSG and 660 % for BSO. By knowing the properties, which are more susceptible to thermal degradation, future studies can focus on viscosity and acid value, for instance. The change in all the studied property values with the thermal degradation time indicates fuel quality deterioration, making these fuels inadequate for use in engines. The caloric value corroborates this information, as a 12 % reduction indicates a decrease in the combustion capacity of these samples subsequent to the thermal degradation process.
References
S. Jain, M.P. Sharma, Renew. Sust. Energy Rev. 14, 667 (2010)
G. Knothe, Prog. Energy Combust. 36, 364 (2010)
G. Knothe, Fuel Process. Technol. 88, 669 (2007)
S. Jain, M.P. Sharma, Renew. Sustain. Energy Rev. 15, 438 (2011)
J. Pullen, K. Saeed, Renew. Sustain. Energy Rev. 16, 5924 (2012)
Z. Yaakob, B. Narayanan, S. Padikkaparambil, S. Unni, M. Akbar, Renew. Sustain. Energy Rev. 35, 136 (2014)
R. Dunn, J. Am. Oil Chem. Soc. 79, 915 (2009)
R. Dunn, Energy Fuel 22, 657 (2008)
M.M. Conceio, V.J. Fernandes Jr., A.S. Araujo, M.F. Farias, I.M.G. Santos, A.G. Souza, Energy Fuel 21, 1522 (2007)
F. Lacoste, L. Lagardere, Eur. J. Lipid. Sci. Tech. 105, 149 (2003)
H. Li, S. Niu, C. Lu, S. Cheng, Manage. Energy Convers. 98, 81 (2015)
R. Ferrari, V. Oliveira, A. Scabio, Sci. Agric. (Piracicaba, Braz.) 62, 291 (2005)
G. Knothe, R0. Dunn, J. Am. Oil. Chem. Soc. 80, 1021 (2003)
A. Monyem, J.H. Van Gerpen, Biomass Bioenerg. 20, 317 (2001)
A. Monyem, J.H. Van Gerpen, M. Canakci, Trans. ASAE 44, 35 (2001)
A. Monyem, M. Canakci, J.H. Van Gerpen, Appl. Eng. Agric. 16, 373 (2000)
W.C. Silva, M.P.P. Castro, V.H. Perez, F.A. Machado, L. Mota, M.S. Sthel, Energy 114, 1093 (2016)
R. Lin, Y. Zhu, L.L. Tavlarides, Fuel 117, 981 (2014)
A.M. Ashraful, H.H. Masjuki, M.A. Kalam, S.M.A. Rahman, M. Habibullah, M. Syazwan, Energy Fuels 28, 1081 (2014)
J. Zhou, Y. Xiong, Y. Gong, X. Liu, Fuel 202, 23 (2017)
E.L. Savi, L.S. Herculano, G.V.B. Lukasievicz, A.S. Torquato, M.L. Baesso, N.G.C. Astrath, L.C. Malacarne, Energy Fuel 31, 7 (2017)
Brazilian Agency of Petroleum, Natural Gas and Biodiesel (ANP). Resolução ANP 45, 25.8.2014 (2015)
L. Grunberg, A. Nissan, Nature 164, 799 (1949)
A. Steimacher, A.N. Medina, A.C. Bento, J.H. Rohling, M.L. Baesso, V.C.S. Reynoso, S.M. Lima, M.N. Petrovich, D.W. Hewak, J. Non-Cryst, Solids 348, 240 (2004)
Q. Wen, J. Shen, R. Gieleciak, K.H. Michaelian, J.H. Rohling, N.G.C. Astrath, M.L. Baesso, Int. J. Thermophys. 35, 930 (2014)
J. Shen, G.C. Astrath, P.R.B. Pedreira, F.B. Guimares, R. Gieleciak, Q. Wen, K.H. Michaelian, C. Fairbridge, L.C. Malacarne, J.H. Rohling, M.L. Baesso, Fuel 163, 324 (2016)
B. Pelegrini, E. Sudati, F. R, A. Moreira, I. Ferreira, A. Sampaio, N. Kimura, M. Lima, Fuel 199, 627 (2017)
D.M.M. Pinho, V.O. Santos Jr., V.M.L. Santos, M.C.S. Oliveira, M.T. Silva, P.G.T. Piza, A.C. Pinto, M.J.C. Rezende, P.A.Z. Suares, Fuel 136, 136 (2014)
S.V. Araújo, B.S. Rocha, F.M.T. Luna, E.M. Rola Jr., D.C.S. Azevedo, C.L. Cavalcante Jr., Fuel Process. Technol. 92, 1152 (2011)
Acknowledgements
We would like to acknowledge the Brazilian agencies: Coordination for the Improvement of Higher Education Personnel Foundation (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)), National Board for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ)), and State of Paraná Araucária Foundation for Support to Scientific and Technological Development (Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná (FA)) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the selected papers presented at the 19th International Conference on Photoacoustic and Photothermal Phenomena.
Rights and permissions
About this article
Cite this article
Regatieri, H.R., Savi, E.L., Lukasievicz, G.V.B. et al. Investigation of Thermophysical Properties of Thermal Degraded Biodiesels. Int J Thermophys 39, 73 (2018). https://doi.org/10.1007/s10765-018-2393-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-018-2393-0