Abstract
Thermal properties such as thermal conductivity, heat capacity, and thermal diffusivity and physical properties such as calorific value, density, and kinematic viscosity of liquid fuels play a significant role in the combustion process. These properties vary with the chemical structures and the fatty acid profiles of biodiesels. Making correlations for various properties with respect to fatty acid profile helps to analyze the combustion process. Most of the reported works generated correlations using data taken from the literature, and the correlations based on the fatty acid profile for thermal properties of biodiesel are unavailable. As coconut oil, sunflower oil, and palm oil have entirely different fatty acid profiles, in this study, biodiesels produced from these three waste cooking oils and their three hybrids (each in a 1:1 ratio) were used for biodiesel production, followed by correlation formulation. Higher thermal conductivity (0.2211 W mK−1) and thermal diffusivity (0.2459 mm2 s−1) and lower heat capacity (0.8993 MJ m−3 K−1), density (862.8 kg m−3), kinematic viscosity (2.71 cSt), and calorific value (36.73 cSt) were observed for coconut-based biodiesels compared with other biodiesels. The addition of coconut content to hybrid biodiesel enhanced thermal conductivity and thermal diffusivity and reduced heat capacity, density, and kinematic viscosity. From the experimental data of thermal conductivity, heat capacity, thermal diffusivity, density, kinematic viscosity, and calorific value, empirical correlations were proposed with the fatty acid profile. Good agreement was obtained between experimental and calculated values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a fascinating alternative to petroleum diesel as it is renewable, biodegradable, less polluting, and non-toxic and can be used in the available diesel engines without any alterations [1,2,3]. Biodiesel can be produced from different vegetable oils and animal fats using the transesterification reaction, which converts triglycerides of oils and fats to mono-alkyl esters (biodiesel) [4, 5]. Biodiesel is composed of complex mixtures of fatty acid esters with various carbon chain lengths and degrees of saturation, and their chemical structure varies from biodiesel to biodiesel. Thermal conductivity is a significant thermo-physical property with respect to the design, optimization, modeling, and simulation [6, 7] and is also vital to determining the combustion efficiency of diesel engines [8]. The thermal and physical properties of biodiesel vary for different biodiesels due to their varying chemical structures [9]. Alviso et al. [10] formulated correlations for physicochemical properties such as kinematic viscosity, pour point, cold filter plugging point, flash point, iodine number, and cetane number as functions of fatty acid profile. Alviso et al. [10] collected details of biodiesels produced from 48 origins, which include edible and non-edible sources, and since the data collected were dispersed, genetic programming was used instead of simple regression strategies, which prevents the usage of simple regression strategies. The authors concluded that, even though generic regression models with third-degree terms predicted the properties, these models were not optimal and suggested using simple models to predict physicochemical properties of biodiesels. Agarwal et al. [11] produced biodiesel from soybean, sunflower, safflower, mustard, linseed, groundnut, jatropha, palm, karanja, and mahua oils and determined properties such as cloud point, pour point, flash point, fire point, viscosity, density, saponification value, and iodine value using linear regression analysis. Scatter plots for all the properties, excluding flash and fire points, were observed closer to a straight line.
Giakoumis et al. [12] formulated correlations for biodiesel properties such as density, lower and higher heating values, kinematic viscosity, and cetane number in terms of fatty acid profile. The primary data from the literature were used to make correlations using multiple linear regression analysis. Derived correlations for cetane number and density were observed successful in prediction. For kinematic viscosity and both the heating values, the correlations were weaker. Kumbhar et al. [13] performed statistical analysis to formulate mathematical models to predict biodiesel properties such as heating value, cetane number, density, and viscosity in terms of fatty acid composition, applying multiple linear regression analysis. The correlations were validated with the data from the literature and found that models developed for density and cetane number were successful in predicting, and models developed for heating value and kinematic viscosity were observed to be ineffective.
Hong et al. [14] formulated correlations to predict biodiesel properties such as oxidation stability, heating value, and kinematic viscosity using various fatty acid alkyl esters, which are building blocks of biodiesel. The predicted results were compared with the properties of methyl and ethyl esters of soybean oil, canola oil, olive oil, grape seed oil, corn oil, beef tallow, and lard. Average absolute error ranges from 0.14 to 1.42, 0.2 to 4.3, and 0.4 to 7.5% were observed for heating value, kinematic viscosity, and oxidation stability, respectively. Ramírez-Verduzco et al. [15] produced biodiesel from soybean and beef tallow and characterized. Also, they have formulated four correlations for density, higher heating value, viscosity, and cetane number in terms of molecular mass and degree of unsaturation and reported good agreement between experimental and calculated properties.
Ustra et al. [6] investigated the influence of temperature and chemical composition on the density, viscosity, and thermal conductivity of biodiesels from castor, soybean, and jatropha oils. The experimental results were used to make empirical relations and found similarities between calculated and experimental values. Soybean and jatropha biodiesels exhibit similar, and castor oil biodiesel showed higher thermal conductivity values. Fan et al. [7] determined the thermal conductivity of binary blends of methyl laurate with 1-pentanol, 1-butanol, and 1-propanol at a temperature range of 287–358 K. A correlation for estimating thermal conductivity was also proposed. Fan et al. [8] determined the thermal conductivity of methyl caprate, methyl laurate, and methyl myristate, which are the significant biodiesel components, at various pressures ranging from 0.1 to 15 MPa and temperatures ranging from 292 to 372 K. The obtained data were correlated as a polynomial function of pressure and temperature. Kumbhar et al. [13] predicted biodiesel properties such as density, kinematic viscosity, cetane number, and heating value using multiple linear regression analysis from the fatty acid composition. The proposed models were also validated with reported data from the literature. Giakoumis et al. [12] correlated density, kinematic viscosity cetane number, and heating value with fatty acid composition using multiple linear regression analysis. Density and cetane number correlations were highly statistical and successful in prediction. However, correlations for heating value and kinematic viscosity were weaker.
From the literature, most of the researchers have used reported data for the preparation of correlations. Correlations for thermal properties are also rare in the literature. In the present work, biodiesels were prepared from coconut waste cooking oil, sunflower waste cooking oil, palm waste cooking oil, and their binary hybrid waste cooking oils in the ratio of 1:1. Coconut oil has a short chain and saturated fatty acid as the major constituent, sunflower oil has a long chain with poly-unsaturated fatty acid as the major constituent, and palm oil has medium- to long-chain fatty acid chains with saturated and mono-unsaturated compounds as major constituents. Thus, the six biodiesel samples produced will have different compositions of fatty acid with various chain lengths and saturation. Thermal properties such as thermal conductivity, heat capacity, and thermal diffusivity and physical properties such as density, kinematic viscosity, and calorific value were determined and compared. Also, the fatty acid profiles of the six biodiesel samples obtained were determined and compared. Then, by using the obtained data, three correlations were formulated with respect to fatty acid composition by applying multiple linear regression analysis.
Materials and methods
Preparation of oil samples
Coconut oil, sunflower oil, and palm oil were purchased from the local market. 1:1 blends of coconut–sunflower, coconut–palm, and sunflower–palm were also made. These six oil samples were used to prepare waste cooking oils. Each oil sample is used for frying wheat dough slices 1 to 2 mm thick and 4 to 5 cm in diameter in batches at 180 °C. The frying process continued for 1 h by removing the fried food after it turned light brown color and adding a new batch of slices to the oil. Then, the oil was allowed to cool to room temperature after removing all traces of food. The same procedure was repeated three times for all three oils and labeled as HCO3 (three-time-heated coconut oil), HSO3 (three-time-heated sunflower oil), HPO3 (three-time-heated palm oil), produce HCSO3 (three-time-heated blend of coconut oil and sunflower oil), HCPO3 (three-time-heated blend of coconut oil and palm oil), and HSPO3 (three-time-heated blend of sunflower oil and palm oil).
Biodiesel production from different waste cooking oils
Six samples of biodiesels were prepared from HCO3, HSO3, HPO3, HCSO3, HCPO3, and HSPO3 by using methanol (6:1 methanol-to-oil ratio) and KOH (1% mass of oil) and labeled as HCME, HSME, HPME, HCSME, HCPME, and HSPME, respectively. Transesterification reaction was carried out using a magnetic stirrer reactor and an electrical heater, keeping a temperature of 60 °C for 1 h. Then, the product was settled in a separating funnel, and the glycerol was removed. After water washing, the obtained biodiesel was heated above 100 °C to remove moisture.
Determination of fatty acid profile
Fatty acid profiles of six biodiesel samples were determined using GCMS (Agilent Technologies, 7890 A GC with 5975C MS with a triple-axis detector). The oven temperature was initially kept at 80 °C and increased at the rate of 4 °C min−1 up to 230 °C, and the injector temperature was kept at 250 °C.
Measurement of thermal properties
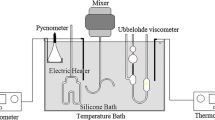
Thermal properties of biodiesel such as thermal conductivity, heat capacity, and thermal diffusivity were measured using the hot disk thermal constants analyser (Hot Disk TPS 500 S), which works based on the Transient Plane Source (TPS) method (ISO 22007-2). To avoid natural convection, specially designed sample holder for liquids, which consists of a small cell for liquid, was used. The relatively large metallic volume of the container ensures a stable temperature and negligible evaporation of the liquid.
Measurement of thermal properties
Density, kinematic viscosity, and calorific value of the biodiesel samples were determined using the portable digital density meter (ASTM D7777), Cannon–Fenske viscometer (ASTM D446), and bomb calorimeter (ASTM D5865-13), respectively.
Results and discussion
Fatty acid profile of biodiesel
Biodiesels are composed of saturated, mono-unsaturated, and poly-unsaturated fatty acid esters with different chain lengths and numbers of double bonds, which are dependent on the feedstocks. Molecules with double bonds are more fluid-like structures, and random molecular interaction increases with increased chain length. Thus, the kinematic viscosity of biodiesel increases with the number of carbon atoms and decreases with the number of double bonds in the fatty acid chain [16, 17]. The calorific value (CV) of biodiesel increases with the number of carbon atoms and decreases with the number of double bonds in the fatty acid chain [17, 18]. Density increases with the decrease in chain length and increases with unsaturation [17]. Saturated fatty acid molecules have higher melting points compared with unsaturated molecules and thereby poor cold flow properties [19].
Figure 1 demonstrates the chromatogram of all six biodiesel samples. The X-axis indicates the response time, and the Y-axis indicates the detector response (the abundance of the ions that reach the detector according to their mass-to-charge ratio). The relative abundance reveals information about the structure of the molecule. As shown in Table 1, HCME is composed of caprylic acid (4.64%), capric acid (4.62%), lauric acid (43.61%), myristic acid (22.76%), palmitic acid (10.93%), stearic acid (3.84%), oleic acid (8.04%), linoleic acid (1.48%), and eicosanoic acid (0.08%). The major constituent of HCME is lauric acid, a saturated fatty acid chain with 12 carbon atoms. HSME is composed of palmitic acid (7.49%), stearic acid (15.78%), oleic acid (17.24%), linoleic acid (58.02%), linolenic acid (0.12%), eicosanoic acid (0.37%), and behenic acid (0.98%). The major constituent of HSME is linoleic acid, an unsaturated fatty acid chain with 18 carbon atoms and two double bonds. HPME is composed of lauric acid (0.24%), myristic acid (1.02%), palmitic acid (40.33%), stearic acid (17.57%), oleic acid (30.81%), linoleic acid (9.59%), and eicosanoic acid (0.45%). The major constituent of HPME is palmitic acid (40.33%), a saturated fatty acid chain with 16 carbon atoms.
Hybrid biodiesels (HCSME, HCPME, and HSPME) showed new compositions of fatty acid chains. Adding sunflower and palm contents to coconut cooking oil enhanced the concentration of long-chain molecules such as stearic acid (3.64% and 4.54%, respectively), oleic acid (7.32% and 15.49%, respectively), and linoleic acid (30.72% and 4.51%, respectively). The addition of coconut and sunflower contents to palm oil reduced the concentration of palmitic acid (13.13% and 15.7%, respectively). The addition of coconut and palm contents to sunflower oil reduced the concentration of linoleic acid (25.82% and 25.07%, respectively) and enhanced the concentration of lauric acid, myristic acid, and palmitic acid.
Thermal properties of biodiesel samples
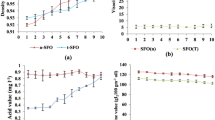
The thermal conductivity of a material is its ability to transfer heat by conduction [22]. Fuels with higher thermal conductivity transfer heat quickly throughout the medium and easily heat up uniformly, enhancing the evaporation rate [21]. Experimental and theoretical values of various thermal properties of six biodiesel samples are shown in Table 2. Thermal conductivity of HCME, HSME, HPME, HCSME, HCPME, and HSPME was observed as 0.2211 W mK−1, 0.1980 W mK−1, 0.2072 W mK−1, 0.2089 W mK−1, 0.2117 W mK−1, and 0.2020 W mK−1, respectively. A higher thermal conductivity was observed for HCME, and the addition of coconut oil content also improved the thermal conductivity of hybrid biodiesels HCSME (+ 0.0109 W mK−1) and HCPME (+ 0.0045 W mK−1) compared with HSME and HPME, respectively.
The heat capacity indicates the heat required to increase its temperature by unity [22]. Liquid fuels with lower heat capacity reach boiling temperature with minimum heat, which improves air–fuel mixing [23]. Heat capacity of HCME, HSME, HPME, HCSME, HCPME, and HSPME was observed as 0.8993 MJ m−3 K−1, 1.115 MJ m−3 K−1, 1.060 MJ m−3 K−1, 1.008 MJ m−3 K−1, 1.002 MJ m−3 K−1, and 1.082 MJ m−3 K−1, respectively. Lower heat capacity was observed for HCME, which may be due to the presence of lower chain length as heat capacity increases with chain length (molar mass) [24, 25]. Also, the addition of coconut content reduced the heat capacity of hybrid biodiesels HCSME (− 0.107 MJ m−3 K−1) and HCPME (− 0.058 MJ m−3 K−1) compared with HSME and HPME, respectively.
Thermal diffusivity is a measure of heat transport quality, and heat can transfer easily through the liquid medium with higher thermal diffusivity [26]. It is the ratio of heat conducted to heat stored, mathematically the ratio of thermal conductivity to the product of density and heat capacity (K/ρCp) [27]. Thus, liquid fuels, with high thermal diffusivity, injected into the combustion chamber of an internal combustion engine can transfer heat and evaporate easily. Thermal diffusivity of HSME, HPME, HCSME, HCPME, and HSPME was observed as 0.2459 mm2 s−1, 0.1776 mm2 s−1, 0.1955 mm2 s−1, 0.2073 mm2 s−1, 0.2114 mm2 s−1, and 0.1868 mm2 s−1, respectively. Among the biodiesels tested, HCME was observed with higher thermal diffusivity, which may be due to reduced chain length, and the addition of coconut content enhanced the thermal diffusivity of hybrid biodiesels HCSME (+ 0.0297 mm2 s−1) and HCPME (+ 0.0159 mm2 s−1) compared with HSME and HPME, respectively.
Physical properties of biodiesel samples
Density is an important physical property of liquid fuels, which decides the quantity of fuel injected and injection timing, influencing the engine characteristics [28]. Higher density leads to the quick transmission of the pressure wave from the fuel pump to the injector resulting in advanced injection timing [29,30,31]. Experimental and theoretical values of physical properties such as density, kinematic viscosity, and CV of six biodiesel samples are shown in Table 3. The density of HCME was found to be minimum (862.8 kg m−3) compared with HSME (875.8 kg m−3) and HPME (864.8 kg m−3). Also, the addition of coconut content reduced the density of sunflower biodiesel (− 5.4 kg m−3) and palm biodiesel (− 0.7 kg m−3).
Fuel injection, atomization, and air–fuel mixture formation in the combustion chamber depend on the kinematic viscosity of the liquid fuel. Fuels with lower kinematic viscosity lead to better fuel atomization and air–fuel mixture formation [28, 30], whereas fuels with higher viscosity lead to advanced injection timing with mechanical injection system [29, 30, 32]. Among the biodiesels tested, HCME (2.71 cSt) was observed with lower kinematic viscosity compared with HSME (4.39 cSt) and HPME (4.56 cSt). The addition of coconut content reduced the kinematic viscosity of hybrid biodiesel by 0.84 cSt for sunflower-based biodiesel and 0.92 cSt for palm-based biodiesels. Hence, atomization and air–fuel mixing will be better for coconut-based biodiesels and their hybrids.
CV represents the heat content in the fuel and increases with the length of the carbon chain and reduces with the number of double bonds [17,18,19]. Among the biodiesels tested, HPME (39.75 MJ kg−1) was observed with the maximum CV compared with HCME (36.73 MJ kg−1) and HSME (38.88 MJ kg−1). The addition of palm and sunflower contents to coconut content improved the CV by 1.09 MJ kg−1 and 1.8 MJ kg−1, respectively.
Empirical correlations
Linear regression analysis was used to determine three correlations for thermal conductivity (K), heat capacity (c), thermal diffusivity (α), density (ρ), kinematic viscosity , and calorific value (CV). The correlations in terms of the different fatty acids are shown in Eqs. (1–6):
where (C12:0) is the percentage concentration of lauric acid methyl ester, (C16:0) is the percentage concentration of palmitic acid methyl ester, (C18:1) is the percentage concentration of oleic acid methyl ester, (C18:2) is the percentage concentration of linoleic acid methyl ester, and (C20:0) is the percentage concentration of behenic acid methyl ester. Other fatty acid methyl esters present were found to be not significant. The obtained regression models are plotted for thermal conductivity, thermal diffusivity, heat capacity, density, kinematic viscosity, and CV in Figs. 2, 3, 4, 5, 6, 7, respectively. R2 values were observed as 1 for the thermal conductivity, thermal diffusivity, heat capacity, kinematic viscosity, and CV and 0.94 for the density.
Conclusions
Biodiesels were produced from coconut waste cooking oil, sunflower waste cooking oil, palm waste cooking oil, and their binary hybrids of 1:1 ratio. Fatty acid profiles and thermal properties such as thermal conductivity, heat capacity, thermal diffusivity, density, kinematic viscosity, and calorific value were determined and compared. Also, the empirical correlations were proposed using linear regression analysis. The subsequent conclusion can be made from the investigation:
-
The substantial constituent of HCME is lauric acid (43.61%), a saturated fatty acid chain with 12 carbon atoms. The major constituent of HSME is linoleic acid (58.02%), an unsaturated fatty acid chain with 18 carbon atoms and two double bonds. Two significant constituents of HPME were observed as palmitic acid (40.33%), a saturated fatty acid chain with 16 carbon atoms, and oleic acid (30.81%), an unsaturated fatty acid chain with 18 carbon atoms and one double bond.
-
A higher thermal conductivity was observed for HCME compared with other biodiesels, and the addition of coconut oil content to hybrid biodiesel improved the thermal conductivity.
-
Lower heat capacity was observed with HCME, which may be due to the presence of lower chain length as heat capacity increases molecular mass. Also, the addition of coconut content reduced the heat capacity of hybrid biodiesels.
-
Among the biodiesels tested, HCME was observed with higher thermal diffusivity, which is due to reduced chain length and the addition of coconut content enhanced the thermal diffusivity of hybrid biodiesels.
-
The minimum density and kinematic viscosity were observed for HCME compared with other biodiesels. Also, the addition of coconut content reduced the density and kinematic viscosity of hybrid biodiesels. Hence, atomization and air–fuel mixing will be better for coconut-based biodiesels and their hybrids.
-
Among the biodiesels tested, HPME was observed with the maximum calorific value. The addition of palm and sunflower contents to coconut content improved the calorific value of hybrid biodiesels.
-
It was found that lauric acid methyl ester (C12:0), palmitic acid methyl ester (C16:0), oleic acid methyl ester (C18:1), linoleic acid methyl ester (C18:2), and behenic acid methyl ester (C20:0) were significant components for predicting the properties.
Obtained correlations can be used to predict physical and thermal properties of various biodiesels from their fatty acids profiles. Also, it helps to blend different biodiesels from various sources to obtain required physical and thermal properties.
References
Kathirvelu B, Subramanian S, Govindan N, Santhanam S. Emission characteristics of biodiesel obtained from jatropha seeds and fish wastes in a diesel engine. Sustain Environ Res. 2017;27(6):283–90. https://doi.org/10.1016/j.serj.2017.06.004.
Raman LA, Deepanraj B, Rajakumar S, Sivasubramanian V. Experimental investigation on performance, combustion and emission analysis of a direct injection diesel engine fuelled with rapeseed oil biodiesel. Fuel. 2019;246:69–74. https://doi.org/10.1016/j.fuel.2019.02.106.
Srinivasan GR, Shankar V, Sekharan SC, Munir M, Balakrishnan D, Mohanam A, Jambulingam R. Influence of fatty acid composition on process optimization and characteristics assessment of biodiesel produced from waste animal fat. Energy Source Part A. 2020. https://doi.org/10.1080/15567036.2020.1771477.
Adewale P, Dumont MJ, Ngadi M. Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew Sustain Energy Rev. 2015;45:574–88. https://doi.org/10.1016/j.rser.2015.02.039.
Ashok A, Gugulothu SK, Reddy RV, Gurel AE, Deepanraj B. Prediction-optimization of the influence of 1-pentanol/jatropha oil blends on RCCI engine characteristics using multi-objective response surface methodology. Renew Energy Focus. 2022;42:8–23. https://doi.org/10.1016/j.ref.2022.05.006.
Ustra MK, Silva JR, Ansolin M, Balen M, Cantelli K, Alkimim IP, Mazutti MA, Voll FA, Cabral VF, Cardozo-Filho L, Corazza ML. Effect of temperature and composition on density, viscosity and thermal conductivity of fatty acid methyl esters from soybean, castor and Jatropha curcas oils. J Chem Thermodyn. 2013;58:460–6. https://doi.org/10.1016/j.jct.2012.10.007.
Fan J, Cui B, Liu S, Song F, Wang X. Experimental studies on the thermal conductivity of methyl laurate component of biodiesel with three alcohols. J Chem Thermodyn. 2019;139:105881. https://doi.org/10.1016/j.jct.2019.105881.
Fan J, Liu S, Meng Q, Song F. Liquid thermal conductivity of three biodiesel compounds: methyl myristate, methyl laurate and methyl caprate. J Chem Thermodyn. 2021;155:106374. https://doi.org/10.1016/j.jct.2020.106374.
Fan J, Liu Q, Song F, Wang X, Zhang L. Experimental investigations on the liquid thermal conductivity of five saturated fatty acid methyl esters components of biodiesel. J Chem Thermodyn. 2018;125:50–5. https://doi.org/10.1016/j.jct.2018.05.019.
Alviso D, Artana G, Duriez T. Prediction of biodiesel physico-chemical properties from its fatty acid composition using genetic programming. Fuel. 2020;264:116844. https://doi.org/10.1016/j.fuel.2019.116844.
Agarwal M, Singh K, Chaurasia SP. Prediction of biodiesel properties from fatty acid composition using linear regression and ANN techniques. Indian Chem Eng. 2010;52(4):347–61. https://doi.org/10.1080/00194506.2010.616325.
Giakoumis EG, Sarakatsanis CK. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel. 2018;222:574–85. https://doi.org/10.1016/j.fuel.2018.02.187.
Kumbhar V, Pandey A, Sonawane CR, El-Shafay AS, Panchal H, Chamkha AJ. Statistical analysis on prediction of biodiesel properties from its fatty acid composition. Case Stud Therm Eng. 2022;30:101775. https://doi.org/10.1016/j.csite.2022.101775.
Hong IK, Jeon GS, Lee SB. Prediction of biodiesel fuel properties from fatty acid alkyl ester. J Ind Eng Chem. 2014;20(4):2348–53. https://doi.org/10.1016/j.jiec.2013.10.011.
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, del Rayo J-J. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel. 2012;91(1):102–11. https://doi.org/10.1016/j.fuel.2011.06.070.
Yalcin H, Toker OS, Dogan M. Effect of oil type and fatty acid composition on dynamic and steady shear rheology of vegetable oils. J Oleo Sci. 2012;61(4):181–7. https://doi.org/10.5650/jos.61.181.
Folayan AJ, Anawe PA, Aladejare AE, Ayeni AO. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019;5:793–806.
Knothe G. “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels. 2008;22(2):1358–64. https://doi.org/10.1021/ef700639e.
Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86(10):1059–70. https://doi.org/10.1016/j.fuproc.2004.11.002.
Asadi I, Shafigh P, Hassan ZF, Mahyuddin NB. Thermal conductivity of concrete: a review. J Build Eng. 2018;20:81–93. https://doi.org/10.1016/j.jobe.2018.07.002.
Aboalhamayie A, Festa L, Ghamari M. Evaporation rate of colloidal droplets of jet fuel and carbon-based nanoparticles: effect of thermal conductivity. Nanomaterials. 2019;9(9):1297. https://doi.org/10.3390/nano9091297.
Zhou LP, Wang BX, Peng XF, Du XZ, Yang YP. On the specific heat capacity of CuO nanofluid. Adv Mech Eng. 2010;2:172085. https://doi.org/10.1155/2010/172085.
Emmons HW. The film combustion of liquid fuel. ZAMM J Appl Math Mech. 1956;36(1–2):60–71. https://doi.org/10.1002/zamm.19560360105.
Crosthwaite JM, Muldoon MJ, Dixon JK, Anderson JL, Brennecke JF. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J Chem Thermodyn. 2005;37(6):559–68. https://doi.org/10.1016/j.jct.2005.03.013.
Blume A. Apparent molar heat capacities of phospholipids in aqueous dispersion. Effects of chain length and head group structure. Biochemistry. 1983;22(23):5436–42. https://doi.org/10.1021/bi00292a027.
Erdoğdu F. A review on simultaneous determination of thermal diffusivity and heat transfer coefficient. J Food Eng. 2008;86(3):453–9. https://doi.org/10.1016/j.jfoodeng.2007.10.019.
Biyikli S, Modest MF, Tarr R. Measurements of thermal properties for human femora. J Biomed Mater Res. 1986;20(9):1335–45. https://doi.org/10.1002/jbm.820200908.
Pratas MJ, Freitas S, Oliveira MB, Monteiro SC, Lima AS, Coutinho JA. Densities and viscosities of fatty acid methyl and ethyl esters. J Chem Eng Data. 2010;55(9):3983–90. https://doi.org/10.1021/je100042c.
Boehman AL, Morris D, Szybist J, Esen E. The impact of the bulk modulus of diesel fuels on fuel injection timing. Energy fuels. 2004;18(6):1877–82. https://doi.org/10.1021/ef049880j.
Sun J, Caton JA, Jacobs TJ. Oxides of nitrogen emissions from biodiesel-fuelled diesel engines. Prog Energy Combust Sci. 2010;36(6):677–95. https://doi.org/10.1016/j.pecs.2010.02.004.
Chauhan BS, Kumar N, Cho HM, Lim HC. A study on the performance and emission of a diesel engine fueled with Karanja biodiesel and its blends. Energy. 2013;56:1–7. https://doi.org/10.1016/j.energy.2013.03.083.
Palash SM, Kalam MA, Masjuki HH, Masum BM, Fattah IR, Mofijur M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew Sustain Energy. 2013;23:473–90. https://doi.org/10.1016/j.rser.2013.03.003.
Funding
No funding was received for conducting this study. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
MNM was involved in conceptualization, methodology, validation, investigation, resources, and writing—original draft. AS was involved in supervision and writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niyas, M.M., Shaija, A. Effect of fatty acid profiles of waste cooking oil biodiesels on their thermal and physical properties. J Therm Anal Calorim 148, 9225–9235 (2023). https://doi.org/10.1007/s10973-023-12279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12279-x