Abstract
Endotoxemia induced by lipopolysaccharide (LPS) is an extremely severe syndrome identified by global activation of inflammatory responses. Neutrophil extracellular traps (NETs) play an important role in the development of endotoxemia. Histone hypercitrullination catalyzed by peptidylarginine deiminases (PADs) is a key step of NET formation. We have previously demonstrated that simultaneous inhibition of PAD2 and PAD4 with pan-PAD inhibitors can decrease NETosis and improve survival in a mouse model of LPS-induced endotoxic shock. However, the effects of PAD2 specific inhibition during NETosis and endotoxic shock are poorly understood. Therefore, in the present study, we aimed to investigate the effect of the specific PAD2 or PAD4 inhibitor on LPS-induced endotoxic shock in mice. We found that PAD2 inhibition but not PAD4 inhibition improves survival. Also, the levels of proinflammatory cytokines and NETosis were significantly reduced by PAD2 inhibitor. To our knowledge, this study demonstrates for the first time that PAD2 inhibition can reduce NETosis, decrease inflammatory cytokine production, and protect against endotoxin-induced lethality. Our findings provided a novel therapeutic strategy for the treatment of endotoxic shock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Lipopolysaccharide (LPS), also known as endotoxin, is a part of the exterior cell wall of gram-negative bacteria [1]. LPS can induce excessive activation of inflammatory responses and cause endotoxemia or endotoxic shock marked by fever, dyspnea, hypotension, multiple organ dysfunctions, and disseminated intravascular coagulation [2]. Endotoxemia is a life-threatening condition where the mortality rate can be as high as 30% [3]. Although the pathophysiological mechanisms underlying LPS-induced endotoxemia remain elusive, dysregulated neutrophil activation is considered to be an essential step [4].
Neutrophils are the most abundant immune cells in humans and exert indispensable antimicrobial functions [5]. After stimulation with LPS, neutrophils can extrude DNA, myeloperoxidase (MPO), neutrophil elastase (NE), and histones to form web-like structures called neutrophil extracellular traps (NETs); this process is termed NETosis [6]. NETosis is an inflammatory form of neutrophil death, which is an important antimicrobial strategy at the early stages of infections. However, excessive NETosis can lead to severe inflammatory responses and tissue damage [7]. As such, dysregulated NETosis has been implicated in the development of endotoxemia and endotoxemia-related organ dysfunction [8].
Studies have shown that histone citrullination is a vital process during NETosis [9, 10]. The citrullination of histones is catalyzed by a group of enzymes called peptidylarginine deiminases (PADs). PADs are calcium-dependent enzymes that citrullinate proteins via converting arginine residues into citrullines [11]. The PAD family consists of 5 members: PAD1, PAD2, PAD3, PAD4, and PAD6. However, only PAD2 and PAD4 have been found to be expressed in immune cells and enter the nucleus to citrullinate histones in adults [12, 13]. These findings indicate that PAD2 and PAD4 probably serve as inflammatory mediators and play important roles in the development of sepsis. In fact, our recent studies demonstrated that simultaneous inhibition of PAD2 and PAD4 with YW3-56, a pan-PAD inhibitor, rescued mice from LPS-induced lethal endotoxemia [14]. However, a study on PAD4 by Martinod et al. revealed that PAD4 deficiency does not protect mice from either CLP-induced sepsis or LPS-induced endotoxic shock [15]. The remained question is whether inhibition of PAD2 could improve animal survival in endotoxic shock.
In the present study, we first investigated whether inhibition of PAD2 with selective PAD2 inhibitor AFM32a [16], instead of PAD4 inhibitor (GSK484), could improve survival. We then determined whether AFM32a could decrease proinflammatory cytokine production, ameliorate acute lung injury in a mouse model of endotoxic shock, and reduce NETosis in vivo and in vitro.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (8–10 weeks) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and housed for 3 days before being used. All animal experiments were conducted in compliance with the animal protocol approved by the University of Michigan Institutional Animal Care and Use Committee.
Reagents
Lipopolysaccharide (LPS), dimethyl sulfoxide (DMSO), 4% paraformaldehyde, and 4′,6-diamidino-2-2phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO); rabbit anti-mouse myeloperoxidase (MPO) polyclonal antibody, HRP-conjugated goat anti-rabbit IgG antibody, mouse interleukin-1 beta (IL-1β) enzyme-linked immunosorbent assay (ELISA) kit, and mouse tumor necrosis factor-alpha (TNFα) ELISA kit were purchased from Abcam (Cambridge, MA); Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody, RPMI 1640 medium, and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA); neutrophil isolation kit for mouse was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany); Quant-iT™ PicoGreen™ dsDNA Assay Kit was purchased from Invitrogen (San Diego, CA); anti-citrullinated histone H3 (CitH3) rabbit polyclonal antibody was purchased from Abcam (Ab5103, Cambridge, MA); GSK484 was purchased from Cayman Chemical (Ann Arbor, MI); AFM32a was synthesized according to previously established methods [16].
LPS-Induced Endotoxic Model
Mice were randomly divided into four groups:
-
1)
Control: this group only received intraperitoneal (i.p.) administration of normal saline (NS) followed by DMSO (1 h later) injection i.p. as normal controls.
-
2)
LPS + DMSO: mice were injected with i.p. LPS (35 mg/kg, dissolved in NS), and DMSO was administered 1 h later.
-
3)
LPS + AFM32a: mice were administered (i.p.) with AFM32a (20 mg/kg) which is dissolved in DMSO (1 μL per kg mouse body weight) 1 h after LPS insult.
-
4)
LPS + GSK484: mice were subjected to GSK484 (40 mg/kg in DMSO) treatment 1 h after LPS administration.
In non-survival studies, animals were euthanized by CO2 24 h after LPS administration, and then serum and organs were harvested and stored at − 80 °C. In survival observational studies, mice were monitored for 10 consecutive days and then euthanized with CO2 at the endpoint of observation or whenever they were found moribund.
Mouse Neutrophil Isolation
The neutrophils were isolated from mouse bone marrow cells (tibiae and femora) by negative magnetic cell sorting using the neutrophil isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions. The purity of isolated neutrophils was determined by flow cytometry (CD45+/Ly6G+ expression profile), and consistently > 95% was considered no batch to batch variation.
NETosis Induction In Vitro
Isolated mouse neutrophils were induced to undergo NETosis as follows. In brief, mouse neutrophils were resuspended in RPMI 1640 medium with 5% FBS and then seeded in 4-well chamber slides or 24-well plates at concentration of 106/mL. After incubation for 30 min at 37 °C, the cells were challenged by LPS (10 μg/ml) with or without 2 μM AFM32a followed by another 1-h or 3-h incubation.
Immunofluorescent (IF) Staining of NETs In Vitro
IF staining of NETs was performed as previously described [17]. In short, after a 3-h incubation in 4-well chamber slides, medium containing LPS and AFM32a was added. The remaining cells were then washed with PBS and fixed with 4% paraformaldehyde for 20 min, followed by 1-h reaction at room temperature with rabbit anti-mouse MPO polyclonal antibody at 5 μg/mL. Thereafter, unbound antibody was removed, and Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody was used as secondary antibody (1:2000) for 1 h at room temperature. For staining of neutrophil DNA, the slides were mounted with solution of DAPI after being washed with PBS. NET formation was observed under an Olympus DP70 microscope with Olympus BH2-RFL-T3 burner (Olympus, Tokyo, Japan) at the magnification of × 400. DAPI filter set and FITC filter set were applied to visualize DNA and MPO, respectively.
Quantification of NETs
NETs in supernatants of LPS-treated mouse neutrophils or blood were quantified using a Quant-iT™ PicoGreen™ dsDNA Assay Kit following manufacturer’s instructions. Briefly, the supernatants of neutrophil cell culture or mouse sera were prepared and mixed with Tris-EDTATE buffer at the ratio of 1:3. The mixtures were then added into different cuvettes (1.0 mL per cuvette). Working solution of the PicoGreen reagent was then added into the cuvettes (1.0 mL each), followed by 5-min incubation at room temperature. Fluorescence were then read at 490 nm and interpolated using standard curve.
Determination of IL-1β and TNF-α In Vivo
To determine the levels of IL-1β and TNF-α in serum and lung, a mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit and a mouse TNF- α ELISA kit were used following the manufacturer’s instructions.
Acute Lung Injury Assessment
Mouse lung sections were stained with hematoxylin and eosin (H&E) to observe the histological alterations. Acute lung injury (ALI) scores [18] were graded by a licensed pathologist who was blinded to the experiments.
Western Blot
Serum proteins were loaded and separated by SDS-PAGE. The samples were then transferred to nitrocellulose membranes. After blocking at room temperature for 20 min, the membranes were incubated with anti-CitH3 rabbit polyclonal antibody overnight at 4 °C. Then the membranes were washed and incubated with HRP-conjugated goat anti-rabbit IgG antibody for 1 h at room temperature. The blots were imaged using chemiluminescent substrate.
Sample Size Calculation and Statistical Analysis
Power analysis is used to determine the minimum numbers of mice to be used. To achieve two-sided α = 0.05, a power of 0.8, and expected difference between experimental and control groups of 0.50, a minimum of 4 mice/group was required. Analysis was performed using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). Kaplan-Meier curves and log-rank tests were performed to analyze the survival curve. To make comparisons between three or more groups, one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test was performed. All data are presented as mean ± SEM in figures. All the experiments were conducted three independent times with 4 replicates each time. P value less than 0.05 was considered statistically significant.
RESULTS
PAD2 Inhibition Improved Survival in Lethal Endotoxic Shock
Mice were treated with DMSO or AFM32a 1 h after LPS insult. Mice in the sham group received DMSO as a vehicle control. Survival was monitored for 240 h. No mortality was observed in sham group. Six out of eight animals survived in the LPS + AFM32a group, while LPS + DMSO mice and LPS + GSK484 mice had 100% mortality rate (Fig. 1). The results indicate that a PAD2-selective inhibitor improved survival. Since treatment with specific PAD4 inhibitor GSK484 had no survival benefits, we did not study it furthermore.
PAD2 inhibition improved survival in lethal endotoxic shock. C57BL/6J mice were randomly divided into 4 groups: (1) DMSO (control, n = 8), (2) LPS (35 mg/kg) + DMSO (n = 8), (3) LPS (35 mg/kg) + AFM32a (20 mg/kg) (n = 8), and (4) LPS (35 mg/kg) + GSK484 (40 mg/kg). Survival was monitored for 10 consecutive days. AFM32a remarkably improved survival compared with LPS + DMSO (P = 0.0052) and LPS + GSK484 (P = 0.0466). DMSO, dimethyl sulfoxide; LPS, lipopolysaccharide.
PAD2 Inhibition Reduced Systemic Proinflammatory Cytokines
To determine the effect of PAD2 inhibition on systemic inflammatory responses, the levels of IL-1β and TNF-α in serum were measured after treatment. The concentration of serum IL-1β in the LPS + DMSO group (2715 ± 521.9 pg/mL) was approximately 5 times higher than that in LPS + AFM32a group (487.5 ± 210.5 pg/mL, P < 0.0001) (Fig. 2a). AFM32a also decreased serum levels of TNF-α dramatically compared with LPS + DMSO group (173.5 ± 4.7 vs 353 ± 18.7 pg/mL, P < 0.001, respectively) (Fig. 2b). These findings indicate that selective inhibition of PAD2 suppresses systemic inflammatory responses.
PAD2 inhibition decreased proinflammatory cytokines in serum. Mouse were randomly divided into 3 groups (n = 3): group DMSO, group LPS + DMSO, and group LPS + AFM32a. a ELISA results show that AFM32a greatly reduced IL-1β in serum 24 h after LPS insult. b ELISA results show that TNF-α in serum 24 h after LPS insult was reduced by AFM32a. Data were shown as a representative of three independent experiments. All data in figures were presented as mean ± SEM. IL, interleukin; TNF, tumor necrosis factor; DMSO, dimethyl sulfoxide; LPS, lipopolysaccharide; ns, no significance; **P < 0.01; ***P < 0.001; ****P < 0.0001.
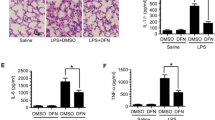
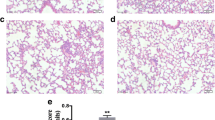
PAD2 Inhibition Alleviates Endotoxic Shock-Induced Acute Lung Injury
Due to the unique blood supply, the lung is one of the most vulnerable organs during endotoxemia [19]. Therefore, we further investigated therapeutic effects of AFM32a on LPS-induced pulmonary injury. The levels of IL-1β and TNF-α in lung tissues were determined. Similar to the results in serum, AFM32a significantly reduced IL-1β (333 ± 125.1 vs 559.6 ± 32.2 pg/mg, P = 0.0104, respectively) and TNF-α (25.1 ± 1.2 vs 40.9 ± 6.6 pg/mg, P = 0.0020, respectively) compared with LPS + DMSO group (Fig. 3a, b). Additionally, AFM32a notably alleviated acute lung injury according to morphological analyses. In the LPS + DMSO group, increased inflammatory cell infiltration, thickening of alveolar wall, and erythrocyte leakage were observed which were significantly reversed by AFM32a treatment (Fig. 3c). These findings indicate that PAD2 inhibition alleviates endotoxic shock-induced lung inflammation and restores pulmonary function.
PAD2 inhibition alleviated endotoxic shock-induced lung inflammation. Lung tissues were harvested 24 h after LPS insult. a and b ELISA results showed that proinflammatory cytokines (IL-1β and TNF-α) in the lung 24 h after LPS insult were significantly reduced by AFM32a. All data in figures were presented as mean ± SEM. c H&E staining of lung sections and ALI scores showed that AFM32a alleviated lung injury caused by LPS-induced endotoxic shock. Data were shown as a representative of three independent experiments. Scale bar, 20 μm. DMSO, dimethyl sulfoxide; LPS, lipopolysaccharide; ALI, acute lung injury; IL, interleukin; TNF, tumor necrosis factor; ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
PAD2 Inhibition Decreases NET Formation In Vitro
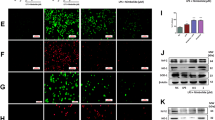
Next, we investigated the effect of PAD2 inhibition on NET formation, which may mechanistically explain how PAD2 inhibition suppresses inflammation. Neutrophils isolated from mice were challenged with LPS (100 ng/mL) and then treated with AFM32a or DMSO (2 μM). Supernatants were collected to measure extracellular DNA levels, an indicator of NETosis [20]. We found that LPS stimulation for 3 h significantly increases NETosis compared with the sham group [(4695 ± 1979) vs (2081 ± 132.1) arbitrary units (au), P = 0.0007, respectively] and that AFM32a treatment leads to a remarkable decrease in extracellular DNA levels compared with the LPS + DMSO group [(2831 ± 275.5) vs (4695 ± 1979) au, P = 0.0115, respectively] (Fig. 4a). We also observed increased extracellular DNA in the control group. This increase could be attributed to the neutrophils undergoing other forms of death such as apoptosis and necrosis. To directly demonstrate that NETosis was decreased by PAD2 inhibition, we co-localized DNA and MPO (a component of NETs) to visualize NETs using immunofluorescence. As shown in Fig. 4b, LPS-induced NET formation was obviously diminished by AFM32a treatment, whereas there was limited NETosis in sham group. These results suggest that PAD2 inhibition prevents LPS-stimulated neutrophils from releasing NETs.
PAD2 inhibition decreased NET formation in vitro. a The graph showed the levels of extracellular DNA in supernatant of cultured mouse neutrophils 1 h and 3 h after LPS treatment (100 ng/ml) with or without AFM32a (2 μM). Extracellular DNA was significantly reduced by AFM32a compared with group LPS + DMSO. b Results of immunofluorescence staining showed that AFM32a significantly prevented NET formation. DNA was stained with DAPI (blue), and MPO was stained with rabbit anti-mouse MPO antibody and Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (green). NETs were recognized as scattered DNA co-localized with MPO (red arrows). Scale bar, 50 μm. DMSO, dimethyl sulfoxide; LPS, lipopolysaccharide; MPO, myeloperoxidase; au, arbitrary units; ns, no significance; *P < 0.05; ***P < 0.001.
PAD2 Inhibition Decreases NET Formation In Vivo
Next, we evaluated the inhibitory effect of AFM32a on NETosis in vivo. Circulating double strand DNA (dsDNA) is a widely used marker of NETosis [21]. The results show that serum levels of circulating dsDNA in LPS + DMSO group are notably increased (6.483 ± 0.522 μg/mL) compared with DMSO group (0.589 ± 0.131 μg/mL) (P < 0.0001). A modest but significant decrease in circulating dsDNA was observed in the LPS + AFM32a group (4.817 ± 0.679 μg/mL), relative to the LPS + DMSO group (P < 0.05) (Fig. 5a). We further detected the levels of serum CitH3, a marker of NETosis in vivo. Consistent with the levels of dsDNA, LPS-induced CitH3 production was significantly reduced by AFM32a (Fig. 5b). These results suggest that PAD2 inhibition also reduces NET formation in vivo.
DISCUSSION
Host responses to LPS-induced endotoxic shock are marked by systemic inflammation [22]. These responses are predominantly mediated by the activation of neutrophils. Neutrophils represent the foremost line of the innate immune system, and dysregulated neutrophil activation plays an important role in the development of endotoxic shock [23, 24]. During endotoxic shock, neutrophils can overproduce proinflammatory cytokines and cast excessive NETs, which subsequently cause multiple organ dysfunction and death [25, 26]. In the present study, we sought to investigate the effects of selective PAD2 inhibition in a mouse model of LPS-induced endotoxic shock. Consistent with the previous findings, LPS-induced endotoxic shock notably increased the level of circulating NETs and proinflammatory cytokines and resulted in severe acute lung injury and high mortality [15, 27]. Notably, selective PAD2 inhibition instead of PAD4 inhibition improved survival. Moreover, we showed that AFM32a also alleviates systemic inflammation and acute lung injury and decreases NET levels in vivo and in vitro. These data imply that PAD2 plays a key role in the onset of endotoxic shock and secondary acute lung injury.
NETs are regarded as “a double-edged sword” [6, 28]. On the one hand, NETs entrap pathogens (e.g., bacteria and fungi) to immobilize and eliminate them, which serves as a vital mechanism of pathogen clearance [7]. On the other hand, DNA and citrullinated histones in NETs are implicated in causing damage to the host [20, 29,30,31]. Dysregulated NETosis was observed during endotoxemia, which is responsible for the progression of systemic inflammation to organ injury [32, 33]. As such, previous studies have found that decreasing the NET levels can improve survival and attenuate organ injury [30, 34, 35]. NET formation is generally associated with chromatin decondensation and histone hypercitrullination, which is catalyzed by PADs [12, 36]. Within recent years, it has been displayed that both PAD2 and PAD4 can translocate into nucleus and citrullinate histones [12, 13]. The effect of PAD4 inhibition on NETosis has been studied frequently [37,38,39], whereas the effect of PAD2 inhibition on NETosis has barely been explored. Here, we used AFM32a, a PAD2-selective inhibitor developed by the Thompson lab [16], and showed that this compound reduces NET formation in LPS-treated neutrophils. Previous studies showed that PAD4 plays an important role in NET formation and the knockout of pad4 gene can largely reduce the production of NETs [38, 39]. However, pad4 deficiency cannot improve survival during endotoxic shock in the absence of NETs [15], which is consistent with our findings. Besides, notable levels of extracellular DNA and histones were still detected in LPS-insulted pad4−/− animals, which were likely from necrotic cells [15]. In our study, we observed a significant decrease of circulating dsDNA after PAD2 inhibition in mice with endotoxic shock, suggesting that PAD2 inhibition not only suppresses NETosis but also is likely to enhance the clearance of dsDNA in circulation, which is possibly why PAD2 inhibition has a survival benefit.
PAD2 is the most widely expressed member among the PAD family and can citrullinate hundreds of proteins beyond histones [40, 41]. These citrullinated proteins often serve as inflammatory mediators that trigger inflammatory responses in vivo. PAD2 hyper-activation can result in dysregulation of immune responses in the host and is associated with multiple diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and even cancer [42,43,44]. Endotoxic shock is also caused by dysregulated inflammatory responses to LPS. Thus, inhibition of PAD2 can possibly decrease the citrullination of certain proteins and result in attenuated systemic inflammation [45]. This is another possible reason why inhibition of PAD2 can decrease levels of IL-1β and TNF-α in serum of AFM32a-treated endotoxic mice than those non-treated endotoxic shock mice.
IL-1β and TNF-α, as proinflammatory cytokines, can mediate the innate immune response and cause systemic inflammation that contribute to the pathogenesis of many diseases [46] as well as pulmonary damage (e.g., ALI). In this study, we demonstrated that PAD2 inhibition significantly reduced the levels of IL-1β and TNF-α in the lung and blood, which may explain why AFM32a can attenuate LPS-induced ALI.
In conclusion, our results show that selective PAD2 inhibition with AFM32a can improve survival and alleviate lung injury during LPS-induced endotoxic shock via attenuating systemic inflammation and decreasing levels of circulating NETs. Our findings provide the evidence that inhibition of PAD2 could be a novel therapeutic strategy for treatment of endotoxic shock.
References
Kulp, A., and M.J. Kuehn. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Review of Microbiology 64: 163–184. https://doi.org/10.1146/annurev.micro.091208.073413.
Opal, S.M. 2010. Endotoxins and other sepsis triggers. Contributions to Nephrology 167: 14–24. https://doi.org/10.1159/000315915.
Ramnath, R.D., S.W. Ng, A. Guglielmotti, and M. Bhatia. 2008. Role of MCP-1 in endotoxemia and sepsis. International Immunopharmacology 8 (6): 810–818. https://doi.org/10.1016/j.intimp.2008.01.033.
Grigoleit, J.S., J.R. Oberbeck, P. Lichte, P. Kobbe, O.T. Wolf, T. Montag, A. del Rey, E.R. Gizewski, H. Engler, and M. Schedlowski. 2010. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiology of Learning and Memory 94 (4): 561–567. https://doi.org/10.1016/j.nlm.2010.09.011.
Hirz, T., and C. Dumontet. 2016. Neutrophil isolation and analysis to determine their role in lymphoma cell sensitivity to therapeutic agents. Journal of Visualized Experiments 109: e53846. https://doi.org/10.3791/53846.
Yipp, B.G., and P. Kubes. 2013. NETosis: How vital is it? Blood 122 (16): 2784–2794. https://doi.org/10.1182/blood-2013-04-457671.
Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D.S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303 (5663): 1532–1535. https://doi.org/10.1126/science.1092385.
Camicia, G., R. Pozner, and G. de Larranaga. 2014. Neutrophil extracellular traps in sepsis. Shock 42 (4): 286–294. https://doi.org/10.1097/shk.0000000000000221.
Wong, S.L., M. Demers, K. Martinod, M. Gallant, Y. Wang, A.B. Goldfine, C.R. Kahn, and D.D. Wagner. 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine 21 (7): 815–819. https://doi.org/10.1038/nm.3887.
Li, R.H.L., G. Ng, and F. Tablin. 2017. Lipopolysaccharide-induced neutrophil extracellular trap formation in canine neutrophils is dependent on histone H3 citrullination by peptidylarginine deiminase. Veterinary Immunology and Immunopathology 193-194: 29–37. https://doi.org/10.1016/j.vetimm.2017.10.002.
Wang, S., and Y. Wang. 2013. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochimica et Biophysica Acta 1829 (10): 1126–1135. https://doi.org/10.1016/j.bbagrm.2013.07.003.
Wang, Y., M. Li, S. Stadler, S. Correll, P. Li, D. Wang, R. Hayama, L. Leonelli, H. Han, S.A. Grigoryev, C.D. Allis, and S.A. Coonrod. 2009. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. The Journal of Cell Biology 184 (2): 205–213. https://doi.org/10.1083/jcb.200806072.
Zhang, X., M. Bolt, M.J. Guertin, W. Chen, S. Zhang, B.D. Cherrington, D.J. Slade, C.J. Dreyton, V. Subramanian, K.L. Bicker, P.R. Thompson, M.A. Mancini, J.T. Lis, and S.A. Coonrod. 2012. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proceedings of the National Academy of Sciences of the United States of America 109 (33): 13331–13336. https://doi.org/10.1073/pnas.1203280109.
Liang, Y., B. Pan, H.B. Alam, Q. Deng, Y. Wang, E. Chen, B. Liu, Y. Tian, A.M. Williams, X. Duan, Y. Wang, J. Zhang, and Y. Li. 2018. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. European Journal of Pharmacology 833: 432–440. https://doi.org/10.1016/j.ejphar.2018.07.005.
Martinod, K., T.A. Fuchs, N.L. Zitomersky, S.L. Wong, M. Demers, M. Gallant, Y. Wang, and D.D. Wagner. 2015. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125 (12): 1948–1956. https://doi.org/10.1182/blood-2014-07-587709.
Muth, A., V. Subramanian, E. Beaumont, M. Nagar, P. Kerry, P. McEwan, H. Srinath, K. Clancy, S. Parelkar, and P.R. Thompson. 2017. Development of a selective inhibitor of protein arginine deiminase 2. Journal of Medicinal Chemistry 60 (7): 3198–3211. https://doi.org/10.1021/acs.jmedchem.7b00274.
Park, S.Y., S. Shrestha, Y.J. Youn, J.K. Kim, S.Y. Kim, H.J. Kim, S.H. Park, W.G. Ahn, S. Kim, M.G. Lee, K.S. Jung, Y.B. Park, E.K. Mo, Y. Ko, S.Y. Lee, Y. Koh, M.J. Park, D.K. Song, and C.W. Hong. 2017. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis. American Journal of Respiratory and Critical Care Medicine 196 (5): 577–589. https://doi.org/10.1164/rccm.201603-0596OC.
Deng, Q., T. Zhao, B. Pan, I.S. Dennahy, X. Duan, A.M. Williams, B. Liu, N. Lin, U.F. Bhatti, E. Chen, H.B. Alam, and Y. Li. 2018. Protective effect of tubastatin A in CLP-induced lethal sepsis. Inflammation 41 (6): 2101–2109. https://doi.org/10.1007/s10753-018-0853-0.
Gotts, J.E., and M.A. Matthay. 2016. Sepsis: Pathophysiology and clinical management. Bmj 353: i1585. https://doi.org/10.1136/bmj.i1585.
Pan, B., H.B. Alam, W. Chong, J. Mobley, B. Liu, Q. Deng, Y. Liang, Y. Wang, E. Chen, T. Wang, M. Tewari, and Y. Li. 2017. CitH3: A reliable blood biomarker for diagnosis and treatment of endotoxic shock. Scientific Reports 7 (1): 8972. https://doi.org/10.1038/s41598-017-09337-4.
Gloude, N.J., P. Khandelwal, N. Luebbering, D.T. Lounder, S. Jodele, M.N. Alder, A. Lane, A. Wilkey, K.E. Lake, B. Litts, and S.M. Davies. 2017. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood 130 (10): 1259–1266. https://doi.org/10.1182/blood-2017-05-782870.
van den Boogaard, M., B.P. Ramakers, N. van Alfen, S.P. van der Werf, W.F. Fick, C.W. Hoedemaekers, M.M. Verbeek, L. Schoonhoven, J.G. van der Hoeven, and P. Pickkers. 2010. Endotoxemia-induced inflammation and the effect on the human brain. Critical Care 14 (3): R81. https://doi.org/10.1186/cc9001.
Okeke, E.B., Z. Mou, N. Onyilagha, P. Jia, A.S. Gounni, and J.E. Uzonna. 2017. Deficiency of phosphatidylinositol 3-kinase delta signaling leads to diminished numbers of regulatory T cells and increased neutrophil activity resulting in mortality due to endotoxic shock. Journal of Immunology 199 (3): 1086–1095. https://doi.org/10.4049/jimmunol.1600954.
Stiel, L., F. Meziani, and J. Helms. 2018. Neutrophil activation during septic shock. Shock 49 (4): 371–384. https://doi.org/10.1097/SHK.0000000000000980.
Delabranche, X., L. Stiel, F. Severac, A.C. Galoisy, L. Mauvieux, F. Zobairi, T. Lavigne, F. Toti, E. Anglès-Cano, F. Meziani, and J. Boisramé-Helms. 2017. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock 47 (3): 313–317. https://doi.org/10.1097/SHK.0000000000000719.
Czaikoski, P.G., J.M. Mota, D.C. Nascimento, F. Sonego, F.V. Castanheira, P.H. Melo, G.T. Scortegagna, et al. 2016. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One 11 (2): e0148142. https://doi.org/10.1371/journal.pone.0148142.
Pieterse, E., N. Rother, C. Yanginlar, L.B. Hilbrands, and J. van der Vlag. 2016. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Frontiers in Immunology 7: 484. https://doi.org/10.3389/fimmu.2016.00484.
Gupta, S., and M.J. Kaplan. 2016. The role of neutrophils and NETosis in autoimmune and renal diseases. Nature Reviews. Nephrology 12 (7): 402–413. https://doi.org/10.1038/nrneph.2016.71.
Porto, B.N., and R.T. Stein. 2016. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Frontiers in Immunology 7: 311. https://doi.org/10.3389/fimmu.2016.00311.
McDonald, B., R.P. Davis, S.J. Kim, M. Tse, C.T. Esmon, E. Kolaczkowska, and C.N. Jenne. 2017. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129 (10): 1357–1367. https://doi.org/10.1182/blood-2016-09-741298.
Shen, X.F., K. Cao, J.P. Jiang, W.X. Guan, and J.F. Du. 2017. Neutrophil dysregulation during sepsis: An overview and update. Journal of Cellular and Molecular Medicine 21 (9): 1687–1697. https://doi.org/10.1111/jcmm.13112.
Kaufman, T., D. Magosevich, M.C. Moreno, M.A. Guzman, L.P. D'Atri, A. Carestia, M.E. Fandino, C. Fondevila, and M. Schattner. 2017. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clinical Immunology 183: 254–262. https://doi.org/10.1016/j.clim.2017.08.014.
Sakurai, K., T. Miyashita, M. Okazaki, T. Yamaguchi, Y. Ohbatake, S. Nakanuma, K. Okamoto, et al. 2017. Role for neutrophil extracellular traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo 31 (6): 1051–1058. https://doi.org/10.21873/invivo.11169.
Jimenez-Alcazar, M., C. Rangaswamy, R. Panda, J. Bitterling, Y.J. Simsek, A.T. Long, R. Bilyy, et al. 2017. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358 (6367): 1202–1206. https://doi.org/10.1126/science.aam8897.
Biron, B.M., C.S. Chung, X.M. O'Brien, Y. Chen, J.S. Reichner, and A. Ayala. 2017. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. Journal of Innate Immunity 9 (1): 22–32. https://doi.org/10.1159/000448808.
Leshner, M., S. Wang, C. Lewis, H. Zheng, X.A. Chen, L. Santy, and Y. Wang. 2012. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Frontiers in Immunology 3: 307. https://doi.org/10.3389/fimmu.2012.00307.
Kolaczkowska, E., C.N. Jenne, B.G. Surewaard, A. Thanabalasuriar, W.Y. Lee, M.J. Sanz, K. Mowen, G. Opdenakker, and P. Kubes. 2015. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nature Communications 6: 6673. https://doi.org/10.1038/ncomms7673.
Li, P., M. Li, M.R. Lindberg, M.J. Kennett, N. Xiong, and Y. Wang. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. The Journal of Experimental Medicine 207 (9): 1853–1862. https://doi.org/10.1084/jem.20100239.
Rohrbach, A.S., D.J. Slade, P.R. Thompson, and K.A. Mowen. 2012. Activation of PAD4 in NET formation. Frontiers in Immunology 3: 360. https://doi.org/10.3389/fimmu.2012.00360.
Asaga, H., K. Nakashima, T. Senshu, A. Ishigami, and M. Yamada. 2001. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. Journal of Leukocyte Biology 70 (1): 46–51.
Takahara, H., M. Tsuchida, M. Kusubata, K. Akutsu, S. Tagami, and K. Sugawara. 1989. Peptidylarginine deiminase of the mouse. Distribution, properties, and immunocytochemical localization. The Journal of Biological Chemistry 264 (22): 13361–13368.
Horibata, S., K.E. Rogers, D. Sadegh, L.J. Anguish, J.L. McElwee, P. Shah, P.R. Thompson, and S.A. Coonrod. 2017. Role of peptidylarginine deiminase 2 (PAD2) in mammary carcinoma cell migration. BMC Cancer 17 (1): 378. https://doi.org/10.1186/s12885-017-3354-x.
Knight, J.S., V. Subramanian, A.A. O'Dell, S. Yalavarthi, W. Zhao, C.K. Smith, J.B. Hodgin, P.R. Thompson, and M.J. Kaplan. 2015. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Annals of the Rheumatic Diseases 74 (12): 2199–2206. https://doi.org/10.1136/annrheumdis-2014-205365.
Muller, S., and M. Radic. 2015. Citrullinated autoantigens: From diagnostic markers to pathogenetic mechanisms. Clinical Reviews in Allergy and Immunology 49 (2): 232–239. https://doi.org/10.1007/s12016-014-8459-2.
Mishra, N., L. Schwerdtner, K. Sams, S. Mondal, F. Ahmad, R.E. Schmidt, S.A. Coonrod, P.R. Thompson, M.M. Lerch, and L. Bossaller. 2019. Cutting edge: Protein arginine deiminase 2 and 4 regulate NLRP3 inflammasome-dependent IL-1beta maturation and ASC speck formation in macrophages. Journal of Immunology 203 (4): 795–800. https://doi.org/10.4049/jimmunol.1800720.
Ott, L.W., K.A. Resing, A.W. Sizemore, J.W. Heyen, R.R. Cocklin, N.M. Pedrick, H.C. Woods, J.Y. Chen, M.G. Goebl, F.A. Witzmann, and M.A. Harrington. 2007. Tumor necrosis factor-alpha- and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. Journal of Proteome Research 6 (6): 2176–2185. https://doi.org/10.1021/pr060665l.
Funding
This work was funded by grants from Kickstart N022142 to Dr. Yongqing Li, UMHS-PUHSC Joint Institute U050150 and the National Institutes of Health Grant 5 R01 GM084127 to Dr. Hasan B. Alam and by the National Institutes of Health Grant R35 GM118112 to Paul R. Thompson.
Author information
Authors and Affiliations
Contributions
YL and HA designed the study. QD and BP carried out the experiments. YL and ZW wrote the manuscript, and YL and HA made a critical revision. QD, BP, YT, and UFB reviewed manuscript, and XD, BL, YT, and ZW provided experimental support. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The protocol for the animal experiments was approved by the University of Michigan Institutional Animal Care and Use Committee (PRO00008861). All experiments complied with animal welfare and research regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Z., Deng, Q., Pan, B. et al. Inhibition of PAD2 Improves Survival in a Mouse Model of Lethal LPS-Induced Endotoxic Shock. Inflammation 43, 1436–1445 (2020). https://doi.org/10.1007/s10753-020-01221-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01221-0