Abstract
We have found earlier that Tubastatin A (TubA), a selective inhibitor of histone deacetylase 6 (HDAC6), improves survival in a mouse model of lethal cecal ligation and puncture (CLP)-induced sepsis. However, the underlying mechanisms have not been fully established. This study sought to test the hypothesis that TubA could affect both lung and splenic functions. C57BL/6J mice were subjected to CLP, and randomized to receive either TubA (70 mg/kg) dissolved in dimethyl sulfoxide (DMSO), or DMSO alone, 1 h following CLP. Sham animals acted as control. Twenty-four hours later, lung tissue was harvested for pathological examination, and splenic tissue was harvested for bacterial colonization. In a parallel study, the spleen was collected 48 h following CLP, and single cell suspension was prepared. Splenocytes then underwent flow cytometry to analyze the immune cell population. RAW264.7 macrophages were treated with lipopolysaccharide (LPS) with or without the presence of TubA (10 μM) at 37 °C for 3 h to assess the effect on macrophage phagocytosis. We found that acute lung injury secondary to lethal sepsis was attenuated by TubA. Treatment with TubA restored the percentage of B lymphocytes, and significantly increased percentages of innate immune cells and macrophages compared to the vehicle-treated CLP group. Moreover, TubA significantly decreased the bacterial load in the spleen, and improved the phagocytic ability of RAW264.7 murine macrophages in vitro. Such findings may help to explain the beneficial effects of TubA treatment in a model of lethal sepsis, as previously reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis is the most common cause of death in hospitalized patients that results in millions of deaths yearly [1]. Sepsis has been newly defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection” [2]. Septic shock is further described as a subclass of sepsis in which the level of physiologic derangements increases the risk of mortality [3].
Multiple organ failure (MOF) accounts for the large majority of early deaths in septic shock [4]. One of the first organs to fail is the lung; acute lung injury (ALI) is one of the leading causes of mortality in sepsis and septic shock [5]. Another important organ in the consideration of sepsis is the spleen. As a major component of the reticuloendothelial system, the spleen is involved in monitoring and regulating the mononuclear phagocyte system. In particular, splenic macrophages act as key mediators of the innate immune response. In sepsis, however, the inflammatory response can cause macrophage dysfunction and apoptosis, compromising the host response and contributing to mortality [6].
Histone deacetylases (HDACs) are a class of enzymes that catalyze the removal of acetyl groups from the nuclear histone proteins, tightening their association with the deoxyribonucleic acid (DNA). This causes condensation of chromatin (composed of histones and DNA), with a resultant decrease in accessibility of the DNA to various transcription factors. Conversely, a class of drugs called histone deacetylase inhibitors (HDACIs) can be used to induce acetylation of histones and an increase in transcription. Histone acetylation status can govern the amplitude of the immune response by moderating the transcription of pathways involved in immune cells [7]. HDACs have been classified into four subclasses: three Zn2+-dependent classes (I, II, and IV), and one NAD+-dependent class III. Class II is subdivided into class IIa and class IIb. HDAC6 belongs to HDAC class IIb and is mostly cytoplasmic in location. It is associated with non-histone substrates, including α-tubulin, heat shock protein 90 (HSP90), and cortactin [8,9,10]. HDAC6 has been found to be involved in many disease processes, such as breast cancer, leukemia, multiple myeloma [11,12,13], chronic hypertension [14], and nervous system injury [15].
We have recently demonstrated that inhibition of HDAC6 with Tubastatin A (TubA) can improve survival in a mouse model of cecal ligation and puncture (CLP)-induced lethal sepsis [16]. Moreover, TubA treatment can restore populations of innate immune cells and macrophage populations, and increase the neutrophil composition in mouse bone marrow [17]. The present study was designed to provide further insight into whether and how inhibition of HDAC6 with TubA can affect critical organs such as the lung and spleen.
MATERIALS AND METHODS
The protocol for this study was approved by the University of Michigan Institutional Animal Care and Use Committee. All experiments complied with animal welfare and research regulations.
Animals
Male C57BL/6J mice (18–26 g; Jackson Laboratory, Bar Harbor, ME) were used for this study. They underwent a 3-day period of acclimation before participation in experiments.
Cell Culture and Reagents
RAW 264.7(American Type Culture Collection, Manassas, VA), a murine macrophage cell line, were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY). The medium was supplemented with 100 U/mL penicillin, 100 U/mL streptomycin, 292 mg/L l-glutamine, and 10% fetal bovine serum (Invitrogen, Grand Island, NY). Macrophages were maintained at the condition of 37 °C and 5% CO2.
Cecal Ligation and Puncture
CLP was utilized to induce fecal peritonitis, as described in a previous study [18].
We have previously shown that this CLP model is 100% lethal, and that Tub A treatment improves survival [16]. Therefore, we used the same CLP model in the present study to investigate the effects of TubA on immune system functions as well as the development of acute lung injury.
Under inhaled anesthesia with isoflurane, 1% bupivacaine was injected at the operative site for local analgesia, and then a midline incision was made to obtain access to the peritoneal cavity. The cecum was eviscerated and 75% of the cecum was ligated using 5-0 silk suture, and punctured through-and-through using a 20-gauge needle as described previously [16]. 1–2 mm3 of fecal material was expelled from the cecum into the peritoneal cavity, and the cecum returned to its normal location. The abdomen was closed in two layers separately using 4-0 silk sutures, and 1 ml normal saline was injected subcutaneously for resuscitation. Sham animals were manipulated in the same way, without the CLP component.
Treatment
Animals were randomized to the following groups: (a) sham; (b) CLP + vehicle (Veh; DMSO, 1 μL/g), and (c) CLP + TubA (70 mg/kg, dissolved in 1 μL/g DMSO; intraperitoneal injection). Treatment was administered 1 h following the CLP.
Experiment I (24-h Model)
A time point of 24 h post-CLP was chosen to assess the degree of acute lung injury, in line with our previous study [16]. Splenic tissue was also harvested at this time point to measure the spleen bacterial load during early sepsis.
Acute Lung Injury
Animals were euthanized and tissues were harvested (n = 3/group). Histological analysis of lung tissue was performed per our previous study [19]. Briefly, the lung tissue was embedded in paraffin, sliced into 5-μm sections, and stained with hematoxylin and eosin (H&E). A pathologist, blinded to group allocation of the specimen, evaluated the sections and graded six parameters: (1) septal mononuclear cell/lymphocyte infiltration, (2) septal hemorrhage and congestion, (3) neutrophils, (4) alveolar macrophages, (5) alveolar hemorrhage, and (6) alveolar edema. Each individual parameter of lung injury was graded on a scale of 0–3, with 0 meaning “absent,” 1 meaning “mild,” 2 meaning “moderate,” and 3 meaning “severe.” The total injury score was expressed as the sum of the scores for all parameters, with the maximum score (representing maximal injury) being 18 [20].
Spleen Bacterial Load
Splenic tissue was homogenized in 1 mL of normal saline and centrifuged for 5 min at 500×g (n = 3–5/group). The supernatant was collected and further diluted with sterile normal saline to 10−5. Fifty microliters of the dilute was evenly spread on tryptic soy agars (BD Difco Inc., Franklin Lakes, NJ) and then incubated at 37 °C for 24 h. Bacterial population was calculated as the number of colony-forming units (CFU). Data were then log transformed for statistical analysis.
Experiment II (48-h Model)
A later time point of 48 h was chosen to measure the delayed immune response to sepsis. Flow cytometry was performed on splenic tissue to assess the relative proportions of different immune cells (n = 7/group).
Animals were euthanized and tissues harvested. Splenic tissue was homogenized through 70-μm cell strainers, after which erythrocyte lysis was performed using Red Blood Cell Lysis Buffer (Sigma Aldrich Inc., St. Louis, MO). Splenocytes were washed with phosphate-buffered saline, blocked with anti-mouse CD16/32 antibody (eBiosicence Inc., San Diego, CA), and then labeled with anti-mouse CD11b FITC, Gr-1 PerCP-Cy5.5, F4/80 Antigen APC, B220 PE-Cy7, and CD3 APC-eFluor® 780 antibodies (eBiosicence Inc., San Diego, CA). Cells were washed prior to flow cytometry analysis. Data were acquired on an LSRII (BD Biosciences Inc., San Jose, CA) and analyzed using FlowJo (Tree Star Inc., Ashland, OR).
Experiment III (In Vitro Assay)
The phagocytosis assay was performed as previously described [16]. RAW264.7 macrophages (American Type Culture Collection, Manassas, VA) were seeded in 96-well plates at a density of 1 × 106/mL. Plates were assigned to the following three groups: (1) sham (no LPS, no treatment), (2) LPS (1 μg/mL) + Veh, (3) LPS + TubA, and cultured at 37 °C for 3 h, before removing the medium and adding 100 μL Live Cell Imaging Solution (Life Technologies Corporation, Eugene, OR) containing 1 mg/mL pHrodo E. coli bioparticles (Life Technologies Corporation, Eugene, OR). The assay plate was then transferred to an incubator warmed to 37 °C for 1 h to allow phagocytosis, and then analyzed on a SpectraMax plate reader (Molecular Devices, Sunnyvale, CA). The net phagocytosis was calculated by subtracting the baseline fluorescence from wells containing 100 μL of 1 mg/mL pHrodo E. coli bioparticles without cells.
Statistical Analysis
All of the analyses were performed utilizing GraphPad Prism 6.00 (GraphPad Software Inc., La Jolla, CA). Differences among the three groups were assessed using one-way analysis of variance (ANOVA), followed by Bonferroni post hoc test for multiple comparison. Data are presented as mean ± standard error of the mean (SEM) unless mentioned otherwise. A p value of < 0.05 was considered to be statistically significant.
RESULTS
TubA Treatment Attenuates Acute Lung Injury
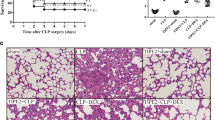
We first investigated the mechanism of action of TubA by determining the effect of TubA on CLP-induced acute lung injury. As shown in Fig. 1, sham animals given DMSO vehicle (sham + DMSO) had normal lung histology. In contrast, sepsis caused significant damage to the lung tissue—histology from the CLP + DMSO group showed significant interstitial edema, alveolar hemorrhage, thickening of the alveolar wall, and infiltration of tissue by mononuclear cells/lymphocytes. Administration of TubA attenuated these changes and decreased the acute lung injury score significantly (CLP + TubA = 2.48 ± 0.41 vs. CLP + DMSO = 5.97 ± 0.13, p = 0.0002).
Tubastatin A alleviated acute lung injury 24 h after cecal ligation and puncture. Mice were intraperitoneally administered 70 mg/kg Tubastatin A, or vehicle (DMSO) 1 h after CLP. Twenty-four hours after operation, lung tissues were harvested and then sectioned for H&E staining. Representative images were chosen from different experimental groups. Semi-quantitative pathology scores were determined by a scoring system as described in the “Materials and Methods” (means ± SEM, n = 3 animals/group). CLP cecal ligation and puncture, DMSO dimethyl sulfoxide, TubA Tubastatin A, Sham no cecal ligation and puncture.
TubA Treatment Decreases Spleen Bacterial Load
We then sought to determine whether administration of TubA could modulate the immune response to CLP by examining spleen bacterial load. Sham-operated mice had no bacteria in the spleen. However, CLP significantly increased the spleen bacterial load compared to sham (Sham + DMSO = 0 ± 0 log10 CFU/g spleen vs. CLP + DMSO = 6.78 ± 0.56 log10 CFU/g spleen, p < 0.0001). Treatment with TubA significantly reduced the bacterial load compared to CLP + DMSO (CLP + DMSO = 6.78 ± 0.56 log10 CFU/g spleen vs. CLP + TubA = 4.95 ± 0.40 log10 CFU/g spleen, p = 0.036) (Fig. 2).
Tubastatin A increased spleen bacteria clearance in lethal sepsis. Spleens of animals from the 3 groups were harvested at 24 h after CLP. Supernatant was made after homogenization and centrifugation. Equal amount of supernatant was spread on agar plates for colony formation. Bacteria number was calculated as colony-forming units per gram of spleen, and data were log transformed for presentation (means ± SEM, n = 3–5 animals/group). CFU colony-forming units, CLP cecal ligation and puncture, DMSO dimethyl sulfoxide, TubA Tubastatin A, Sham no cecal ligation and puncture.
Effect of TubA Treatment on Innate Immune Cell Populations in Splenic Tissue
To determine how TubA affects the innate immune system, we performed flow cytometry to analyze the immune cell populations in the spleen. As shown in Fig. 3, there were no statistical differences in the percentages of neutrophils (CD11b+ Gr-1+) and T lymphocytes (CD3+) among the three groups (Fig. 3c, e, respectively). There were statistical differences in the percentage of splenic B lymphocytes (B220+, p = 0.0125; Fig. 3d) among the three groups analyzed by ANOVA. Bonferroni post hoc test for multiple comparisons revealed that the significant differences were between the sham and CLP + DMSO groups. Vehicle-treated CLP animals showed decreased percentage of B lymphocytes compared to sham (sham = 59.1 ± 2.9% vs. CLP + DMSO = 44.3 ± 2.6%, p < 0.05; Fig. 3d), and TubA treatment normalized the percentage of B lymphocytes. There were significant differences in the percentages of innate immune cells (CD11b+, p = 0.0444; Fig. 3a) and macrophages (CD11b+ F4/80+, p < 0.01; Fig. 3b) among the three groups. As compared to the vehicle-treated CLP group, TubA treatment significantly increased the percentages of innate immune cells (CLP + DMSO = 6.2 ± 1.3% vs. CLP + TubA = 10.8 ± 0.4, p < 0.05; Fig. 3a) and macrophages (CLP + DMSO = 2.9 ± 0.3% vs. CLP + TubA 7.0 ± 1.1, p < 0.01; Fig. 3b). There were no significant differences in innate immune cells, macrophages, neutrophils, B lymphocytes, and T lymphocytes between the sham and CLP + TubA groups.
Tubastatin A treatment increased the percentage of innate immune cells and macrophages in spleen in the lethal septic model. Animals were randomly assigned into 3 groups, and given intraperitoneal vehicle DMSO or Tubastatin A (70 mg/kg) dissolved in DMSO or 1 h after the CLP. The DMSO mice were used as sham. Animals were sacrificed and spleens were harvested 48 h after CLP, and homogenized into single cell suspension, blocked with anti-mouse CD16/32, and stained with anti-mouse CD11b FITC, F4/80 antigen APC, anti-mouse B220 PE-Cy7, Gr-1 PerCP-Cy5.5, or anti-mouse CD3 APC-eFluor® 780. Flow cytometry was employed to study the different cell populations. Representative plots of innate immune cells, macrophages, neutrophils, B lymphocytes, and T lymphocytes are shown on the left side of the panel (CD11b+, CD11b+ F4/80+, CD11b+ Gr-1+, B220+, CD3+). Tubastatin A treatment significantly increased the percentage of innate immune cells and macrophages as compared to the vehicle-treated CLP group. There were no significant differences in innate immune cells, macrophages, neutrophils, B lymphocytes, and T lymphocytes between the sham and CLP + TubA groups. Data were presented as group means ± SEM (n = 7/group). CLP cecal ligation and puncture, DMSO dimethyl sulfoxide, TubA Tubastatin A, Sham no cecal ligation and puncture, SSC side scatter.
TubA Increases Phagocytic Activity of Macrophages
To determine whether TubA could affect phagocytic activity of the macrophages, we cultured mouse RAW264.7 macrophages and performed a phagocytosis assay as previously described [16]. As shown in Fig. 4, LPS insult decreased the macrophage phagocytosis compared to sham (sham = 373.7 ± 7.20 RFU vs. LPS + DMSO 289.6 ± 1.76 RFU, p = 0.0002). However, TubA treatment resulted in significantly increased phagocytic activity compared to LPS + DMSO (LPS + DMSO = 289.6 ± 1.76 RFU vs. LPS + TubA 344.6 ± 9.68 RFU, p = 0.004). No difference was seen between the sham and LPS + TubA, suggesting that TubA helped restore phagocytic activity to normal.
Tubastatin A enhanced phagocytosis of RAW264.7 in vitro. Macrophages were treated at 37 °C in the absence or presence of LPS (1 μg/mL) or Tubastatin A (10 μM) for 3 h, then incubated with 1 mg/mL pHrodo E. coli bioparticles and the fluorescence analyzed on a plate reader. Mean values are relative fluorescence units (RFU), calculated from 3 to 5 wells per group subtracting the baseline fluorescence from wells containing pHrodo E. coli bioparticles but no cells (means ± SEM, n = 3–5/group). LPS lipopolysaccharides, DMSO dimethyl sulfoxide, TubA Tubastatin A, Sham no LPS, no treatment.
DISCUSSION
The present study is a logical continuation of our previous research. Our laboratory has previously shown that treatment with the HDAC6 inhibitor TubA improves survival in a lethal CLP model, attenuates bone marrow atrophy, and restores the bone marrow’s macrophage population [16, 17]. However, it remained to be determined whether and how TubA treatment would further affect key organs such as the lung and spleen. In this study, we have demonstrated that inhibition of HDAC6 with TubA significantly attenuates acute lung injury and decreases spleen bacterial load. In addition, we have discovered that TubA increases the splenic macrophage and innate immune cell proportions and enhances phagocytic activity of the macrophages.
We chose to examine lung injury and inflammation because lungs are highly susceptible to the systemic inflammatory response in sepsis, and our previous work had not evaluated the effects of TubA on acute lung injury. The present study reveals that TubA treatment attenuates acute lung injury in polymicrobial sepsis, which may be due to a reduction in primary inflammation and protection of the endothelial barrier. Thangavel et al. showed that pan-HDAC inhibitor Trichostatin A (TSA) treatment significantly reduced mRNA levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, meanwhile increasing mRNA levels of anti-inflammatory IL-10, in bone marrow-derived macrophages that had been challenged with LPS [21]. Whether TubA has similar immunomodulatory effects needs further validation. However, TubA has been proven to inhibit TNF-α (a pro-inflammatory cytokine)-induced endothelial barrier disruption [22], which suggests that HDAC6 inhibition could potentially prevent inflammation-mediated vascular injury. TubA has also been shown to reduce pulmonary edema in a mouse model of endotoxemia, which may be associated with α-tubulin and β-catenin acetylation, thus strengthening the cytoskeleton and adherens junctions [22]. These mechanisms should be evaluated in the context of sepsis-induced ALI in the future.
Sepsis can be caused by a combination of factors related to the invading pathogen and the host’s immune system. The early stages of sepsis are characterized by an exaggerated inflammatory response that results in a “cytokine storm.” This cytokine storm is followed by a prolonged period of relative immunosuppression [23]. Both phases contribute to mortality in sepsis. Innate immune cells, especially macrophages, play a key role in the immune responses against infection. Macrophages act as pathogen scavengers and are the predominant source of inflammatory cytokines. In fact, macrophage dysfunction has been implicated in the immunosuppression that is often witnessed during the late stages of sepsis [24]. The effects of HDAC inhibitors on immune cell functions have been extensively studied; however, only a few studies have addressed the effects of HDAC inhibitors on the immune cell numbers in sepsis, and to our knowledge, this is the first paper to assess the effects on the splenic reservoir.
In the current study, we have shown that inhibition of HDAC6 significantly increased the proportions of splenic macrophages and innate immune cells in a model of lethal sepsis. In addition, we found that TubA treatment can restore the ability of macrophages to phagocytose pHrodo E. coli bioparticles in the presence of LPS. It has been reported that human macrophages treated with an HDAC inhibitor at the time of bacterial infection can enhance the production of mitochondrial reactive oxygen species and promote the clearance of intracellular bacteria from the macrophages [25]. Our results indicate that TubA induces an increase in both the macrophage population and the macrophage phagocytic activity, which may enhance the host’s ability to eliminate the foreign pathogens from the circulation. These findings may explain the significantly decreased bacterial load in the spleens in the present study, as well as the increased survival rates that we have previously reported with TubA treatment in this model [16]. In our former study, we also found that TubA treatment can enhance phagocytosis of mouse primary splenocytes [16]. The spleen has mixed populations of immune cells, but macrophages are the main cells that play a role in pathogen elimination in the spleen [26]. Therefore, we further took RAW264.7 murine macrophages to evaluate phagocytic ability in the current study and received the same result.
The percentage of macrophages in the spleen was reduced compared to the sham following CLP; however, it did not reach statistical significance. The trend of macrophage reduction may be due to apoptosis in macrophages when invading pathogens evade host defenses by eliminating these phagocytic cells [27]. The increased proportions of macrophages in the spleen after TubA treatment as noted in the current study could be due to several reasons. One possibility is that TubA could inhibit macrophage apoptosis. Our previous study has shown that TubA decreases cleaved caspase-3 expression in RAW264.7 macrophages after LPS treatment [16]. Another possibility is that the HDAC6 inhibition increases the differentiation of myeloid stem cells into macrophages in the bone marrow via epigenetic modulation. Theoretically, HDAC6 inhibitors may activate the gene promoters of myeloid stem cells and facilitate their proliferation and differentiation. It was recently reported that the pan-HDAC inhibitor valproic acid can induce bone marrow stromal stem cell differentiation into hepatocytes both in vitro and in vivo by gene regulation of fibroblast growth factor receptors and c-Met through core histone post-translational modification [28]. CUDC-907, a dual PI3K and HDAC inhibitor for PI3Kα and HDAC, has been shown to promote bone marrow adipocyte differentiation through inhibition of histone deacetylase and regulation of cell cycle [29].
This study has also shown that LPS exposure decreases phagocytic activity of the macrophages, while TubA restores it towards baseline. This could be due to a decrease in TNF-α production; TNF-α production in sepsis inhibits phagocytosis [30], and production of TNF-α has been shown to be dramatically decreased by another HDAC6 inhibitor, Tubacin, in both RAW264.7 cell line and primary bone marrow-derived macrophages [30]. Moreover, co-treatment of human macrophages with HDAC inhibitors vorinostat (suberoylanilide hydroxamic acid, SAHA) or TSA at the time of infection promotes bacterial clearance in vitro [25].
This study has certain limitations that should be acknowledged. This was a highly lethal model, and survival beyond 48 h in the untreated animals was impossible. Therefore, our observations are limited to this 48-h window and the long-term implications of our findings are unclear. Follow-up studies with a sub-lethal model (and multiple sampling time points) will be needed to fill in this gap. Also, we do not have direct evidence to show where the increased splenic innate immune cells and macrophages come from after TubA treatment. In addition, for logistical reasons, we focused on splenic cellular populations and function which may only be one of many mechanisms contributing to improved outcomes with HDAC inhibitors in sepsis. Further investigation of TubA needs to be carried out to develop the whole picture of its mechanism of action.
In summary, we have demonstrated for the first time that inhibition of HDAC6 significantly attenuates acute lung injury and modulates sepsis-induced immunosuppression by increasing the immune cell populations and improving immune cell functions, which may be responsible for the improved outcomes. These results may help further explain, in part, the beneficial effects of TubA treatment in models of sepsis.
References
Hicks, P., D.J. Cooper, and Australian, New Zealand Intensive Care Society B, Clinical Trials Group Executive C. 2008. The Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care and Resuscitation 10 (1): 8.
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA : the journal of the American Medical Association. 315 (8): 801–810.
Shankar-Hari, M., G.S. Phillips, M.L. Levy, C.W. Seymour, V.X. Liu, C.S. Deutschman, D.C. Angus, G.D. Rubenfeld, M. Singer, and for the Sepsis Definitions Task Force. 2016. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA : the journal of the American Medical Association. 315 (8): 775–787.
Daviaud, F., D. Grimaldi, A. Dechartres, J. Charpentier, G. Geri, N. Marin, J.D. Chiche, A. Cariou, J.P. Mira, and F. Pène. 2015. Timing and causes of death in septic shock. Annals of Intensive Care 5 (1): 16.
Varisco, B.M. 2011. The pharmacology of acute lung injury in sepsis. Advances in Pharmacological Sciences 2011: 254619.
Luan, Y.Y., Y.M. Yao, X.Z. Xiao, and Z.Y. Sheng. 2015. Insights into the apoptotic death of immune cells in sepsis. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 35 (1): 17–22.
Mortaz, E., M.R. Masjedi, P.J. Barnes, and I.M. Adcock. 2011. Epigenetics and chromatin remodeling play a role in lung disease. Tanaffos. 10 (4): 7–16.
Zhang, X., Z. Yuan, Y. Zhang, S. Yong, A. Salas-Burgos, J. Koomen, N. Olashaw, J.T. Parsons, X.J. Yang, S.R. Dent, T.P. Yao, W.S. Lane, and E. Seto. 2007. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Molecular Cell 27 (2): 197–213.
Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X.F. Wang, and T.P. Yao. 2002. HDAC6 is a microtubule-associated deacetylase. Nature 417 (6887): 455–458.
Ai, J., Y. Wang, J.A. Dar, J. Liu, L. Liu, J.B. Nelson, and Z. Wang. 2009. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Molecular Endocrinology 23 (12): 1963–1972.
Hackanson, B., L. Rimmele, M. Benkisser, M. Abdelkarim, M. Fliegauf, M. Jung, et al. 2012. HDAC6 as a target for antileukemic drugs in acute myeloid leukemia. Leukemia research. 36 (8): 1055–1062.
Hideshima, T., J. Qi, R.M. Paranal, W. Tang, E. Greenberg, N. West, M.E. Colling, G. Estiu, R. Mazitschek, J.A. Perry, H. Ohguchi, F. Cottini, N. Mimura, G. Görgün, Y.T. Tai, P.G. Richardson, R.D. Carrasco, O. Wiest, S.L. Schreiber, K.C. Anderson, and J.E. Bradner. 2016. Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America 113 (46): 13162–13167.
Medler, T.R., J.M. Craig, A.A. Fiorillo, Y.B. Feeney, J.C. Harrell, and C.V. Clevenger. 2016. HDAC6 deacetylates HMGN2 to regulate Stat5a activity and breast cancer growth. Molecular Cancer Research 14 (10): 994–1008.
Lemon, D.D., T.R. Horn, M.A. Cavasin, M.Y. Jeong, K.W. Haubold, C.S. Long, D.C. Irwin, S.A. McCune, E. Chung, L.A. Leinwand, and T.A. McKinsey. 2011. Cardiac HDAC6 catalytic activity is induced in response to chronic hypertension. Journal of Molecular and Cellular Cardiology 51 (1): 41–50.
Rivieccio, M.A., C. Brochier, D.E. Willis, B.A. Walker, M.A. D’Annibale, K. McLaughlin, A. Siddiq, A.P. Kozikowski, S.R. Jaffrey, J.L. Twiss, R.R. Ratan, and B. Langley. 2009. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proceedings of the National Academy of Sciences of the United States of America 106 (46): 19599–19604.
Li, Y., T. Zhao, B. Liu, I. Halaweish, R. Mazitschek, X. Duan, and H.B. Alam. 2015. Inhibition of histone deacetylase 6 improves long-term survival in a lethal septic model. The journal of trauma and acute care surgery. 78 (2): 378–385.
Zhao T, Li Y, Liu B, Pan B, Cheng X, Georgoff P, et al. Inhibition of histone deacetylase 6 restores innate immune cells in the bone marrow in a lethal septic model. The journal of trauma and acute care surgery. 2016;80(1):34-40; discussion -1.
Zhao, T., B. Pan, H.B. Alam, B. Liu, R.T. Bronson, Q. Deng, E. Wu, and Y. Li. 2016. Protective effect of Cl-amidine against CLP-induced lethal septic shock in mice. Scientific Reports 6: 36696.
Liu, Z., Y. Li, W. Chong, D.K. Deperalta, X. Duan, B. Liu, I. Halaweish, P. Zhou, and H.B. Alam. 2014. Creating a prosurvival phenotype through a histone deacetylase inhibitor in a lethal two-hit model. Shock 41 (2): 104–108.
Kim, K., Y. Li, G. Jin, W. Chong, B. Liu, J. Lu, K. Lee, M. deMoya, G.C. Velmahos, and H.B. Alam. 2012. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation 83 (2): 243–248.
Thangavel, J., S. Samanta, S. Rajasingh, B. Barani, Y.T. Xuan, B. Dawn, and J. Rajasingh. 2015. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. Journal of Cell Science 128 (16): 3094–3105.
Yu, J., Z. Ma, S. Shetty, M. Ma, and J. Fu. 2016. Selective HDAC6 inhibition prevents TNF-alpha-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. American journal of physiology Lung cellular and molecular physiology. 311 (1): L39–L47.
Shukla, P., G.M. Rao, G. Pandey, S. Sharma, N. Mittapelly, R. Shegokar, and P.R. Mishra. 2014. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. British Journal of Pharmacology 171 (22): 5011–5031.
Boomer, J.S., To K, K.C. Chang, O. Takasu, D.F. Osborne, A.H. Walton, et al. 2011. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA : the journal of the American Medical Association. 306 (23): 2594–2605.
Ariffin JK, das Gupta K, Kapetanovic R, Iyer A, Reid RC, Fairlie DP, et al. Histone deacetylase inhibitors promote mitochondrial reactive oxygen species production and bacterial clearance by human macrophages. Antimicrobial Agents and Chemotherapy 2015;60(3):1521–1529.
Borges da Silva H, Fonseca R, Pereira RM, Cassado Ados A, Alvarez JM, D’Imperio Lima MR. Splenic macrophage subsets and their function during blood-borne infections. Frontiers in immunology. 2015;6:480.
Roger T LJ, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson AL, Reymond MK, Miconnet I, Schrenzel J, François P, Calandra T. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection.pdf. Blood 2011;117:1205–1217.
Chen, Y., R.L. Pan, X.L. Zhang, J.Z. Shao, L.X. Xiang, X.J. Dong, and G.R. Zhang. 2009. Induction of hepatic differentiation of mouse bone marrow stromal stem cells by the histone deacetylase inhibitor VPA. Journal of Cellular and Molecular Medicine 13 (8B): 2582–2592.
Ali, D., H. Alshammari, R. Vishnubalaji, E.P. Chalisserry, R. Hamam, M. Alfayez, et al. 2016. CUDC-907 promotes bone marrow adipocytic differentiation through inhibition of histone deacetylase and regulation of cell cycle. Stem Cells and Development.
Yan, B., S. Xie, Z. Liu, J. Ran, Y. Li, J. Wang, Y. Yang, J. Zhou, D. Li, and M. Liu. 2014. HDAC6 deacetylase activity is critical for lipopolysaccharide-induced activation of macrophages. PLoS One 9 (10): e110718.
Funding
This work was funded by a grant from NIH RO1 GM084127 to H.B.A. Data was presented at the 12th Annual Academic Surgical Congress, Clinical Congress Las Vegas, NV (February 2017).
Author information
Authors and Affiliations
Contributions
Q.D., T.Z., Y.L., and H.B.A. designed this study, for which H.B.A. secured funding. Q.D. and T.Z. performed the experiments and collected and analyzed data. B.L. and B.P provided experimental support. N.L. performed statistical analysis. E.C. presented the data at the 12th Annual Academic Surgical Congress. Q.D., T.Z., I.S.D., A.M.W., U.F.B., and Y.L. wrote the manuscript, which was critically reviewed and revised by Y.L. and H.B.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The protocol for this study was approved by the University of Michigan Institutional Animal Care and Use Committee. All experiments complied with animal welfare and research regulations.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Deng, Q., Zhao, T., Pan, B. et al. Protective Effect of Tubastatin A in CLP-Induced Lethal Sepsis. Inflammation 41, 2101–2109 (2018). https://doi.org/10.1007/s10753-018-0853-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0853-0