Abstract
Acute kidney injury (AKI) is one of the most common complications of sepsis, which largely contributes to the high mortality rate of sepsis. Honokiol, a natural polyphenol from the traditional Chinese herb Magnolia officinalis, is known to possess anti-inflammatory and antioxidant activity. Here, the underlying mechanism of honokiol-induced amelioration of sepsis-associated AKI was analyzed. The expression patterns of oxidative stress moleculars and TLRs-mediated inflammation pathway were examined to identify the response of NRK-52E cells incubated with septic rats’ serum to honokiol. The levels of iNOS, NO, and myeloperoxidase in NRK-52E cells were increased during sepsis, which could be reversed by honokiol. The production of GSH and SOD as in vivo antioxidant was increased after honokiol treatment. The administration of honokiol significantly inhibited TLR2/4/MyD88 signaling pathway in AKI-induced NRK-52E cells. Furthermore, ZnPPIX, the HO-1 inhibitor, weakened honokiol-mediated morphological amelioration, and the reduced level of TNF-α, IL-1β, and IL-6 in kidneys of rats subjected to CLP. Finally, Honokiol was shown to connect with the Nrf2-Keap1 dimensionally. These findings suggest that honokiol plays its protective role on sepsis-associated AKI against oxidative stress and inflammatory signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Severe sepsis with organ dysfunction is a leading cause of multiple organ failure [1, 2]. Acute kidney injury (AKI) is one of the primary complications of sepsis, which is characterized by inappropriate hyperinflammatory and oxidative stress [3,4,5]. Understanding the process mechanisms of sepsis-associated acute kidney injury would be of significant clinical value. Inflammatory reactions triggered by cytokine production and tubular dysfunction induced by oxidative stress have been implicated in the process of sepsis-induced AKI [6]. It has positive significance to develop the hypotoxicity and efficient drug which has an anti-inflammatory and antioxidant effect and then uses it in clinical treatment of sepsis-derived AKI.
Honokiol, which is isolated from the traditional Chinese herb Magnolia officinalis, might be considered as a potential therapeutic agent with anti-inflammatory and antioxidant activities [7,8,9,10]. One study suggests that honokiol can inhibit oxidative stress and inflammation happened in renal ischemia/reperfusion injury [11]. It can reduce the mortality of sepsis in mice [12]. Now, there is no report about how honokiol exhibited the protective effect in sepsis-induced AKI via the mechanism of suppression of oxidative stress and inflammation. We hypothesized that honokiol could alleviate sepsis-induced AKI through its anti-inflammatory and anti-oxidative activities.
Activated nuclear transcription factor (Nrf2) regulates intracellular oxidation-reduction homeostasis in oxidative stress by dissociating from its kelch-like ECH-associated protein (Keap1), to promote the expression of downstream endogenous antioxidant heme oxygenase-1 (HO-1). Nrf2/HO-1 pathway has a pivotal role in the field of anti-oxidative stress, anti-inflammation, anti-aging, anti-apoptosis, neuroprotection, and anti-tumor [13,14,15]. Our previous reports have shown that the administration of honokiol effectively reduced cecal ligation and puncture (CLP)-induced oxidative stress and inflammatory cytokine production. We also observed that the levels of nitric oxide (NO) and inducible NO synthetase (iNOS) were attenuated by honokiol in septic rats [12].
In order to study the molecular mechanisms of anti-inflammatory and anti-oxidative effects of honokiol, we cultured NRK-52E cells of rats incubated with normal rats’ serum and septic rats’ serum. The levels of iNOS were tested, and activities of NO, MPO, GSH, and SOD were determined. At the same time, we studied its effects on TLR2/4/MyD88 signaling pathways. Given the hypothesis that the dissociation of Nrf2-Keap1 initials the protection process of oxidative stress from sepsis-induced AKI, we boldly connected honokiol with Nrf2-Keap1 dimensionally to investigate the possible function of honokiol during the process of the dissociation of Nrf2-Keap1 to provide experimental support for the application of the Chinese medicine honokiol in the treatment of sepsis-induced AKI which also can be considered as a new approach for the application of Chinese medicine in the research of treatments of sepsis.
MATERIALS AND METHODS
Cell Culture and Treatment
NRK-52E cells were grown in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) under 37 °C and 5% CO2 condition. 12.5 mg Honokiol was dissolved in 100 μl DMSO and diluted to appropriate concentrations with growth medium immediately before use. 15.7 mg ZnPPIX was dissolved in 5 ml NaOH and diluted to neutral with HCL. Then, 25 mg/10 ml ZnPPIX was treated with 5 ml honokiol. Cultured NRK-52E cells were divided into five groups: control, normal rat serum control (cells cultured in normal rat serum for 24 h), normal rat serum + honokiol (normal rat serum + 20 μmol honokiol), septic serum (cells cultured in septic serum for 24 h), and septic serum + honokiol (septic serum + 20 μmol honokiol).
Septic serum in this article is the serum from sepsis rat model. Based on the methods we used in the published article [12], sepsis was induced in rat by cecal ligation and puncture, which was used to induce polymicrobial sepsis in rats. Blood samples were obtained from the peripheral vessels of rats under anesthesia.
Animals and CLP Procedures
Most of the animal experiment listed here have been published previously but are presented here for clarity [12, 16].
ELISA Assay
The levels of TNF-α, IL-1β, and IL-6 were measured using ELISA kit (USCN, Shanghai, China) following the manufacturer’s protocol. The samples were centrifuged at 1000 rpm for 20 min at 4 °C, then the supernatant was collected for ELISA according to the manufacturer’s instructions. Quantitative analysis is performed by spectrophotometry at 450 nm. The reading at 450 nm is directly proportional to TNF-α, IL-1β, and IL-6 and serves as a convenient method to determine activation of serum inflammatory factors.
Protein Preparation and Western Blot Analysis
Total cellular proteins were prepared from NRK-52E cells under culture conditions. The protein (15 μg/well) was collected following lysis buffer (Beyotime, Shanghai, China), then centrifuged at 12000 rpm for 10 min at 4 °C. The proteins were transferred to a fresh tube, and the concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). The proteins were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The membrane was blocked with skimmed milk in TBS-T at room temperature and followed by incubation with primary antibodies (Beyotime, Shanghai, China) at 4 °C overnight, including anti-iNOS, anti-TLR4, anti-TLR2, anti-MyD88, anti-TRIF, anti-p-IκBα, anti-IκBα, and anti-β-actin antibodies. After being rinsed three times with TBS-T, the membrane was incubated with peroxidase-linked secondary antibody (Beyotime, Shanghai, China). The bound antibody was detected using the chemiluminescence system. The label signal was removed by using stripping buffer and followed by being reprobed with another antibody. The binding of specific antibodies was visualized using a Gel Imaging System and analyzed by Gel-Pro Analyzer software.
Immunocytochemical Staining
Immunocytochemical (ICC) staining was performed on the different experimental groups following the standard protocol. The antibody used was rabbit anti-rat iNOS (Beyotime, Shanghai, China) at dilutions of 1:100. The section without the first antibody incubation was used as the background control.
Molecular Docking Study
The crystallographic structure of keap1/Nrf2 complex was obtained from Protein Data Bank (http://www.rcsb.org/pdb). The two-dimensional structures of honokiol were drawn by MDL ISIS Draw 2.5 standalone software and converted into three-dimensional structures using the Prepare Ligands of Accelrys Discovery Studio (DS) 2.5 software (http://www.accelrys.com/). After defining and editing the bind site by using DS, we predicted the docking procedures involved adding the hydrogens and correcting the chemistry of the protein. The parameters of input receptor and input binding site were rebuilt followed by energy grid parameters set. The Ligand Fit module was used for molecular docking modeling. The analysis of molecular dock was performed by DS software.
Statistics Analysis
All data are expressed as means ± SD. Statistical significance was determined by ANOVA Bonferroni test. Differences with P < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Results
Honokiol attenuates oxidative stress of NRK-52E cell incubated with septic serum.
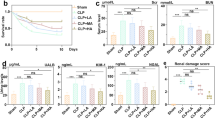
The expression patterns of iNOS in the NRK-52E cells were analyzed using immunohistochemical staining. The intensive signal of NRK-52E cells in septic serum group was observed relative to those in the control group, serum control group, and serum control group plus honokiol. However, the treatment with honokiol reduced the increase of iNOS in NRK-52E cells incubated with septic serum (Fig. 1d, e). Based on western blot results, honokiol given after septic serum effectively reverse the septic serum-induced increase in iNOS protein expression (Fig. 1f). After evaluating the supernatant fluid of cultured NRK-52E cells, we find that honokiol effectively lowered the expression of oxidative stress molecules NO and MPO and significantly increased the activity of antioxidant molecules GSH and SOD (Fig. 2).
a–e Immunocytochemical staining of iNOS expression in NRK-52E cells. a Control (normal NRK-52E cell), b NC (normal rat serum control), c NC+H (normal rat serum + honokiol), d SC (septic rat serum control), and e SC+H (septic rat serum + honokiol). f Western blot analyses of iNOS in NRK-52E cell cultured with a–e treatment.
The contents of NO, GSH, SOD, and MPO in supernatant of NRK-52E cells under the following conditions: control (normal NRK-52E cell), NC (normal rat serum control), NC+H (normal rat serum + honokiol), SC (septic rat serum control), and SC+H (septic rat serum + honokiol). ###P < 0.001 and ##P < 0.01 vs. NC+H, **P < 0.01 and *P < 0.05 vs. SC.
Honokiol Attenuates Septic AKI-Induced TLR2/4/MyD88 Signal Pathway
It has been demonstrated that TLR2/4/MyD88/NF-κB signal pathway was activated in septic AKI. Western blotting showed that there were little trace expressions of TLR4, TLR2, MyD88, TRIF, and p-IκBα in the control group, serum control group, and serum control group plus honokiol. Conversely, in the group incubated with septic serum, these protein levels of TLR4, TLR2, MyD88, TRIF, and p-IκBα were significantly upregulated. The expressions of all these proteins were markedly downregulated by honokiol treatment (Fig. 3).
Western blot profiling of the expression of TLR4, TLR2, MyD88, TRIF, and p-IκBα in NRK-52E cells under the following conditions: a Control (normal NRK-52E cell), b NC (normal rat serum control), c NC+H (normal rat serum + honokiol), d SC (septic rat serum control), and e SC+H (septic rat serum + honokiol). **P < 0.01 vs. control and #P < 0.05 vs. SC.
ZnPPIX Weakened the Amelioration of Honokiol
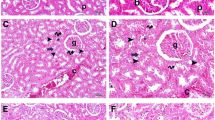
PAS staining was used to observe the morphological change of renal tissue after application of honokiol and ZnPPIX (HO-1 inhibitor) for CLP rat. As shown in Fig. 3(A), (B), no obvious morphological changes were observed in normal rats treated with honokiol relative to the control group. However, dilated renal tubules and swollen tubular cells were found in the CLP group (Fig. 4c). The treatment with honokiol effectively restored the morphological changes in kidneys of rats subjected to CLP (Fig. 4d). Additionally, ZnPPIX as the HO-1 inhibitor dramatically weakened the honokiol amelioration (Fig. 4e).
a–e Periodic acid-Schiff (PAS) staining of renal tissue injury in sepsis. a Control (normal rat), b SO+H (sham operation group with honokiol treatment), c SR (septic rat), d SR+H (septic rat group with honokiol treatment), e SR+H+Z (septic rat group with 10 μmol/kg ZnPPIX followed by 5 mg/kg honokiol treatment). f Tubule pathology score of renal tissue injury in sepsis with a–e treatment. **P < 0.01 vs. SR and #P < 0.05 vs. SR+H.
The inflammatory cytokine TNF-α, IL-1β, and IL-6 showed little change in honokiol group relative to the control group. It was found that the production of TNF-α, IL-1β, and IL-6 was increased in the CLP group and reversed following honokiol treatment. However, ZnPPIX as the HO-1 inhibitor reversed the level of these inflammatory cytokine against the honokiol effects (Fig. 5).
The concentrations of TNF-α, IL-6, and IL-1β in peripheral blood of rat under the following conditions: Control (sham operation group), H (sham operation group with honokiol treatment), CLP (septic rat group), CLP+H (septic rat group with honokiol treatment honokiol), CLP+H+Z (septic rat group with ZnPPIX followed by honokiol treatment). **P < 0.01 and *P < 0.05 vs. control, ##P < 0.01 and #P < 0.05 vs. CLP.
Molecular Docking Confirmed the Interaction of Honokiol with Keap1/Nrf2 Complex
The molecular modeling and docking simulation methods were used to confirm the interaction between honokiol with keap1/Nrf2 complex. The results revealed that two benzene ring structures of honokiol were basically vertical. The structure of Nrf2 was computationally predicted to dock to the binding sites of Keap1. After dimensionally connecting honokiol with Nrf2-Keap1, we preliminary think that honokiol is able to activate Nrf2 and then activate Nrf2/HO-1 channel, which is probably by combining with keap1 to displace Nrf2 (Fig. 6).
Molecular docking of honokiol to keap1/Nrf2 complex. a Keap1 is on the left side and Nrf2 (red) on the other side. b Fig. a is rotated by 180°. c, d Docking conformation of honokiol (green molecular skeleton) to the crystal structure keap1 is identified with DS software. The hydrogen bond (green dotted line) is formed by hydroxyl portion of honokiol and Arg415 of keap1.
DISCUSSION
The effect of honokiol on sepsis has been investigated, with the aim of finding a method to improve the therapeutic outcome of sepsis. AKI is one of the most common complications of sepsis. Reactive oxygen species (ROS) generated during oxidative stress is the major factor that leads to the occurrence of sepsis-induced AKI [17,18,19]. There are two well-characterized free radical defense systems. One is the anti-oxidative enzyme system, such as superoxide dismutase (SOD). The other is non-anti-oxidative enzyme system, such as glutathione peroxidase (GSH). Both kinds of systems are able to remove part of the reactive oxygen species in time, which avoided excessive oxidative stress injury. SOD and GSH, as important antioxidants, can block the lipid peroxidation and remove the harmful oxygen-free radicals. As demonstrated in the current study, SOD and GSH in supernatant fluid of NRK-52E cells were clearly downregulated after incubation with septic serum, and both levels were restored by honokiol treatment. Moreover, the amount of myeloperoxidase (MPO) released from azurophil granule of neutrophils was increased during oxidative stress [20,21,22]. In the present study, we also demonstrate that MPO was downregulated in NRK-52E cells after honokiol treatment compared to sepsis group. It is thus revealed that honokiol can effectively alleviate sepsis-derived AKI in order to protect kidney cells by alleviation of oxidative stress injury.
The presence of iNOS has been thought to play an important role in the pathogenesis of renal injury. Aberrant induction and activation of iNOS act to produce large amount of NO, which has been contributed to organ injury during sepsis [23,24,25]. Excess NO has been shown to cause mitochondrial protein damage and mitochondrial respiratory function injury. A strong increase of NO during sepsis can lead to tissue hypoxia-associated high mortality and low blood pressure. For this reason, the iNOS expression pattern was profiled immunohistochemically. It was revealed that honokiol reduced iNOS expression in NRK-52E cells. Meanwhile, we found that honokiol treatment weakened the aberrant level of NO induced by sepsis. These results are consistent with the previous reports that honokiol can significantly reduce the level of NO in vivo and in vitro [26, 27]. The currently working hypothesis on the effects of honokiol indicates that honokiol plays a favorable role in the protective effect of response to oxidative stress in AKI via modulation of iNOS-derived NO decrease.
Inhibition of NF-κB activation can improve the survival rate of mice with sepsis [28, 29]. It was previously reported that NF-κB can regulate the expression of multiple genes related to the pathophysiology of sepsis [30,31,32]. According to our previous research, honokiol significantly reduced the level of NF-κB p65 in the nuclear and inhibited activation of the NF-κB signaling pathway in the kidney of septic rats, suggesting that honokiol might be used as a potential therapeutic agent for complications of sepsis-induced AKI [12]. Given these data, it is necessary to explore the molecular mechanism underlying attenuation of honokiol to enable reliable application for sepsis-induced AKI.
A body of evidence has revealed that TLR2/4/MyD88/NF-κB pathway is involved in the process of sepsis [33, 34]. Some reports demonstrate that the activation of Nrf2/HO-1 can effectively inhibit the activation of TLRs in inflammatory diseases. For example, it was reported that activation of Nrf2/HO-1 had the protective effect in the ischemic brain by modulation of TLR4 and NF-κB decrease [35]. Knockdown of keap1 enabled Nrf2 to inhibit the activation of TLR4 involved in innate immune [36]. In addition, knockdown of Nrf2 improved the production of NADPH oxidase-derived ROS to enhance TLR4-mediated excessive inflammatory response and promote the septic death.
The antioxidant enzyme HO-1 played a favorable role of the endogenous antioxidant system against oxidative stress [37, 38]. This strategy has been attempted on human pulmonary vascular endothelial cells and liver Kupffer cells as well. One study found that extract of Ginkgo biloba downregulated ROS level to inhibit the apoptosis of pulmonary vascular endothelial cells in order to enable the protective effect on the pulmonary function [39]. One report demonstrated that the expressions of Nrf2 and HO-1 were increased in the liver and kidney after the activation of liver Kupffer cells during hepatic ischemia-reperfusion (I-R) in wild-type SD rats. Moreover, Nrf2 nuclear translocation was promoted and serum creatinine clearance rate reduced. Nevertheless, the protective effect in Nrf2−/− rats disappeared [40]. In the current study, the HO-1 inhibitor ZnPPIX was used to investigate whether honokiol exerted protective effect on the sepsis-derived AKI via Nrf2/HO-1 antioxidant pathway. Our data clearly revealed that honokiol-induced protective property of Nrf2/HO-1 pathway could be weakened after HO-1 inhibitor treatment. The in vivo results showed that honokiol effectively alleviated the change of renal tubular expansion and renal tubular epithelial cell necrosis in CLP model rats. It further supports this notion underlying the improvement of honokiol to morphological changes of renal tissue of septic rats.
Consequently, our results suggest that honokiol has a protective effect on renal injury in sepsis by inducing HO-1 expression in CLP rat. This hypothesis was therefore tested using Discovery Studio software to calculate the possible molecular mechanism underlying molecular docking models of honokiol and Nrf2. It was found that honokiol was dimensionally connected with Nrf2-Keap1 complex. Therefore, we preliminary think that honokiol is able to activate Nrf2 and then activate Nrf2/HO-1 channel, which could probably combine with keap1 followed by displacing Nrf2. Current data has suggested that keap1 binding with Nrf2 is able to promote Nrf2 ubiquitin degradation [41, 42].
Taken together, the current study has revealed that levels of iNOS, NO, and MPO in NRK-52E cells are decreased by honokiol. In contrast, the activity of GSH and SOD had a significant rise after honokiol treatment. Honokiol can increase the antioxidant property of HO-1 in rats subjected to CLP and ameliorates the morphological changes in kidneys of rats subjected to CLP. The antioxidant effect can be weakened by ZnPPIX as the HO-1 inhibitor. Given these data, it is demonstrated that honokiol ameliorates oxidative stress in sepsis-induced AKI. Meanwhile, the protein detection of TLR2, TLR4, TRIF, MyD88, IκBα, and p-IκBα is performed that honokiol can inhibit the aberrant activation of TLRs signaling pathway. The inhibition of TLRs signaling pathway with the application of honokiol further supports the notion that honokiol has anti-inflammatory effect.
CONCLUSIONS
These results provide a new insight into the role of honokiol as protective role on sepsis-derived AKI via the modulation of levels of oxidative stress and expression of inflammatory cytokines in the kidney. Future clinical studies are needed to confirm the therapeutic efficacy of honokiol.
Funding Statement
This research was supported by the National Natural Science Foundation of China Grant 81703871, China Postdoctoral Science Foundation 2017M611240, and Liaoning Province S&T Project 20170520408.
References
Goncalves, G.M., D.S. Zamboni, and N.O. Camara. 2010. The role of innate immunity in septic acute kidney injuries. Shock 34 (Suppl 1): 22–26.
Mehta, R.L., J. Bouchard, S.B. Soroko, et al. 2011. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Medicine 37: 241–224.
Pinto, C.F., M. Watanabe, C.D. da Fonseca, et al. 2012. The sepsis as cause of acute kidney injury: an experimental model. Revista da Escola de Enfermagem da USP 46: 86–90.
Lentini, P., M. de Cal, D. Cruz, A. Chronopoulos, S. Soni, F. Nalesso, M. Zanella, F. Garzotto, A. Brendolan, P. Piccinni, and C. Ronco. 2010. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. Journal of Critical Care 25: 605–609.
Okazaki, Y., and A. Matsukawa. 2009. Pathophysiology of sepsis and recent patents on the diagnosis, treatment and prophylaxis for sepsis. Recent Patents on Inflammation & Allergy Drug Discovery 3: 26–32.
Doi, K., A. Leelahavanichkul, P.S. Yuen, and R.A. Star. 2009. Animal models of sepsis and sepsis-induced kidney injury. The Journal of Clinical Investigation 119: 2868–2878.
Liou, K.T., Y.C. Shen, C.F. Chen, C.M. Tsao, and S.K. Tsai. 2003. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. European Journal of Pharmacology 475: 19–27.
Kim, B.H., and J.Y. Cho. 2008. Anti-inflammatory effect of honokiol is mediated by PI3K/Akt pathway suppression. Acta Pharmacologica Sinica 29: 113–122.
Wang, Y., Z.Z. Zhang, Y. Wu, et al. 2013. Honokiol protects rat hearts against myocardial ischemia reperfusion injury by reducing oxidative stress and inflammation. Experimental and Therapeutic Medicine 5: 315–319.
Weng, T.I., H.Y. Wu, C.W. Kuo, and S.H. Liu. 2011. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Medicine 37: 533–541.
Yu, Y., M. Li, N. Su, et al. 2016. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Molecular Medicine Reports 13: 1353–1360.
Li, N., H. Xie, L. Li, J. Wang, M. Fang, N. Yang, and H. Lin. 2014. Effects of honokiol on sepsis-induced acute kidney injury in an experimental model of sepsis in rats. Inflammation 37: 1191–1199.
Qaisiya, M., Z.C.D. Coda, C. Bellarosa, and C. Tiribelli. 2014. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cellular Signalling 26: 512–520.
Kim, H.S., E.J. Park, S.W. Park, H.J. Kim, and K.C. Chang. 2013. A tetrahydroisoquinoline alkaloid THI-28 reduces LPS-induced HMGB1 and diminishes organ injury in septic mice through p38 and PI3K/Nrf2/HO-1 signals. International Immunopharmacology 17: 684–692.
Hao, E., F. Lang, Y. Chen, H. Zhang, X. Cong, X. Shen, and G. Su. 2013. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One 8: e69452.
Souza, A.C., R.A. Volpini, M.H. Shimizu, et al. 2012. Erythropoietin prevents sepsis-related acute kidney injury in rats by inhibiting NF-kappaB and upregulating endothelial nitric oxide synthase. American Journal of Physiology. Renal Physiology 302: F1045–F1054.
Parikh, S.M. 2013. Therapeutic targeting of the mitochondrial dysfunction in septic acute kidney injury. Current Opinion in Critical Care 19: 554–559.
Kong, X., R. Thimmulappa, P. Kombairaju, and S. Biswal. 2010. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. Journal of Immunology 185: 569–577.
Wu, Z., P. Puigserver, U. Andersson, C. Zhang, G. Adelmant, V. Mootha, A. Troy, S. Cinti, B. Lowell, R.C. Scarpulla, and B.M. Spiegelman. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124.
Vilela, F.M., Y.M. Fonseca, F.T. Vicentini, and M.J. Fonseca. 2013. Sunscreen protection against ultraviolet-induced oxidative stress: evaluation of reduced glutathione levels, metalloproteinase secretion, and myeloperoxidase activity. Pharmazie 68: 872–876.
Anatoliotakis, N., S. Deftereos, G. Bouras, G. Giannopoulos, D. Tsounis, C. Angelidis, A. Kaoukis, and C. Stefanadis. 2013. Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Current Topics in Medicinal Chemistry 13: 115–138.
Nakazato, T., M. Sagawa, K. Yamato, M. Xian, T. Yamamoto, M. Suematsu, Y. Ikeda, and M. Kizaki. 2007. Myeloperoxidase is a key regulator of oxidative stress mediated apoptosis in myeloid leukemic cells. Clinical Cancer Research 13: 5436–5445.
Heemskerk, S., P. Pickkers, M.P. Bouw, A. Draisma, J. van der Hoeven, W.H. Peters, P. Smits, F.G. Russel, and R. Masereeuw. 2006. Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury. Clinical Journal of the American Society of Nephrology 1: 853–862.
Aksu, U., C. Demirci, and C. Ince. 2011. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contributions to Nephrology 174: 119–128.
Fortin, C.F., P.P. McDonald, T. Fulop, and O. Lesur. 2010. Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 33: 344–352.
Hu, Z., X. Bian, X. Liu, Y. Zhu, X. Zhang, S. Chen, K. Wang, and Y. Wang. 2013. Honokiol protects brain against ischemia-reperfusion injury in rats through disrupting PSD95-nNOS interaction. Brain Research 1491: 204–212.
Singh, T., and S.K. Katiyar. 2011. Honokiol, “a phytochemical from Magnolia spp., inhibits breast cancer cell migration by targeting nitric oxide and cyclooxygenase-2”. International Journal of Oncology 38: 769–776.
Fang, J.P., Y. Liu, J. Li, W.F. Liao, Y.H. Hu, and K. Ding. 2012. A novel small molecule, HK-156, inhibits lipopolysaccharide-induced activation of NF-kappaB signaling and improves survival in mouse models of sepsis. Acta Pharmacologica Sinica 33: 1204–1216.
O'Sullivan, A.W., J.H. Wang, and H.P. Redmond. 2009. NF-kappaB and p38 MAPK inhibition improve survival in endotoxin shock and in a cecal ligation and puncture model of sepsis in combination with antibiotic therapy. The Journal of Surgical Research 152: 46–53.
Zhao, H., S. Li, H. Zhang, et al. 2015. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-kappaB activation. Experimental and Therapeutic Medicine 10: 823–827.
Thair, S., and J.A. Russell. 2013. Noncanonical nuclear factor kappa B (NF-kappaB) signaling and potential for therapeutics in sepsis. Current Infectious Disease Reports 15: 364–371.
Raspe, C., K. Hocherl, S. Rath, C. Sauvant, and M. Bucher. 2013. NF-kappaB-mediated inverse regulation of fractalkine and CX3CR1 during CLP-induced sepsis. Cytokine 61: 97–103.
Kalakeche, R., T. Hato, G. Rhodes, K.W. Dunn, T.M. el-Achkar, Z. Plotkin, R.M. Sandoval, and P.C. Dagher. 2011. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. Journal of the American Society of Nephrology 22: 1505–1516.
Hu, Y.M., M.H. Pai, C.L. Yeh, Y.C. Hou, and S.L. Yeh. 2012. Glutamine administration ameliorates sepsis-induced kidney injury by downregulating the high-mobility group box protein-1-mediated pathway in mice. American Journal of Physiology. Renal Physiology 302: F150–F158.
Li, L., X. Zhang, L. Cui, L. Wang, H. Liu, H. Ji, and Y. du. 2013. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Research 1497: 32–39.
Kong, X., R. Thimmulappa, F. Craciun, C. Harvey, A. Singh, P. Kombairaju, S.P. Reddy, D. Remick, and S. Biswal. 2011. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. American Journal of Respiratory and Critical Care Medicine 184: 928–938.
Wang, F., J. Liu, Y. Ye, X. Zhang, and L. Wan. 2014. Mechanism of cardiac function changes in rat models of Sjogren’s syndrome based on Keap1-Nrf2/ARE signaling pathways. Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology 30: 497–501.
Kensler, T.W., N. Wakabayashi, and S. Biswal. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology 47: 89–116.
Hsu, C.L., Y.L. Wu, G.J. Tang, T.S. Lee, and Y.R. Kou. 2009. Ginkgo biloba extract confers protection from cigarette smoke extract-induced apoptosis in human lung endothelial cells: role of heme oxygenase-1. Pulmonary Pharmacology & Therapeutics 22: 286–296.
Tanaka, Y., J.M. Maher, C. Chen, and C.D. Klaassen. 2007. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Molecular Pharmacology 71: 817–825.
Martinez, V.D., E.A. Vucic, L.A. Pikor, K.L. Thu, R. Hubaux, and W.L. Lam. 2013. Frequent concerted genetic mechanisms disrupt multiple components of the NRF2 inhibitor KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex in thyroid cancer. Molecular Cancer 12: 124.
Hast, B.E., D. Goldfarb, K.M. Mulvaney, M.A. Hast, P.F. Siesser, F. Yan, D.N. Hayes, and M.B. Major. 2013. Major, proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Research 73: 2199–2210.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shilin Xia and Hongli Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xia, S., Lin, H., Liu, H. et al. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation 42, 826–834 (2019). https://doi.org/10.1007/s10753-018-0937-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0937-x