Abstract
Acute kidney injury (AKI) resulting from septic shock caused by sepsis is an important health problem encountered at rates of 55–73%. Increasing oxidative stress and inflammation following sepsis is a widely observed condition with rising mortality rates. The purpose of this study was to determine whether perindopril (PER) can prevent sepsis-associated AKI with its antioxidant, anti-inflammatory, and anti-apoptotic effects. The control group received an oral saline solution only for 4 days. Cecal ligation and puncture (CLP)–induced sepsis only was applied to the CLP group, while the CLP + PER (2 mg/kg) received CLP-induced sepsis together with 2 mg/kg PER via the oral route for 4 days before induction of sepsis. Finally, all rats were euthanized by anesthesia and sacrificed. TBARS, total SH levels and NF-κβ, TNF-α, and Caspase-3 expression were then calculated for statistical analysis. TBARS, total SH, NF-kβ/p65, TNF-a, and Caspase-3 levels increased in the CLP group. In contrast, oral administration of PER (2 mg/kg) to septic rats reduced TBARS levels and NF-kβ/p65, TNF-α, and Caspase-3 immunopositivity at biochemical analysis. PER treatment appears to be a promising method for preventing sepsis-induced acute kidney injury through its antioxidant anti-inflammation and anti-apoptotic activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis is an important health problem involving severe complications that emerges when microorganisms in the host trigger antioxidant and anti-inflammatory mechanisms in favor of oxidants and inflammation [1]. Research has shown that sepsis progresses with high morbidity and mortality by causing dysfunction of more than one organ in the host [2]. Acute kidney injury (AKI) is the most important problem caused by sepsis [3]. Research has shown that mortality rates resulting from sepsis-related kidney damage should not be underestimated [4].
Although the cause of AKI is not fully understood, the main causes include increased endothelial dysfunction with sepsis-induced inflammation and renal tubular cell damage [5]. Decreased tissue oxygenation also plays an important role in the development of damage [6]. The glomerular filtration rate decreases in particular with damage, while blood urea nitrogen and creatinine levels increase significantly [7, 8]. In addition, increasing oxidative stress is one of the important mechanisms contributing to worsening AKI damage [9]. Thiobarbituric acid reactive substances (TBARS) are one of the most important markers of oxidative stress [9]. Previous studies have shown a significant increase in TBARS concentrations in sepsis-induced AKI compared with control groups [10, 11]. Oxidative stress can be eliminated with glutathione (GSH), which is endogenously present and exhibits antioxidant effects [12]. Rousta MA et al. showed that decreased GSH levels resulting from sepsis-associated AKI increased with the application of agents exhibiting antioxidant effects [13]. In addition, an excessive increase in oxidative stress under conditions of damage also triggers the inflammatory mechanism [14].
Inflammation is one of the factors caused by sepsis that play a central role in the development of AKI [15]. Previous research has shown that proinflammatory cytokine levels increase with sepsis [16, 17]. The production of pro-inflammatory cytokines, particularly interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α), increases during the resulting inflammatory process, and if this is not halted, the scale of the damage increases continually [10]. Zhaoheng Lin et al. and Yi-Zhan Cao et al. showed an increase in the synthesis of these proinflammatory cytokines in AKI associated with sepsis induced by cecal ligation puncture (CLP) in case groups [10, 14]. In addition, transcription factors such as nuclear factor-kappa beta/mitogen-activated protein kinase (NF-κβ/MAPK) are the most important pathway causing an inflammatory response in CLP-induced AKI [18]. Previous research has shown that expression of NF-κβ/p65 in the kidney triggers an oxidant and inflammatory effect, but that proinflammatory cytokine levels decrease with inhibition [10, 19]. On the other hand, inflammation occurring in the renal tissue can induce apoptosis in kidney cells [20]. In that context, it is most important to reduce apoptosis in sepsis-related AKI. The present study aimed to use immunohistochemical methods to determine levels of the important apoptosis marker Caspase-3 expression.
Sepsis is known to cause organ dysfunction by establishing endothelial damage [21]. The discovery of novel agents capable of reducing oxidative stress and inflammation that increase with damage and that can reverse potential AKI has led to new hopes in treatment. This suggested that perindopril (PER), the anti-oxidant and anti-inflammatory efficacy of which has been demonstrated in previous studies, may also exhibit beneficial effects in sepsis-related AKI [22, 23]. PER is a classic angiotensin-converting enzyme inhibitor (ACEi) with known beneficial effects in several cardiovascular diseases through mechanisms involving angiotensin II [24]. Previous studies have also proved that with its antioxidant and anti-inflammatory effects PER can prevent drug-induced renal damage [25, 26].
If oxidative stress and inflammation that increase together in the case of polymicrobial sepsis cannot be prevented, this can lead to renal losses. The purpose of this study was to show, using biochemical, histopathological, and immunohistochemical methods, the positive effects of PER, which was already demonstrated to prevent sepsis-associated lung damage in our previous study, in renal tissue through similar mechanisms.
MATERIALS AND METHODS
Animals
The 3R principle (replacement, reduction, refinement) representing new humane rules concerning animal welfare already implemented in various countries under the European framework was also applied in the present study. The experimental procedures were performed with cadaver tissues leftover from the study titled “The Protective Effects of Angiotensin-Converting Enzyme Inhibitor against Cecal Ligation and Puncture-Induced Sepsis via Oxidative Stress and Inflammation” approved by the local animal ethics committee of Recep Tayyip Erdogan University (decision No. 2020/05 dated 28.02.2020).
Experimental Animals
Twenty-four female Sprague Dawley rats weighing 290 ± 10 g were used for all biochemical, histopathological, and immunohistochemical investigations. Animals were treated in conformity with the principles of the Guide for the Care and Use of Laboratory Animals published by the National Research Council and approved by the local ethical committee. Throughout the study period, rats in all groups were housed in standard plastic cages containing sawdust flooring under normal temperature conditions of 22 ± 1 °C and 55–65% humidity and under controlled lighting (12/12 h dark/light cycle). Ad libitum access was permitted to standard rat chow and tap water. All experiments and other procedures were conducted such as to conform to the national guidelines for the use and care of laboratory animals. The study protocol was approved by the Recep Tayyip Erdogan University animal care committee (approval number: 2019/01-31.01.2019).
Chemicals
PER (Coversyl 10 mg 30 film tablets) was obtained from Servier Ilaç ve Araştırma A.S. (Istanbul, Turkey) under license from Les Laboratory Services (France). All animals were anesthetized using ketamine hydrochloride (Ketalar, 50 mg/kg, Pfizer İlaçları Ltd. Şti., Istanbul, Turkey) and sedative xylazine hydrochloride (Rompun, 10 mg/kg, Bayer, USA). All chemicals employed during laboratory experiments were procured from Sigma Chemical Co. and Merck (Germany).
Experimental Protocol
Rats were randomly assigned into three groups, each containing eight animals. Group 1 (n = 8), the control group, received oral saline solution alone for 4 days. Group 2 (n = 8), the CLP group, underwent only CLP-induced sepsis, while Group 3 (n = 8), the CLP + PER(2 mg/kg) underwent CLP-induced sepsis together with oral administration of 2 mg/kg PER for 4 days prior to induction of sepsis [27].
Cecal Ligation and Puncture-Induced Sepsis Model
Sepsis was induced using the CLP-induced model described by Rittirsch D et al. [28]. All surgical procedures were performed under sterile conditions. Anesthesia was administered with 50 mg/kg ketamine HCL injection and 10 mg/kg xylazine HCL. Following confirmation of anesthesia, a 2.5-cm incision was made to the abdominal midline. Next, the abdominal organs and the cecum were isolated and ligated distal to the ileocecal valve with the help of 3/0 silk sutures. The cecum content was brought into contact with the peritoneum by opening two holes in the distal from the mesentery to the opposite side with a 22-gage needle. The abdominal incision was then closed with two layers of sterile 4/0 synthetic absorbable sutures. The wound was finally washed with 1% lidocaine solution for analgesia. The experiment was terminated 16 h after the related treatments and surgery [29].
Once the experimental procedures had been completed, rats were sacrificed by euthanasia with a high-dose anesthetic. One kidney was stored for biochemical analysis together with the serum specimens at − 80 °C. The other kidney was separated into two halves and stored in 10% neutral formalin.
Biochemical Procedure
Tissue Sampling and Homogenization
We first prepared a mixture of 20 mM 1 L sodium phosphate + 140 mM potassium chloride (pH 7.4) [30]. One hundred milligrams of kidney tissue was homogenized with 1 ml homogenate buffer for 5 min at 30 Hz using a tissue lyser II device (Qiagen, Hilden, Germany). Next, 800g was centrifuged for 10 min at 4° C. The resulting supernatant was removed and used for TBARS and total -SH assays.
Standard Solutions
Briefly, 82.5 μL 1,1,3,3-tetramethoxypropane was added to 0.01 M 50 mL HCl solution. This was then allowed to incubate at 50 °C for 60 min. The concentration of this main stock solution was 10 μmol/mL. Next, 20, 10, 5, 2.5, 1.25, and 0.625 nmol/mL solutions were then prepared from this main stock solution.
TBARS Analysis
TBARS assay was performed following the method described by Ohkawa et al. [31]. A mixture of 200 μL tissue supernatant, 50 μL 8.1% SDS (sodium dodecyl sulfate), 375 μL 20% acetic acid (v/v) pH 3.5, and 375 μL 0.8% thiobarbituric acid (TBA) was vortexed and left to incubate for 1 h in a boiling water bath. The mixture was then cooled in ice water for 5 min and centrifuged for 10 min at 750g. The resulting pink color was read on a spectrophotometer at 532 nm. The results were calculated as nmol/mg tissue.
Total Thiol(-SH) Measurement Assay
–SH groups were assayed using Ellman’s reagent. Briefly, 1000 μL 3 M Na2HPO4 and 250 μL DTNB (4 mg DTNB was prepared in 1% 10 mL sodium citrate solution) were added to 250 μL supernatant. The resulting mixture was then vortexed, and absorbance was measured at 412 nm. The results were calculated using a 1000–62.5 μM reduced glutathione standard curve and were expressed as μmol/mg tissue.
Histopathological Analysis
Renal tissues were separated into 1.5 cm3 volume pieces and fixed for 24 h in 10% neutral formalin (Sigma-Aldrich, Germany) solution. Following fixation, these specimens were placed into tissue processing cassettes and dehydrated by being passed through increasing ethyl alcohol (Merch GmbH, Germany) series (50% (twice), 60%, 70%, 80%, 96%, and 100% (twice)) on a tissue processing device (ThermoScientific™ Citadel 2000, UK). In the following stage, tissues were rendered transparent with xylol (Merck GmbH, Darmstadt, Germany) series. The specimens were then embedded into blocks using tissue embedding cassettes with metal base molds on a paraffin embedding device (Leica EG 1150 H, Germany). Sections 4–5 μm in thickness taken from these blocks using a Rotary microtome (Leica RM2255, Germany) were stained with hematoxylin and eosin G (H&E, Merck GmbH, Darmstadt, Germany) using a staining device (Leica ST5020, Germany).
Immunohistochemical Analysis
NF-kβ/p65 (Rabbit polyclonal, ab16502, Abcam, UK) and TNF-α (Rabbit polyclonal, ab6671, Abcam, UK) primary antibody was used to show the effectiveness of pro-inflammatory cytokines, and cleaved Caspase-3 (Rabbit polyclonal to cleaved Caspase-3, ab2302, Abcam, UK) primary antibodies were used to identify apoptotic tubular cells. Secondary antibodies (Goat Anti-Rabbit IgG H&L (HRP), ab205718, Abcam, UK) compatible with the primary antibodies were also employed. Kidney tissue sections 2–3 μm in thickness were placed onto positively charged slides (Patolab, PRC). Following dehydration, hydrogen peroxide (H2O2) and endogenous peroxide procedures were performed using primary antibody kits in line with the instructions of the manufacturing company. Sections were first incubated with primary antibodies for 1 h, and then for 1 h with secondary antibodies. Counterstaining was subsequently applied with DAB chromogen (Dako, Denmark) and Harris hematoxylin (Merck GmbH, Darmstadt, Germany).
Semi-quantitative Analyses
Tumor necrosis scoring (TNS) was calculated, as shown in Table 1, by modifying the TNS system of Jeong et al. in order to subject H&E-stained sections to histopathological examination. Semi-quantitative analysis was applied to 15 randomly selected different areas of sections from each experimental animal by two histopathologists blinded to the study groups.
Immunohistochemical Analyses
Twenty randomly selected areas of kidney tissue sections incubated with NFk-β/p65, Caspase-3, and TNF-α primary antibodies were subjected to positivity scoring by two histopathologists blinded to the study groups, as shown in Table 2.
Statistical Analysis
Data elicited from the semiquantitative analysis were analyzed on SPSS 18.00 (IBM Corp. IL, Chicago, USA) software. Median and 25% and 75% interquartile ranges were calculated, and differences between groups were evaluated using the Kruskal-Wallis and Bonferroni-corrected Mann-Whitney U tests. p-values ≤ 0.05 were regarded as statistically significant. Parametric data obtained from biochemical analyses were calculated as mean ± standard deviation, and differences between groups were subjected to a one-way analysis of variance (ANOVA), followed by the Tukey test.
RESULTS
Biochemical Results
TBARS and Total -SH Analysis Results
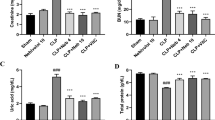
TBARS levels in the control and CLP groups (54.72 ± 1.95 nmol/g tissue and 57.96 ± 4.59 nmol/g tissue, respectively) were higher than in the treatment group (46.01 ± 3.45 nmol/g tissue). TBARS levels were also higher in the CLP group than in the control group, but the difference was not statistically significant. TBARS levels decreased statistically significantly in the PER treatment group compared with the other groups (p < 0.01, Table 3). Similarly, total thiol levels also increased significantly in the treatment group compared with the other groups (p < 0.05, Table 3). Total -SH values were higher in the control and CLP groups (3.05 ± 0.31 μmol/g tissue, 3.23 ± 0.48 μmol/g tissue, respectively) than in the PER treatment group (2.31 ± 0.47 μmol/g tissue). Total -SH was also higher in the CLP group than in the control group, but the difference was not statistically significant.
Histopathological Analysis Results
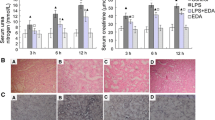
The renal corpuscle in the control group consisted of a normal glomerulus and Bowman’s capsule. Proximal and distal tubular epithelial cells were also normal in appearance (Fig. 1a, b; Table 4; TNS: 1(0-1). In contrast, we detected widespread vacuolization in proximal and distal tubular epithelial cells and loss of connections between epithelial cells in the CLP application group. Tubular lumen debris accumulation and vascular congestion in intertubular spaces were also present. Notable loss of brush border structures was also observed in proximal tubular epithelial cells (Fig. 1c,d; Table 4; TNS: 7(6-7)). In the PER-treatment group, we observed vascular congestion and decreased loss in proximal and distal tubular epithelial cells. We also observed typical brush structures in proximal tubular epithelial cells (Fig. 1e,f; Table 4; TNS: 1(1-2.5)).
Representative light microscopy images of kidney tissue sections exposed to cecal ligation and PER treatment. H&E. A(x20)-B(x40): Control group sections exhibiting renal corpuscles consisting of a normal glomerulus (g) and Bowman’s capsule (b). The brush border in proximal (p) and distal (d) tubules is normal in appearance (TNS:1(0-1). C(x20)-D(x40): CLP group sections exhibit losses in proximal and distal tubular epithelial cells (spiral arrow) and debris accumulation in the tubular lumen (*). In addition, vacuolization in proximal tubular epithelial cells in particular and loss of brush border structures (blue arrow) can also be seen. Vascular congestion can also be seen in the intertubular spaces (c)(TNS: 7(6-7). E(x20)-F(x40): PER treatment group section exhibit decreased losses in proximal and distal tubular epithelial cells and decreased debris accumulation in tubular lumens, together with typical tubular cells (TNS: 1(1-2.5)).
Immunohistochemical Results
Kidney tissue sections incubated with NF-kβ primary antibody were examined under a light microscope. NF-kβ positivity was significantly higher in the CLP application group than in the control group (Fig. 2a,d; Table 5; p = 0.002). In contrast, NF-kβ positivity decreased in the PER treatment group compared with the CLP application group (Fig. 2c,f; Table 5; p = 0.001).
Representative light microscopic images of kidney tissue sections incubated with NFk-β/P65 primary antibody. A(x20)-B(x40): Control group sections exhibit normal renal corpuscles and tubules (NFk-β/P65: positivity score: 0(0-1). C(x20)-D(x40): NFk-β/P65 positivity can be seen in proximal and distal epithelial cells in the CLP treatment group (NFk-β/P65: positivity score: 3(2-3). E(x20)-F(x40): a significant decrease in NFk-β/P65 positivity can be seen in proximal and distal epithelial cells in the PER treatment group (NFk-β/P65: positivity score: 0.5(0-1).
Examination under light microscopy of kidney tissue sections incubated with TNF-α antibody revealed greater TNF-α positivity in proximal and distal tubular epithelial cells in the CLP application group compared with the control group (Fig. 3a–d; Table 5; p = 0.004). However, TNF-α positivity decreased in the PER treatment group compared with that in the CLP application group (Fig. 3 c–f; Table 5; p = 0.005).
Examination under light microscopy of kidney tissue sections incubated with cleaved Caspase-3 antibody revealed mild Caspase-3 positivity in proximal and distal tubular epithelial cells in the CLP application group compared with the control group (Fig. 4a–d; Table 5; p = 0.048).
Representative light microscopic images of kidney tissue sections incubated with cleaved Caspase-3 primary antibody. A(x20)-B(x40): Control group sections exhibit normal renal corpuscles and proximal and distal tubules (cleaved Caspase-3 positivity score: 0(0-1). C(x20)-D(x40): Mild cleaved Caspase-3 positivity can be seen in proximal and distal epithelial cells in the CLP group (cleaved Caspase-3 positivity score: 1(1-1). E(x20)-F(x40): A decrease in cleaved Caspase-3 positivity can be seen in proximal and distal epithelial cells in the PER treatment group (cleaved Caspase-3 positivity score: 0.5(0-1).
Representative light microscopic images of kidney tissue sections incubated with TNF-α primary antibody. A(x20)-B(x40): Control group sections exhibit normal renal corpuscles and proximal and distal tubules (TNF-α positivity score: 0(0-1). C(x20)-D(x40): TNF-α positivity can be seen in proximal and distal epithelial cells in the CLP group (TNF-α positivity score: 1(1-1). E(x20)-F(x40): A decrease in cleaved TNF-α positivity can be seen in proximal and distal epithelial cells in the PER treatment group (TNF-α positivity score: 0.5(0-1).
DISCUSSION
This experimental research revealed the protective effects of PER in sepsis-induced kidney damage. The application of PER before sepsis played an effective role in preventing renal damage.
Kidney damage caused by sepsis, or AKI, is an important pathological problem. The most important mechanisms involved in triggering this damage in previous studies are sepsis-induced oxidative stress and inflammation [32]. The present study also identified AKI by measuring the principal oxidant and pro-inflammatory levels. AKI was thus revealed using both histopathological and immunohistochemical methods.
One of the most important parameters in the present research, and one of the main markers of oxidative damage, is TBARS levels [33]. TBARS and malondialdehyde (MDA) are products of damage that increases with oxidative stress [33, 34]. Zhang Z et al. showed an increase in MDA levels compared with those in a control group following sepsis-induced AKI [35]. Similarly to the present study, Chuang CH, et al. also showed an increase in MDA levels in kidney damage caused by endotoxigenic shock [36]. In the present study, damage developing following lipid peroxidation was expressed as TBARS equivalent nmol/mg tissue, and was in agreement with previous studies. This finding is one of the most important markers of kidney damage emerging with oxidative stress caused by sepsis. TBARS levels decreased in the PER-treated group, and PER was observed to reduce AKI caused by sepsis due to its antioxidant effects. This was consistent with our previous study showing the protective effect of PER on lung tissue [22].
GSH, studied in terms of preventing the effects of oxidative stress and endogenous in origin, occupies an important place in cell defense [37]. GSH is a thiol (-SH)-containing tripeptide molecule [38]. One aim of the present study was to detect antioxidant activity by measuring total thiol levels. Guang-Dao Chen et al. showed that total septic AKI-associated GSH levels decreased compared to a control group [39]. Shilin Xia et al. also reported similar results [40]. In the present study, total thiol levels in tissue were measured, and total –SH levels in the case group increased compared with the control group. This finding was inconsistent with previous studies. This suggests that endogenous GSH stores may have been triggered to protect the cell against oxidative stress caused by damage. In the treatment group, in contrast to previous studies, PER application reduced total–SH levels compared with the case group, suggesting that this resulted from stores being completely depleted during damage prevention.
The induction of oxidative stress in sepsis-induced AKI in previous research has also been shown to cause an increase in the production of such pro-inflammatory cytokines as TNF-α, Il-1β and in particular [32, 41]. Renal dysfunction may therefore be inevitable if the resulting inflammation cannot be brought under control. NF-κB is the most important molecule triggering the synthesis of pro-inflammatory cytokines. Increased ROS activity in favor of oxidation in association with cellular injury triggers NF-κB-mediated inflammation and a vicious circle between them [15, 41]. From that perspective, our data obtained using immunohistochemical methods were consistent with previous studies. Abdel-Gawad S Shalkami et al. showed that PER exhibits antioxidant and anti-inflammatory effects [25, 42]. Similarly in the present study, we observed that PER reduced inflammation caused by sepsis in kidney tissue through its anti-inflammatory activity at the expression level.
Oxidative stress and inflammation, among the principal causes of AKI development, also contribute to AKI by inducing apoptosis [13]. Previous research has shown increased apoptotic enzyme activity on case groups [13, 43]. The Caspase family is one of the most important biomarkers of apoptosis and increased Caspase-3 levels in tissue leads to the triggering of programmed cell death [44]. Lin Liu et al. reported that endotoxigenic sepsis-induced oxidative stress and inflammation also led to AKI by triggering apoptosis [45]. Similarly to those studies, we observed that Caspase-3 expression levels determined using immunohistochemistry increased with CLP and thus contributed to AKI. Caspase-3 levels decreased following PER treatment and were effective in preventing AKI. This finding was consistent with previous research into PER [22, 46].
One limitation of this study is that serum BUN, creatinine, and angiotensin-converting enzyme levels could not be measured. However, this research is an important pilot study showing the antioxidant and anti-inflammatory effects of PER in kidney tissue.
In conclusion, PER prevented kidney injury caused by sepsis-induced AKI through its antioxidant, anti-inflammatory, and anti-apoptotic effects. PER thus exhibited promising results in terms of prevention of AKI. However, further studies assessing other oxidative stress enzymes and molecules, inflammation, and intracellular Ca+2 levels and revealing the clinical effects of these are now needed.
References
Mehta, Ravindra L., Josée Bouchard, Sharon B. Soroko, T. Alp Ikizler, Emil P. Paganini, Glenn M. Chertow, and Jonathan Himmelfarb. 2011. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Medicine 37: 241–248. https://doi.org/10.1007/s00134-010-2089-9.
Cecconi, Maurizio, Laura Evans, Mitchell Levy, and Andrew Rhodes. 2018. Sepsis and septic shock. The Lancet 392: 75–87. https://doi.org/10.1016/S0140-6736(18)30696-2.
Schrier, Robert W., and Wei Wang. 2004. Mechanisms of disease: Acute renal failure and sepsis. New England Journal of Medicine 351: 159–169 + 201. https://doi.org/10.1056/NEJMra032401.
Umbro, Ilaria, Giuseppe Gentile, Francesca Tinti, Paolo Muiesan, and Anna Paola Mitterhofer. 2016. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. Journal of Infection 72: 131–142. https://doi.org/10.1016/j.jinf.2015.11.008.
Jo, Sang Kyung, Su Ah. Sung, Won Yong Cho, Kang Jee Go, and Hyoung Kyu Kim. 2006. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrology, Dialysis, Transplantation 21: 1231–1239. https://doi.org/10.1093/ndt/gfk047.
Langenberg, C., L. Wan, M. Egi, C.N. May, and R. Bellomo. 2006. Renal blood flow in experimental septic acute renal failure. Kidney International 69: 1996–2002. https://doi.org/10.1038/sj.ki.5000440.
Li, Xing, Mu Genhua, Chunmei Song, Liangliang Zhou, Lei He, Qin Jin, and Lu. Zhongqian. 2018. Role of M2 macrophages in sepsis-induced acute kidney injury. Shock 50: 233–239. https://doi.org/10.1097/SHK.0000000000001006.
de Paulo Rodrigues, Francisco Adelvane, Alan Diego da Conceição Santos, Pedro Henrique Quintela Soares de Medeiros, Mara de Moura Gondim Prata, Tailane Caína de Souza Santos, James Almada da Silva, Gerly Anne de Castro Brito, et al. 2018. Gingerol suppresses sepsis-induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production. Scientific Reports 8: 1–10. https://doi.org/10.1038/s41598-018-30522-6.
Pavlakou, Paraskevi, Vassilios Liakopoulos, Theodoros Eleftheriadis, Michael Mitsis, and Evangelia Dounousi. 2017. Oxidative stress and acute kidney injury in critical illness: pathophysiologic mechanisms - biomarkers - interventions, and future perspectives. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2017/6193694.
Lin, Zhaoheng, Jing Jin, and Xiyun Shan. 2019. Fish oils protects against cecal ligation and puncture-induced septic acute kidney injury via the regulation of inflammation, oxidative stress and apoptosis. International Journal of Molecular Medicine 44: 1771–1780. https://doi.org/10.3892/ijmm.2019.4337.
Campos, Renata, Maria Heloísa Massola Shimizu, Rildo Aparecido Volpini, Ana Carolina de Bragança, Lucia Andrade, Fernanda Degobbi Tenório Quirino dos Santos Lopes, Clarice Olivo, Daniele Canale, and Antonio Carlos Seguro. 2012. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. American Journal of Physiology - Lung Cellular and Molecular Physiology 302: 640–650. https://doi.org/10.1152/ajplung.00097.2011.
Lushchak, Volodymyr I. 2012. Glutathione homeostasis and functions: potential targets for medical interventions. Journal of Amino Acids 2012: 1–26. https://doi.org/10.1155/2012/736837.
Rousta, Ali Mohammad, Seyed Mohamad Sadegh Mirahmadi, Alireza Shahmohammadi, Davood Nourabadi, Mohammad Reza Khajevand-Khazaei, Tourandokht Baluchnejadmojarad, and Mehrdad Roghani. 2018. Protective effect of sesamin in lipopolysaccharide-induced mouse model of acute kidney injury via attenuation of oxidative stress, inflammation, and apoptosis. Immunopharmacology and Immunotoxicology 40: 423–429. https://doi.org/10.1080/08923973.2018.1523926.
Cao, Yi Zhan, Yan Yang Tu, Xiang Chen, Bo Liang Wang, Yue Xia Zhong, and Ming Hua Liu. 2012. Protective effect of ulinastatin against murine models of sepsis: inhibition of TNF-α and IL-6 and augmentation of IL-10 and IL-13. Experimental and Toxicologic Pathology 64: 543–547. https://doi.org/10.1016/j.etp.2010.11.011.
Gan, Y., S. Tao, D. Cao, H. Xie, and Q. Zeng. 2017. Protection of resveratrol on acute kidney injury in septic rats. Human and Experimental Toxicology 36: 1015–1022. https://doi.org/10.1177/0960327116678298.
Luo, Cong-Juan, Feng Luo, Bu Quan-Dong, Wei Jiang, Wei Zhang, Xue-Mei Liu, Che Lin, et al. 2019. Protective effects of resveratrol on acute kidney injury in rats with sepsis. Biomedical Papers. 164: 49–56. https://doi.org/10.5507/bp.2019.006.
Wang, Nian, Li Mao, Liu Yang, Jiang Zou, Ke Liu, Meidong Liu, Huali Zhang, Xianzhong Xiao, and Kangkai Wang. 2017. Resveratrol protects against early polymicrobial sepsis-induced acute kidney injury through inhibiting endoplasmic reticulum stress-activated NF-κB pathway. Oncotarget 8: 36449–36461. https://doi.org/10.18632/oncotarget.16860.
Sun, Guodong, Wei Yang, Zhang Yang, and Mingyan Zhao. 2017. Esculentoside A ameliorates cecal ligation and puncture-induced acute kidney injury in rats. Experimental Animals 66: 303–312. https://doi.org/10.1538/expanim.16-0102.
Li, Guofu, Linlin Gao, Jia Jia, Xiaoying Gong, Bin Zang, and Weimin Chen. 2014. α-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clinical and Experimental Pharmacology and Physiology 41: 459–468. https://doi.org/10.1111/1440-1681.12244.
Gao, Li, Wei Feng Wu, Lei Dong, Gui Ling Ren, Hai Di Li, Qin Yang, Xiao Feng Li, et al. 2016. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Frontiers in Pharmacology 7: 1–16. https://doi.org/10.3389/fphar.2016.00479.
Shum, Hoi Ping, Wing Wa Yan, and Tak Mao Chan. 2016. Recent knowledge on the pathophysiology of septic acute kidney injury: a narrative review. Journal of Critical Care 31: 82–89. https://doi.org/10.1016/j.jcrc.2015.09.017.
Kostakoglu, Ugur, Atilla Topcu, Mehtap Atak, Levent Tumkaya, Tolga Mercantepe, and Huseyin Avni Uydu. 2020. The protective effects of angiotensin-converting enzyme inhibitor against cecal ligation and puncture-induced sepsis via oxidative stress and inflammation. Life Sciences 241: 117051. https://doi.org/10.1016/j.lfs.2019.117051.
Ali, Mohammed Ragab Abdel Aziz, Amira Morad Hussein Abo-Youssef, Basim Anwar Shehata Messiha, and Mahmoud Mohamed Khattab. 2016. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn-Schmiedeberg’s Archives of Pharmacology 389: 637–656. https://doi.org/10.1007/s00210-016-1234-6.
Ancion, Arnaud, Julien Tridetti, Mai-Linh Nguyen Trung, Cécile Oury, and Patrizio Lancellotti. 2019. A review of the role of bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: focus on perindopril. Cardiology and Therapy 8: 179–191. https://doi.org/10.1007/s40119-019-00150-w.
Shalkami, Abdel Gawad S., Mohamed I.A. Hassan, and Ahmed A. Abd El-Ghany. 2018. Perindopril regulates the inflammatory mediators, NF-κB/TNF-α/IL-6, and apoptosis in cisplatin-induced renal dysfunction. Naunyn-Schmiedeberg’s Archives of Pharmacology 391: 1247–1255. https://doi.org/10.1007/s00210-018-1550-0.
Tang, S.C.W., J.C.K. Leung, L.Y.Y. Chan, A.A. Eddy, and K.N. Lai. 2008. Angiotensin converting enzyme inhibitor but not angiotensin receptor blockade or statin ameliorates murine adriamycin nephropathy. Kidney International 73: 288–299. https://doi.org/10.1038/sj.ki.5002674.
Mashhoody, Tahereh, Karim Rastegar, and Fatemeh Zal. 2014. Perindopril may improve the hippocampal reduced glutathione content in rats. Advanced Pharmaceutical Bulletin 4: 155–159. https://doi.org/10.5681/apb.2014.023.
Rittirsch, Daniel, Markus S. Huber-lang, Michael A. Flierl, and Peter A. Ward. 2009. Immunodesing of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36. https://doi.org/10.1038/nprot.2008.214.Immunodesign.
Cinar, Irfan, Busra Sirin, Pelin Aydin, Erdem Toktay, Elif Cadirci, Iclal Halici, and Zekai Halici. 2019. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sciences 221: 327–334. https://doi.org/10.1016/j.lfs.2019.02.039.
Rojas, Denise Bertin, Tanise Gemelli, Rodrigo Binkowski De Andrade, Aline Guimarães Campos, Carlos Severo Dutra-Filho, and Clóvis Milton Duval Wannmacher. 2012. Administration of histidine to female rats induces changes in oxidative status in cortex and hippocampus of the offspring. Neurochemical Research 37: 1031–1036. https://doi.org/10.1007/s11064-012-0703-7.
Ohkawa, Hiroshi, Nobuko Ohishi, and Kunio Yagi. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95: 351–358. https://doi.org/10.1016/0003-2697(79)90738-3.
Başol, Nurşah, Oytun Erbaş, Türker Çavuşoğlu, Ayfer Meral, and Utku Ateş. 2016. Sepsis nedenli akut böbrek hasarında agomelatinin etkilerinin değerlendirilmesi. Ulusal Travma ve Acil Cerrahi Dergisi 22: 121–126. https://doi.org/10.5505/tjtes.2015.29499.
Peña-Bautista, Carmen, Miguel Baquero, Máximo Vento, and Consuelo Cháfer-Pericás. 2019. Free radicals in Alzheimer’s disease: lipid peroxidation biomarkers. Clinica Chimica Acta 491: 85–90. https://doi.org/10.1016/j.cca.2019.01.021.
Wheeler, Derek S. 2013. Oxidative stress in critically ill children with sepsis. Open Inflammation Journal 4: 74–81. https://doi.org/10.2174/1875041901104010074.Oxidative.
Zhang, Zhijie, Huatang Zhao, Dongjian Ge, Shanshan Wang, and Bin Qi. 2019. β-casomorphin-7 ameliorates sepsis-induced acute kidney injury by targeting NF-κB pathway. Medical Science Monitor 25: 121–127. https://doi.org/10.12659/MSM.912730.
Chuang, C.H., C.K. Yang, P.H. Wu, Y. Zhang, and P.J. Yang. 2018. Acute renal injury induced by endotoxic shock in rats is alleviated via PI3K/Nrf2 pathway. European Review for Medical and Pharmacological Sciences 22: 5394–5401. https://doi.org/10.26355/eurrev_201808_15742.
Bar-Or, David, Raphael Bar-Or, Leonard T. Rael, and Edward N. Brody. 2015. Oxidative stress in severe acute illness. Redox Biology 4: 340–345. https://doi.org/10.1016/j.redox.2015.01.006.
Lim, Jinhwan, Samiha Ali, Lisa S. Liao, Emily S. Nguyen, Laura Ortiz, Samantha Reshel, and Ulrike Luderer. 2020. Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione deficient mice. Biology of Reproduction: 1–45. https://doi.org/10.1093/biolre/ioaa009.
Chen, Guang Dao, Jun Liang Zhang, Yi Ting Chen, Ju Xing Zhang, Tao Wang, and Qi Yi Zeng. 2018. Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Experimental and Therapeutic Medicine 15: 3967–3975. https://doi.org/10.3892/etm.2018.5890.
Xia, Shilin, Hongli Lin, Han Liu, Zhidan Lu, Hui Wang, Songtao Fan, and Nan Li. 2019. Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation 42: 826–834. https://doi.org/10.1007/s10753-018-0937-x.
Yan, Xi Xiang, Ai Dong Zheng, Zhen En Zhang, Guo Cui Pan, and Wen Zhou. 2019. Protective effect of pantoprazole against sepsis-induced acute lung and kidney injury in rats. American Journal of Translational Research 11: 5197–5211.
Knight, Jasper, Chris Caseldine, and Maxwell T. Boykoff. 2010. Forum review: forum review. Geographical Journal 176: 267–269. https://doi.org/10.1111/j.1475-4959.2010.00371.x.
Qin, Yi, Guizhen Wang, and Zhiyong Peng. 2019. MicroRNA-191-5p diminished sepsis-induced acute kidney injury through targeting oxidative stress responsive 1 in rat models. Bioscience Reports 39: 1–11. https://doi.org/10.1042/BSR20190548.
Zhao, Yuan, Xiujing Feng, Bei Li, Jichen Sha, Chaoran Wang, Tianyuan Yang, Hailin Cui, and Honggang Fan. 2020. Dexmedetomidine protects against lipopolysaccharide-induced acute kidney injury by enhancing autophagy through inhibition of the PI3K/AKT/mTOR pathway. Frontiers in Pharmacology 11: 1–13. https://doi.org/10.3389/fphar.2020.00128.
Liu, Lin, Yijin Song, Ming Zhao, Zhuwen Yi, and Qiyi Zeng. 2015. Protective effects of edaravone, a free radical scavenger, on lipopolysaccharide-induced acute kidney injury in a rat model of sepsis. International Urology and Nephrology 47. 1745–1752. https://doi.org/10.1007/s11255-015-1070-5.
Zhu, Zhenyu, Huihui Li, Wanli Chen, Yameng Cui, Anan Huang, and Xin Qi. 2020. Perindopril improves cardiac function by enhancing the expression of SIRT3 and PGC-1α in a rat model of isoproterenol-induced cardiomyopathy. Frontiers in Pharmacology 11: 1–11. https://doi.org/10.3389/fphar.2020.00094.
Author information
Authors and Affiliations
Contributions
UK, TM, and SB conceived and designed the research. UK and LT conducted the experiments. HKY, HAU, and TM contributed new reagents or analytical tools. UK, HAU, HKY, TM, and SB analyzed the data. UK and TM wrote the manuscript. All authors read and approved the final text.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was approved by the Recep Tayyip Erdogan University animal care committee (approval number. 2020/05 dated 28.02.2020).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kostakoglu, U., Mercantepe, T., Yilmaz, H.K. et al. The Protective Effects of Perindopril Against Acute Kidney Damage Caused by Septic Shock. Inflammation 44, 148–159 (2021). https://doi.org/10.1007/s10753-020-01316-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01316-8