Abstract
Paeoniflorin, a type of bioactive monoterpene glucoside in Paeoniae Radix, possesses anti-oxidative, anti-inflammatory and anti-hyperglycaemic properties. However, the underlying mechanism of paeoniflorin in treating atherosclerosis is unclear. A rat model of high-fat diet-induced atherosclerosis and palmitic acid (PA)-treated vascular smooth muscle cells (VSMCs) were used in this study. The serum concentrations of total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) were determined, and the results indicated that paeoniflorin remarkably lowered the levels of TC, TG and LDL-C induced by a high-fat diet. Histopathological results showed that paeoniflorin significantly improved the pathological changes in the aorta. In addition, paeoniflorin also maintained a normal weight gain speed. Subsequently, the effects of paeoniflorin on the production of inflammatory cytokines (IL-1β, IL-6 and TNF-α) were detected by qPCR and ELISA. The qPCR and ELISA results showed that paeoniflorin decreased the levels of these inflammatory cytokines. Moreover, the expression of TLR4 and its downstream pathway molecules was measured by Western blot. The results indicated that paeoniflorin significantly reduced the expression of TLR4 and MyD88 as well as the phosphorylation of IκBα and NF-κB p65. Taken together, these results suggested that paeoniflorin could alleviate atherosclerotic inflammation by inhibiting the TLR4/MyD88/NF-κB pathway. Therefore, paeoniflorin may be a potential therapy for atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cardiovascular disease is one of the leading causes of death worldwide, and the major cause of this disease is atherosclerosis [1]. Atherosclerosis is a complex disease characterized by chronic inflammation with extensive macrophage and lymphocyte invasion into lesions, thereby promoting pathogenesis [2]. There is growing evidence that inflammation is deeply involved in the progression of atherosclerosis [3]. Inflammation is essential for host defence against invading pathogens, but unchecked inflammation can cause tissue damage and organ failure in the host [4].
NF-κB is a redox-sensitive transcription factor that regulates various aspects of the immune and inflammatory response [5]. It is well known that activation of the NF-κB pathway will contribute to the excessive production of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α [6]. Toll-like receptors (TLRs) are pathogen pattern recognition receptors with a vital role in the activation of both innate and adaptive immunity via controlling the production of cytokines in response to various inflammatory stimuli [7]. TLR4 recognizes endogenous ligands, such as oxidized LDL, fibronectin and heat shock proteins, which are involved in the inflammatory processes of atherosclerosis [8].
There is growing evidence that vascular smooth muscle plays a key role in many physiological and pathological events of the blood vessels, including atherosclerosis [9]. Inflammatory cytokines stimulate the migration and proliferation of vascular smooth muscle cells (VSMCs) and then initiate the inflammatory cascade inside the arterial wall [10]. Proliferation of VSMCs is essential for neointimal development in vessels in vascular diseases [11]. Moreover, palmitic acid, the most abundant saturated fatty acid among free fatty acids, also plays an important role in the development of atherosclerosis [12].
To date, many Chinese herbal medicines have been widely applied in the treatment of inflammatory diseases [4]. Paeoniflorin is one of the bioactive monoterpene glucosides in Paeoniae Radix, the roots of Paeonia lactiflora [13]. Accumulating evidence has shown that paeoniflorin possesses anti-oxidative, anti-inflammatory and anti-hyperglycaemic properties [14,15,16]. Although several studies have reported on the anti-inflammatory activities of paeoniflorin, no research has demonstrated the effect of paeoniflorin on atherosclerosis. Therefore, in the present study, we attempted to investigate whether paeoniflorin could protect against high-fat diet-induced atherosclerosis in rats and elucidate the potential anti-inflammatory mechanisms in palmitic acid (PA)-treated VSMCs. The results of the present study might support the clinical application of paeoniflorin in treating atherosclerosis.
MATERIALS AND METHODS
Reagents
Paeoniflorin (HPLC ≥ 98%) was purchased from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China) (Fig. 1). Palmitic acid was purchased from Sigma (St. Louis, USA). Foetal bovine serum (FBS) and 0.25% trypsin-EDTA were purchased from Gibco (Grand Island, NY, USA). VSMCs were purchased from the American Type Culture Collection (ATCC T/G HA-VSMC). IL-1β, IL-6 and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from BioLegend (Camino Santa Fe, USA). All antibodies used in the study were purchased from Cell Signaling Technology (Danvers, MA). qPCR was carried out using the SYBR green Plus reagent kit (Roche Applied Science, Mannheim, Germany). All other chemicals and reagents were of the highest commercial grade available.
Animals
Six to 8-week-old Sprague-Dawley (SD) rats (200 ± 20 g) were obtained from Guangdong Medical Laboratory Animal Center (Foshan, China). Rats were maintained on a 12-h light/dark cycle at a temperature of 22–24 °C and humidity of 40 ± 50% and fed ad libitum. Animal welfare and experimental procedures were performed strictly in accordance with animal welfare and other related ethical regulations approved by the animal experiment centre of Shenzhen University. Rats were randomly divided into five groups as follows (n = 10):
Atherosclerotic group (AG): The atherosclerosis rat model was established by administration of excessive vitamin D and cholesterol as described by a previous study [17]. Briefly, the rats were first intraperitoneally injected with 600,000 U/kg vitamin D3 and then additional injections of a 300,000 U/kg dosage were repeated every 30 days. At the same time, rats were also intragastrically administered 10 mL/kg fat emulsion (each 500 mL contains 25 g of cholesterol, 5 g of sodium cholate, 75 g lard, 50 mL propylene glycol, 50 mL Tween-80 and distilled water) every day for 15 weeks.
Control group (CG): The control group was intragastrically administered with the same volume of saline as the paeoniflorin group.
Paeoniflorin-treated group: Paeoniflorin-treated groups were intragastrically administered with paeoniflorin (10 or 20 mg/kg) once a day for 15 weeks.
Simvastatin group (SG): The simvastatin group was given simvastatin (5 mg/kg) using the same treatment schedule.
The weight of rats in all groups was recorded every week after the rats were fasted for 12 h. At the end of the 15th weeks, blood samples from all groups were collected and centrifuged (1500×g for 10 min at 4 °C) to obtain the serum. Then, all rats were euthanized by CO2 inhalation, and the aortic tissues were collected and stored at − 80 °C.
Analysis of Serum TC, TG, LDL-C and HDL-C
The concentrations of blood lipids including TC, TG, LDL-C and HDL-C were determined according to the manufactures’ protocols (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histopathologic Evaluation of Aortas
Rats aortic tissues were harvested for histopathological examination. The tissue samples were fixed in 4% paraformaldehyde for 48–72 h, dehydrated with graded alcohol, embedded in paraffin and then stained with haematoxylin (H) and eosin (E). Then, morphological changes in the aortas were observed with an optical microscope (Olympus, Japan).
Cell Culture and Treatment
VSMCs were cultured in DMEM/F12 medium supplemented with 10% FBS, 2 mM L-glutamine, 50 U/mL penicillin and 50 μg/mL streptomycin. The cells were maintained in a 5% CO2 humidified incubator at 37 °C. The cell model of atherosclerosis was established as previously described. In brief, PA powder was dissolved in ethanol to 200 mmol/L at 70 °C, and then diluted with a 10% solution of fatty acid-free bovine serum albumin (BSA, as the formation of BSA complexes is important for decreasing the cell toxicity of fatty acids) to 5 mmol/L at 55 °C for 10 min. The solution was cooled to room temperature, and filtered by a 0.45-μm filter. The cells were pretreated with various concentrations of paeoniflorin (60 and 100 μmol/L) for 1 h and then were stimulated with PA (100 μmol/L) for 24 h. Cells that were not given any treatment were used as the control group.
MTT Assay of Cell Viability
The effect of paeoniflorin on VSMC viability was measured using the standard MTT assay. The cells (1 × 105 cells/mL) were plated in 96-well plates at 37 °C for 12 h. Then, paeoniflorin (5, 10, 30, 60, 100 μmol/L) was added to VSMCs for 24 h. Then, 20 μL of MTT (5 mg/mL) was given for 4 h at 37 °C, the supernatant was removed, and 100 μL of dimethyl sulfoxide was added per well. The absorbance was read at 570 nm with a microplate reader (Thermo, USA).
Cytokine Determination
VSMCs were seeded into six-well plates and grown until 80–85% confluent; paeoniflorin and PA were added or were not added, and incubation was continued for an additional 24 h. Cell-free supernatants and aorta tissues were subsequently used for inflammatory cytokine assays with rat ELISA kits according to the manufacturer’s instructions. Finally, the absorbance was read at 450 nm with a microplate reader (Thermo, USA).
Quantitative PCR Analysis
VSMCs were seeded onto six-well plates and grown until 80–85% confluent. Paeoniflorin and PA were added or were not added, and incubation was continued for an additional 24 h. The cells were washed twice with ice-cold PBS; then, 1 mL of the TRIzol (Molecular Research Center, USA) reagent was added to each well and processed as directed by the manufacturer, and the cell lysates were collected. The purity of total RNA was determined using the 260/280 nm ratio, and then, cDNA was synthesized using the first strand cDNA synthesis kit (Takara, China). The Primer Premier software (PREMIER Biosoft International, USA) was used to design specific primers for TNF-α, IL-1β, IL-6 and GAPDH based on known sequences (Table 1). The expression levels of each target gene were normalized to corresponding GAPDH threshold cycle (CT) values using the 2−△△CT comparative method.
Western Blot Analysis
The total protein of aortic tissues and VSMCs was extracted according to the manufacturer’s recommended protocol (Vazyme, Nanjing, China). Protein concentrations were determined using the BCA Protein Assay Kit (Vazyme, Nanjing, China). Samples with equal amounts of protein (50 μg) were fractionated on 10% SDS polyacrylamide gels, transferred to polyvinylidene difluoride membranes and blocked in 5% skim milk in TBST for 1.5 h at 25 ± 1 °C. Proteins were hybridized with a primary antibody (1:1000 dilution) at 4 °C overnight. Then, the membrane was incubated with secondary antibodies conjugated with horseradish peroxidase (1:4000 dilution) for 1 h at room temperature. The optical density values of immunoblot signals were visualized using the ECL Plus Western Blotting Detection System (ImageQuant LAS 4000mini, USA). Data were normalized against those of the corresponding β-actin values.
Statistical Analysis
All experiments repeated for a minimum of three times, and results were analysed using GraphPad Prism 5 (GraphPad InStat Software). The differences between groups were analysed by one-way ANOVA. A p value of < 0.05 was considered to be statistically significant.
RESULTS
Paeoniflorin Treatment Decreased the Weight of Rats with Atherosclerosis
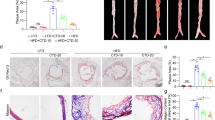
With high-fat intake, rapid weight gain is one of the risk factors for atherosclerosis. Our model rats showed a rapid increase in body weight. To examine the effects of paeoniflorin on the body weight of rats, the animals were weighed. As shown in Fig. 2, compared with the control group, the weight of rats was obviously increased in the atherosclerotic group. In the simvastatin or paeoniflorin treatment groups (10 or 20 mg/kg/day), a normal weight gain speed was maintained (Fig. 2).
The effects of paeoniflorin on the body weight of rats. CG is the control group, AG is the atherosclerotic group, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG.
Effects of Paeoniflorin on Serum TC, TG, LDL-C and HDL-C Levels
As shown in Fig. 3, the levels of TC, TG and LDL-C were dramatically increased in the atherosclerotic group compared with those in the control group. In contrast, treatment with paeoniflorin (10, 20 mg/kg/day) or simvastatin significantly decreased the serum levels of TC, TG and LDL-C. However, the levels of HDL-C in different groups were not significantly different.
The effects of paeoniflorin on levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) in serum. CG is the control group, AG is the atherosclerotic group, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG.
Histological Analysis of the Aorta
Histological analysis showed that there was a clear vessel structure and that the vascular intima, media and adventitia had a clear boundary in the control group (Fig. 4a). Meanwhile, the vessels of the atherosclerotic group showed obviously thickened walls, severely damaged vascular internal elastic membranes and unclear elastic fibre layer structure (Fig. 4b). However, treatment with paeoniflorin evidently reduced the pathological conditions (shown in Fig. 4c, d) and exhibited similar results as simvastatin treatment (Fig. 4e).
Effects of Paeoniflorin on Cell Viability
The potential cytotoxicity of paeoniflorin on VSMCs was estimated using the MTT assay. The results showed that paeoniflorin treatment had little effect on cell viability at the concentrations used in this study (Fig. 5).
Paeoniflorin Decreased the Production of Inflammatory Cytokines
The expression of inflammatory cytokines in the aorta and VSMCs was determined using qPCR (Fig. 6) and ELISA (Fig. 7). The results of both experiments showed markedly increased expression of IL-1β, IL-6 and TNF-α in the atherosclerotic group compared to the control group. However, paeoniflorin treatment decreased the levels of IL-1β, IL-6 and TNF-α.
The effects of paeoniflorin on the expression of inflammatory cytokine mRNA in the aorta and VSMCs. a The expression of IL-1β, IL-6 and TNF-α mRNA was detected by qPCR in the aorta. b The expression of IL-1β, IL-6 and TNF-α mRNA in cells. GAPDH was used as the internal control. CG is the control group, AG is the atherosclerotic group for the animal studies, PG is the palmitic acid group for the cell culture studies, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups for the animal studies, respectively, and the 60 and 100 μmol/L paeoniflorin groups for the cell culture studies, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG or PG.
The effects of paeoniflorin on the production of inflammatory cytokine proteins in the aorta and VSMCs. a The levels of IL-1β, IL-6 and TNF-α proteins in the aorta were measured using ELISA. b The levels of IL-1β, IL-6 and TNF-α proteins in cells. CG is the control group, AG is the atherosclerotic group for the animal studies, PG is the palmitic acid group for the cell culture studies, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups for the animal studies, respectively, and the 60 and 100 μmol/L paeoniflorin groups for the cell culture studies, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG or PG.
Effects of Paeoniflorin on the Expression of TLR4
TLRs, including TLR4, have been reported play a significant role in the progression of atherosclerosis [18]. Thus, we detected the effects of paeoniflorin on the expression of TLR4 in the aorta and VSMCs by qPCR and Western blot. The results showed that paeoniflorin inhibited TLR4 mRNA and protein expression, which was significantly increased in the atherosclerotic group (Fig. 8).
The effects of paeoniflorin on the expression of TLR4 in the aorta and in VSMCs. a TLR4 protein expression in the aorta was measured using Western blot. b TLR4 protein expression in cells. β-actin was used as the internal control. CG is the control group, AG is the atherosclerotic group for the animal studies, PG is the palmitic acid group for the cell culture studies, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups for the animal studies, respectively, and the 60 and 100 μmol/L paeoniflorin groups for the cell culture studies, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG or PG.
Effects of Paeoniflorin on the Expression of TLR4 Downstream Pathway Molecules
The adaptor molecule MyD88 is a downstream protein of the TLR4 pathway during the inflammatory process. Our results also showed increased expression of MyD88 in the atherosclerotic group. However, the level of MyD88 protein was suppressed following the simvastatin or paeoniflorin treatments.
Accumulating evidence has shown that abnormal expression of TLR4 could subsequently activate downstream MyD88-dependent NF-κB pathways. Therefore, the inhibitory effects of paeoniflorin on the NF-κB pathway in the aorta and VSMCs were determined using Western blot. Our results showed that phosphorylation of the IκBα and NF-κB p65 proteins was markedly increased in the atherosclerotic group compared to the control group. However, these trends were greatly inhibited by paeoniflorin treatment (Fig. 9).
The effects of paeoniflorin on the expression of TLR4 downstream pathway molecules. a The protein expression of MyD88, p-IκBα and p-NF-κB p65 in the aorta was measured using Western blot. b The protein expression of MyD88, p-IκBα and p-NF-κB p65 in cells. β-actin was used as the internal control. CG is the control group, AG is the atherosclerotic group for the animal studies, PG is the palmitic acid group for the cell culture studies, SG is the simvastatin group, and the L and H are the 10 and 20 mg/kg paeoniflorin groups for the animal studies, respectively, and the 60 and 100 μmol/L paeoniflorin groups for the cell culture studies, respectively. The values were obtained from three independent experiments and are represented as the mean ± S.E.M. # p < 0.05 vs. CG, and *p < 0.05 vs. AG or PG.
DISCUSSION
Atherosclerosis, the underlying cause of heart attack, stroke and peripheral vascular disease, is a main cause of morbidity and mortality worldwide [17]. As atherosclerosis is a chronic disease requiring lifetime preventive therapy, new anti-atherogenic drugs should meet the criteria of being safe, palatable, inexpensive and of significant benefit beyond currently available therapies [19]. Recently, growing interest has been focused on the development of new anti-inflammatory herbs for their effective properties and their ability to produce fewer side effects [20]. Compared with the existing drugs, paeoniflorin has its own advantages: a wide variety of sources, low cost, simple extraction process and pleiotropic effects [21]. In the present study, the rats in the atherosclerotic group showed considerably higher levels of blood lipids, such as TC, TG and LDL-C, indicating that atherosclerotic rat models were successfully constructed. However, their levels were dramatically decreased by paeoniflorin treatment. In addition, we also observed that paeoniflorin significantly improved the pathological state of the artery and reduced the weight of rats with atherosclerosis.
It is well known that inflammation occurs as a host response to pathogenic challenges or tissue injuries, but acute inflammation is a process with limited benefits and can lead to the development of inflammatory diseases [22]. Pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6, appear in the early phase of the inflammatory response [23]. IL-1β is a pro-inflammatory cytokine that is released from macrophages upon stimulation with inflammatory stimuli [24]. TNF-α has dual roles in both innate immunity and inflammatory pathology, and its gene regulation is challenging [25]. IL-6 plays an important role in mediating the negative feedback on the inflammatory responses [26]. In the present study, we observed that paeoniflorin dose-dependently inhibited the expression of pro-inflammatory cytokines both in vivo and in vitro. The results presented here confirmed that paeoniflorin has anti-inflammatory activities.
To further understand the mechanism by which paeoniflorin exerts its anti-inflammatory actions, we investigated the effects of paeoniflorin on atherosclerosis and the mechanisms involved in inflammation in a mouse model of atherosclerosis and in VSMCs. TLR4 is essential for initiating the activation of innate defences and mediates the activation of the downstream factors MyD88 and NF-κB, leading to the production of a large number of inflammatory cytokines [27]. The expression of TLR4 and its downstream adaptor molecule MyD88 was reduced by paeoniflorin treatment.
NF-κB, a widely expressed nuclear transcription factor, plays a vital role in the regulation of a variety of inflammatory mediators [28]. NF-κB p65 is normally retained in the cytoplasm. Phosphorylation of IκBα allows NF-κB p65 to translocate to the nucleus and induce transcription [29]. In the present study, the phosphorylation of IκBα and NF-κB p65 proteins was dose-dependently inhibited by paeoniflorin. The above results indicated that the beneficial effects of paeoniflorin on atherosclerosis are due to the inhibition of the TLR4-mediated NF-κB signalling pathway.
In conclusion, our study suggests that paeoniflorin has a protective function in atherosclerosis. It could inhibit the secretion of pro-inflammatory cytokines via the suppression of the TLR4-mediated NF-κB signalling pathway. Therefore, paeoniflorin may be a potential drug for the treatment of atherosclerosis.
References
Wang, X., Q. Chen, H. Pu, Q. Wei, M. Duan, C. Zhang, T. Jiang, X. Shou, J. Zhang, and Y. Yang. 2016. Adiponectin improves NF-kappaB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids in Health and Disease 15: 33. doi:10.1186/s12944-016-0202-y.
Kipari, T., P.W. Hadoke, J. Iqbal, T.Y. Man, E. Miller, A.E. Coutinho, Z. Zhang, et al. 2013. 11beta-hydroxysteroid dehydrogenase type 1 deficiency in bone marrow-derived cells reduces atherosclerosis. The FASEB Journal 27 (4): 1519–1531. doi:10.1096/fj.12-219105.
Sueta, D., S. Hokimoto, K. Sakamoto, T. Akasaka, N. Tabata, K. Kaikita, O. Honda, M. Naruse, and H. Ogawa. 2017. Validation of the high mortality rate of malnutrition-inflammation-atherosclerosis syndrome: community-based observational study. International Journal of Cardiology 230: 97–102. doi:10.1016/j.ijcard.2016.12.072.
Wu, H., G. Zhao, K. Jiang, C. Li, C. Qiu, and G. Deng. 2016. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-kappaB activation. Journal of Agricultural and Food Chemistry 64 (31): 6171–6178. doi:10.1021/acs.jafc.6b02304.
Chung, J.H., S.Y. Choi, J.Y. Kim, D.H. Kim, J.W. Lee, J.S. Choi, and H.Y. Chung. 2007. 3-Methyl-1,2-cyclopentanedione down-regulates age-related NF-kappaB signaling cascade. Journal of Agricultural and Food Chemistry 55 (16): 6787–6792. doi:10.1021/jf070952p.
Wu, H., G. Zhao, K. Jiang, X. Chen, Z. Zhu, C. Qiu, C. Li, and G. Deng. 2016. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kappaB and MAPK activation. International Immunopharmacology 35: 315–322. doi:10.1016/j.intimp.2016.04.013.
Li, F., P. Liu, X. Zhang, Q. Zhang, S. Tang, M. Zhu, and M. Qiu. 2013. 1,25(OH)2D3-mediated amelioration of aortic injury in streptozotocin-induced diabetic rats. Inflammation 36 (6): 1334–1343. doi:10.1007/s10753-013-9672-5.
Hodgkinson, C.P., R.C. Laxton, K. Patel, and S. Ye. 2008. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 28 (12): 2275–2281. doi:10.1161/atvbaha.108.175992.
Won, K.J., P. Lee, S.H. Jung, X. Jiang, C.K. Lee, H.Y. Lin, H. Kang, et al. 2011. 3-Morpholinosydnonimine participates in the attenuation of neointima formation via inhibition of annexin A2-mediated vascular smooth muscle cell migration. Proteomics 11 (2): 193–201. doi:10.1002/pmic.200900834.
Skoog, T., W. Dichtl, S. Boquist, C. Skoglund-Andersson, F. Karpe, R. Tang, M.G. Bond, et al. 2002. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. European Heart Journal 23 (5): 376–383. doi:10.1053/euhj.2001.2805.
Ueda, K., Q. Lu, W. Baur, M.J. Aronovitz, and R.H. Karas. 2013. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arteriosclerosis, Thrombosis, and Vascular Biology 33 (8): 1837–1843. doi:10.1161/atvbaha.112.300752.
Wu, D., J. Liu, X. Pang, S. Wang, J. Zhao, X. Zhang, and L. Feng. 2014. Palmitic acid exerts pro-inflammatory effects on vascular smooth muscle cells by inducing the expression of C-reactive protein, inducible nitric oxide synthase and tumor necrosis factor-alpha. International Journal of Molecular Medicine 34 (6): 1706–1712. doi:10.3892/ijmm.2014.1942.
Kim, I.D., and B.J. Ha. 2010. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Archives of Pharmacal Research 33 (6): 959–966. doi:10.1007/s12272-010-0620-8.
Kong, P., R. Chi, L. Zhang, N. Wang, and Y. Lu. 2013. Effects of paeoniflorin on tumor necrosis factor-alpha-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia 91: 44–50. doi:10.1016/j.fitote.2013.08.010.
Tsuboi, H., K. Hossain, A.A. Akhand, K. Takeda, J. Du, M. Rifa'i, Y. Dai, A. Hayakawa, H. Suzuki, and I. Nakashima. 2004. Paeoniflorin induces apoptosis of lymphocytes through a redox-linked mechanism. Journal of Cellular Biochemistry 93 (1): 162–172. doi:10.1002/jcb.20134.
Zhang, L.L., W. Wei, N.P. Wang, Q.T. Wang, J.Y. Chen, Y. Chen, H. Wu, and X.Y. Hu. 2008. Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflammation Research 57 (8): 388–395. doi:10.1007/s00011-007-7240-x.
Li, J., C.X. Chen, and Y.H. Shen. 2011. Effects of total glucosides from paeony (Paeonia lactiflora Pall) roots on experimental atherosclerosis in rats. Journal of Ethnopharmacology 135 (2): 469–475. doi:10.1016/j.jep.2011.03.045.
Yang, L., Y. Chu, L. Wang, Y. Wang, X. Zhao, W. He, P. Zhang, et al. 2015. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-kappaB pathway. International Immunopharmacology 28 (1): 525–530. doi:10.1016/j.intimp.2015.07.001.
Glass, C.K., and J.L. Witztum. 2001. Atherosclerosis. The road ahead. Cell 104 (4): 503–516.
Wu, H., G. Zhao, K. Jiang, X. Chen, Z. Zhu, C. Qiu, and G. Deng. 2016. Puerarin exerts an antiinflammatory effect by inhibiting NF-kB and MAPK activation in Staphylococcus aureus-induced mastitis. Phytotherapy Research 30 (10): 1658–1664. doi:10.1002/ptr.5666.
Zhang, L., B. Yang, and B. Yu. 2015. Paeoniflorin protects against nonalcoholic fatty liver disease induced by a high-fat diet in mice. Biological & Pharmaceutical Bulletin 38 (7): 1005–1011. doi:10.1248/bpb.b14-00892.
Rim, H.K., C.H. Yun, J.S. Shin, Y.W. Cho, D.S. Jang, J.H. Ryu, H. Park, and K.T. Lee. 2013. 5,6,7-Trimethoxyflavone suppresses pro-inflammatory mediators in lipopolysaccharide-induced RAW 264.7 macrophages and protects mice from lethal endotoxin shock. Food and Chemical Toxicology 62: 847–855. doi:10.1016/j.fct.2013.10.025.
Zhang, Xuemei, Keji Song, Huanzhang Xiong, Hongyu Li, Chu Xiao, and Xuming Deng. 2009. Protective effect of abamectin on acute lung injury induced by lipopolysaccharide in mice. Fundamental & Clinical Pharmacology 25 (6): 700–707.
Kim, D.H., M.E. Kim, and J.S. Lee. 2015. Inhibitory effects of extract from G. lanceolata on LPS-induced production of nitric oxide and IL-1β via down-regulation of MAPK in macrophages. Applied Biochemistry and Biotechnology 175 (2): 1–9.
Wu, X., W. Xu, X. Feng, Y. He, X. Liu, Y. Gao, S. Yang, Z. Shao, C. Yang, and Z. Ye. 2015. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. International Journal of Immunopathology and Pharmacology 28 (3): 351–361. doi:10.1177/0394632015593228.
Hopkins, S.J. 2003. The pathophysiological role of cytokines. Legal Medicine (Tokyo) 5 (Suppl 1): S45–S57.
Zhang, Z., N. Chen, J.B. Liu, J.B. Wu, J.B. Zhang, Y. Zhang, and X. Jiang. 2014. Protective effect of resveratrol against acute lung injury induced by lipopolysaccharide via inhibiting the myd88‑dependent Toll-like receptor 4 signaling pathway. Molecular medicine reports 10(1): 101–106. doi:10.3892/mmr.2014.2226.
Zhu, T., W. Zhang, S.J. Feng, and H.P. Yu. 2016. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARgamma-dependent pathway. International Immunopharmacology 34: 16–24. doi:10.1016/j.intimp.2016.02.014.
Sun, Z., and R. Andersson. 2002. NF-kappaB activation and inhibition: a review. Shock 18 (2): 99–106.
Acknowledgments
This work was supported by special funds in the future industry of Shenzhen (JCYJ20150324141711654) and the key project on the basic research in Shenzhen, China, P.R. (JC201005250070A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, H., Jiao, Y. & Xie, M. Paeoniflorin Ameliorates Atherosclerosis by Suppressing TLR4-Mediated NF-κB Activation. Inflammation 40, 2042–2051 (2017). https://doi.org/10.1007/s10753-017-0644-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0644-z