Abstracts
Grateloupia lanceolata is a red alga native to coastal areas of East Asia. In this study, extract from G. lanceolata (EGL) was investigated for suppressive effects on lipopolysaccharide (LPS)-induced inflammatory responses in RAW 264.7 macrophages. EGL was found to have anti-inflammatory properties with the inhibition of nitric oxide (NO), pro-inflammatory cytokine production, and MAPK signaling in LPS-induced RAW 264.7 macrophages. Moreover, treatment of RAW 264.7 macrophage with EGL inhibited LPS-induced IL-1β production in a dose-dependent manner. These inhibitory effects were found with the blockage of p38 mitogen-activated protein kinases (MAPK), extracellular signal regulated kinases 1 and 2 (ERK1/2), and also c-Jun N-terminal kinases 1 and 2 (JNK1/2). These results indicated that anti-inflammatory actions of EGL in RAW 264.7 macrophages involved in the inhibition of LPS-induced p38MAPK/ERK/JNK signaling pathways. In addition, our findings suggest that EGL holds great promise for use in the treatment of various inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a complex biological response to harmful stimuli that is associated with immune response to tissue injury and occurs when the human body attempts to counteract potentially injurious agents, such as viruses, bacteria, and other pathogens [1–3]. Macrophages play an essential role in the local host defense and inflammation [4]. In addition, macrophage plays an important role in triggering inflammation during pathological conditions by overproducing inflammatory mediators via upregulation of inducible genes that contribute to inflammatory response, including asthma and endotoxin-mediated septic shock as well as other inflammatory vascular disease [5, 6]. In response to inflammatory stimuli, activated macrophages produce various pro-inflammatory molecules, including cytokines and nitric oxide (NO) [7]. NO plays an important role in many diseases, such as inflammation, hypertension, diabetes, and atherosclerosis [8, 9]. The inducible nitric oxide synthase (iNOS) is induced in response to various inflammatory stimuli, including lipopolysaccharide (LPS), and is responsible for excessive NO production by macrophages during the inflammatory process [10]. Activation of toll-like receptor by LPS leads to the activation of the MAP kinases (MAPKs). MAPKs are composed of three principal family members with distinct isoforms within each member: extracellular signal-regulated kinases (ERKs), p38 MAPK, and c-Jun NH2-terminal kinases (JNKs) [11]. Activation of MAPKs and NF-κB are important for iNOS activity and cellular responses to extracellular signals and the regulation of pro-inflammatory cytokines, especially interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [12, 13]. Therefore, targeted reduction of NO and pro-inflammatory cytokine production has been proposed as an effective strategy for treating inflammatory diseases.
Grateloupia lanceolata (G. lanceolata) was first discovered in southern California. Typically, seaweeds reside in the seas of northeastern Asia and North America. The distribution of G. lanceolata has been limited to the eastern shores of Asia for a long time. G. lanceolata is a red algae consisting of citrulline and taurine as major components. This seaweed has a short stalk appressorium with vertical width that can be separated into two or three branches. However, the biological functions of G. lanceolata remain poorly understood. Thus, investigation into the biological effect and mechanism of how this seaweed influences anti-inflammation and NO production at the molecular level would be invaluable.
In this study, we showed that an extract from G. lanceolata (EGL) inhibited inflammation during LPS-induced macrophage activation by controlling the production of NO and pro-inflammatory cytokine such as IL-1β via the regulation of MAPKs signaling.
Materials and Methods
Chemicals
LPS from Escherichia coli, Griess reagent, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), ethylenediaminetetraacetic acid (EDTA), 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine hydrochloride (AMT), fetal bovine serum (FBS), and antibiotics were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), and Dulbecco’s Modified Eagle’s Medium (DMEM) from Hyclone (Logan, UT, USA). Polyvinylidene fluoride (PVDF) membrane (Immobilon-P) was obtained from Millipore Co. (Billerica, MA, USA). Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of G. lanceolata Extract
We collected G. lanceolata in Wando, South Korea, during February 2012. After removing salt and debris, the seaweed was incubated at 37 °C to eliminate moisture. The collected samples were washed gently with clean water three times and then dried with hot air (40 °C) for 2 days. The extract was isolated with 10 volumes (v/w) of 70 % ethanol at room temperature overnight; this procedure was repeated three times. The extracts were filtered through Whatman filter paper No. 2 (Whatman Ltd., Maidstone, Kent, England), concentrated with a vacuum evaporator and completely dried with a freeze dryer. The powder was dissolved in 100 % ethanol.
Assay for Cell Viability
The macrophage RAW 264.7 cell line was incubated in DMEM at 37 °C under 5 % CO2 humidified air. The cells were seeded onto a 96-well plate at a density of 1 × 104 cells per well and incubated at 37 °C for 24 h. The cells were then treated with various concentrations of EGL (25, 50, 100 μg/mL for extract). After an additional 24 h of incubation at 37 °C, 100 μL of MTT (0.5 mg/mL in PBS) was added to the wells and mixed well. After incubation in 37 °C for 4 h, MTT solution was removed and formazan product was dissolved in solubilization solution (1:1 = dimethyl sulfoxide:ethanol) into a colored solution. Absorbance of the formazan solution was quantified by an ELISA microplate reader at 570 nm.
Assay for Inhibition of Cellular NO Production
RAW 264.7 macrophages obtained from ATCC were cultured in DMEM supplemented with 10 % FBS and 1 % antibiotics in a 5 % CO2 atmosphere at 37 °C. The cells were activated with LPS as previously described. Briefly, the cells were plated in 96-well plates (5 × 104 cells/well). After pre-incubation for 2 h with various concentration of EGL, LPS (500 ng/mL) was added and incubated for 18 h unless otherwise specified. The samples dissolved in EtOH were diluted to the appropriate concentrations with serum-free DMEM. The final concentration of EtOH was adjusted to 0.1 %. To assess NO production, the stable conversion product of NO was measured using the Griess reagent and the optical density was determined at 540 nm.
Cytokine Producing Assay by ELISA
RAW 264.7 macrophages (5 × 104 cells/well) were seeded in 96-well u-bottom culture plates. Cells were pretreated with various concentrations of EGL (25–100 μg/L) for 2 h, and then stimulated with LPS (500 ng/mL) for 48 h. Cultured cells were separated by microcentrifugation, and the supernatant was used for samples. IL-1β release was measured by murine IL-1β ELISA MAX™ Deluxe Sets (BioLegend, CA, USA), according to the manufacturer’s protocol. Briefly, standards and samples were incubated on a capture antibody-coated plate overnight at 4 °C. Detection antibody was added and samples were incubated for 1 h and then avidin-horseradish peroxidase (HRP) bound to the detection antibody. Substrate solution was added to each well, and then the reaction was stopped by addition of a stop solution (2N H2SO4). Absorbance was measured by ELISA microplate reader at a wavelength of 405 nm.
Real-Time PCR Analysis Assay
To assess the effects of EGL on the transcriptions of genes encoding IL-1β (sense: 5′-GTG TCT TTC CCG TGG ACC TT, anti-sense: 5′-TCG TTG CTT GGT TCT CCT TG-3′), and on the housekeeping enzyme glyceraldehydes-3-phosphate dehydrogenase (GAPDH, 5′-TGC ACC ACC AAC TGC TTA G-3′, anti-sense: 5′-GGA TGC AGG GAT GAT GTT C-3′), RAW 264.7 macrophages grown to 70 % confluence on plates with/without EGL were homogenized using TRIzol reagent (Life Technologies, CA, USA). Total RNA was then isolated, and 0.5 μg RNA of each groups were used for reverse transcription to make cDNA. Reverse transcription was performed using the following conditions: initial incubation at room temperature for 10 min and then subjected to 42 °C for 15 min, 97 °C for 5 min, and at 5 °C for 5 min in a GeneAmp PCR System 2700 (Applied Biosystems, CA, USA). Aliquots of cDNA were amplified in AccuPower® GreenStar qPCR premix (Bioneer Co., Korea) using an ExiCycler™ 96 Real-time Quantitative Thermal Block (Bioneer Co., Korea).
Western Blot Analysis
RAW 264.7 macrophages (2 × 106 cells/well) were seeded in a 60Φ cell culture dish and starved with serum-free DMEM for 6 h. After starvation, cells were pretreated with EGL for 2 h, and then stimulated with 500 ng/mL of LPS, for 15, 30, and 60 min. Treated cells were washed with cold phosphate-buffered saline (PBS) and lysed with modified radioimmunoprecipitation assay (RIPA) buffer (50 mmol/L Tris-HCl, 0.1 % sodium dodecyl sulfate [SDS], 0.5 % sodium deoxycholate, 1 % NP-40, and 150 mmol/L sodium chloride, at pH 8.0) at 4 °C for 30 min. The lysates were centrifuged at 13,000×g for 15 min, and the supernatant was used as protein samples. Protein concentration was measured according to the manufacturers’ instructions by colorimetric bicinchoninic acid (BCA) kit (Thermo Scientific, PA, USA). Equivalent amounts of protein were separated by 12 % SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA). The membranes were incubated with blocking solution (5 % skim milk in Tris-buffered saline [TBS]) for 1 h. After blocking, membranes were probed with anti-ERK1/2, anti-phospho-ERK1/2, anti-JNK1/2, anti-phospho-JNK1/2, anti-p38, and anti-phospho-p38 primary antibodies (Santa Cruz Biotechnology, CA, USA) and then with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (Santa Cruz Biotechnology) for 2 h. Bands were visualized using an enhanced chemiluminescence (ECL) (Bio-Rad, CA, USA) detection system and exposed to radiographic film.
Statistics
All results were expressed as the means ± SD of the indicated number of experiments. Statistical significance was estimated using Student’s t-test for unpaired observations, and the differences were compared with regard to statistical significance by one-way ANOVA, followed by Bonferroni’s post hoc test. The categorical data from the fertility test were subjected to statistical analysis via chi-square test. A P of <0.01 was considered significant.
Results
EGL Inhibits NO Production in LPS-Induced Macrophages Activation
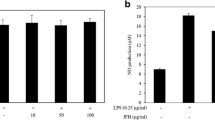
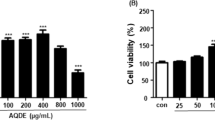
We first examined the cytotoxicity of EGL in macrophages using the MTT assay. The cells were cultured in the presence of various concentrations of EGL (25, 50, and 100 μg/mL). Treatment with 25 to 100 μg/mL of EGL for 24 h did not affect macrophage cytotoxicity (Fig. 1). To investigate whether EGL inhibits NO production, we examined NO levels in LPS-stimulated macrophages following pretreatment with EGL for 2 h. Our data demonstrate that LPS treatment significantly induced NO production compared with control (Fig. 2). However, pretreatment of macrophages with EGL inhibited LPS-induced NO production significantly in a dose-dependent manner. These results suggest that EGL inhibit NO production in LPS-stimulated macrophages are noncytotoxic.
EGL did not affect cell viability in RAW 264.7 murine macrophages. RAW 264.7 cells were plated into 96-well cell culture plates. Various concentrations of EGL and vehicle control (0.1 % ethanol) were added, and viable cell numbers were assessed by MTT assay after 24 h incubation, as described in the “Materials and Methods” section. Data are reported as viable cell numbers expressed as a percentage of control cells that were exposed to 0.1 % ethanol only. The data represent the average (±SD) of four replicate wells and are representative of three independent experiments
EGL inhibited LPS-induced NO production in RAW 264.7 macrophages. RAW 264.7 cells were plated into 96-well cell culture plates. Cells were pretreated with various concentrations of EGL (25–100 μg/mL) for 2 h before incubation in the presence or absence of LPS (500 ng/mL) for 22 h. Supernatants were mixed with Griess reagent, and absorbance was measured using an ELISA microplate reader. The data represent the average (±SD) of four replicate wells and are representative of three independent experiments. (##P < 0.01 vs control (0.1 % ethanol); **P < 0.01 vs LPS only groups)
EGL Suppresses the Expression of IL-1β Genes and Production
IL-1β is a pro-inflammatory cytokine that is released from macrophages upon stimulation with LPS or other inflammatory stimuli. To examine the effects of EGL in IL-1β expression and production, RT-PCR and ELISA were performed to measure IL-1β levels in cell lysate or culture media produced by macrophages pretreated with EGL for 2 h and then stimulated with LPS for 6 and 24 h, respectively. As shown in Fig. 3a, stimulation of macrophages with LPS increased IL-1β expression, which was significantly reduced by treatment of EGL. Moreover, EGL reduced the level of IL-1β secreted by the cells (Fig. 3b). These results suggest that EGL inhibits the production of IL-1β as well as the expression of IL-1β.
EGL inhibited LPS-induced IL-1β expression and production in RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with various concentration of EGL (25–100 μg/mL) for 2 h, and then stimulated with LPS (500 ng/mL) for 6 h. The mRNA level of the indicated inflammation-associated genes was determined by real-time PCR. EGL treatment reduced the level of IL-1β mRNA. The GAPDH was used as the internal control (a). Supernatants were examined for IL-1β secretion by ELISA (b). Data are expressed as the average pg/mL ± SD of triplicate cultures. (##P < 0.01 vs control (0.1 % ethanol); **P < 0.01 vs LPS only groups)
EGL Inhibits LPS-Induced Activation of p38 MAPK/ERK/JNK
The MAPKs such as ERK1/2, JNK1/2, and p38 MAPK are involved in signaling pathways that regulate inflammatory mediators via activation of transcription factors, especially NF-κB. To assess the molecular basis of EGL-mediated inhibition of inflammatory mediators, we determined the effect of EGL on LPS-induced MAP kinase phosphorylation. As shown in Fig. 4a, phosphorylation of ERK1/2, JNK1/2, and p38 MAPK were increased at 30 and 60 min in LPS-induced macrophages. However, treatment with EGL strongly inhibited LPS-induced phosphorylation of ERK1/2 and JNK1/2. In contrast, p38 phosphorylation was slightly decreased by treatment with EGL. This result indicates that the ERK and JNK pathway may contribute to EGL-mediated inhibition of IL-1β gene expression and production in LPS-stimulated macrophages.
EGL inhibited LPS-induced ERK/JNK phosphorylation and NF-κB translocation. RAW 264.7 cells were starved in serum-free DMEM before pretreatment with 100 μg/mL of EGL. After 2 h, the cells were stimulated with 500 ng/mL LPS for 15, 30, and 60 min. a Cells were lysed and equal amounts of protein from whole cell extracts were resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were probed with anti-ERK1/2, anti-phospho-ERK1/2, anti-JNK1/2, anti-phospho-JNK1/2, anti-p38, and anti-phospho-p38 antibodies. b To determine NF-κB translocation, nuclear and cytosolic fractions were probed with an anti-phospho-p65 antibody. β-actin was used as the internal control. The results are from one of four experiments that showed a similar pattern
EGL Attenuates LPS-Stimulated NF-κB Activation
Activated NF-κB plays critical roles in LPS-induced expression of inflammatory mediators and cytokines such as iNOS and IL-1β in macrophages. Therefore, we determined the effects of EGL on LPS-stimulated activation of the NF-κB subunit p65. As shown in Fig. 4b, EGL inhibited LPS-stimulated phosphorylation of NF-κB p65 in macrophages, suggesting the NF-κB pathway, along with the ERK and JNK signaling cascade, may contribute to the inhibitory effects of EGL on downregulating pro-inflammatory mediators such as NO and IL-1β.
Discussion
Macrophages are one of the major cell types that infiltrate the sites of chronic inflammatory disease, such as rheumatoid arthritis, fibrosis, and atherosclerosis [8, 9]. Moreover, macrophage generates reactive oxygen species (ROS) and NO [14]. It is the first line of defense inhaled particles. The imbalance between the exposure to oxidants and the endogenous antioxidants is found to play an important role in various pathological conditions including immunosuppresssion, asthma, and premature aging [15]. In this study, we demonstrated that EGL possesses effective anti-inflammatory properties that inhibit the production of pro-inflammatory mediators such as NO and IL-1β in LPS-stimulated macrophages. Moreover, our results demonstrated that EGL suppressed the expression of IL-1β mRNA and production (Fig. 3). EGL also exhibited inhibitory effects on LPS-stimulated phosphorylation of the ERK1/2 and JNK1/2, which may mediate its anti-inflammatory activity by suppressing NF-κB activation.
NO is a highly reactive molecule with important roles in several biological processes and inflammation [7, 16]. NO production can be mediated by the products of different NO metabolites, but also iNOS expression [17]. Generated by immune-activated macrophages at sites of inflammation, NO plays critical roles in the process of macrophage activation as well as both acute and chronic inflammations. Therefore, suppression of NO production by inhibiting or reducing iNOS protein levels can be a very important therapeutic approach in the development of anti-inflammatory agents. Our data revealed that EGL inhibits NO production in LPS-induced macrophage activation (Fig. 2).
MAP kinases are a highly conserved family of protein serine/threonine kinases with critical roles in iNOS upregulation induced by various stimuli. Several studies have reported that MAP kinase phosphorylation is involved in LPS-induced iNOS expression. Moreover, activation of IκB/NF-κB is associated with the MAP kinase signaling pathway. Previous studies have also shown that degradation of IκB-α following its phosphorylation and the rapid translocation of the p65 and p50 subunits are essential processes for NF-κB activation in response to various stimuli. Upon activation by LPS, this transcription factor controls the expression of genes associated with cell survival, pro-inflammatory enzymes, and cytokines, such as iNOS, TNF-α, COX-2, IL-1β, and IL-6. In LPS-stimulated macrophages, iNOS and COX-2 expression was dependent on NF-κB activation. Therefore, we examined the effect of EGL on the phosphorylation of MAP kinases and the NF-κB subunit p65. As shown in Fig. 4, we found that phosphorylation of ERK1/2, JNK1/2, and the NF-κB subunit p65 were all significantly inhibited in LPS-stimulated macrophages by pretreated with EGL. These results suggest that EGL may not only inhibit the activation of NF-κB, but also upstream signaling proteins such as ERK1/2 and JNK1/2.
In summary, our results demonstrate that EGL suppresses NO synthesis in LPS-stimulated macrophages. Moreover, inhibition of pro-inflammatory cytokine such as IL-1β expression and production is related to the blockade in ERK1/2, JNK1/2, and NF-κB signaling in LPS-stimulated macrophages. Although further investigation is still required to elucidate the exact mechanism, this study provides important information regarding the use of EGL as a candidate therapeutic agent against inflammation.
References
Islam, M. N., Ishita, I. J., Jin, S. E., Choi, R. J., Lee, C. M., Kim, Y. S., Jung, H. A., & Choi, J. S. (2013). Food and Chemical Toxicology, 55, 541–548.
Chiu, I. M., Heesters, B. A., Ghasemlou, N., Von Hehn, C. A., Zhao, F., Tran, J., Wainger, B., Strominger, A., Muralidharan, S., Horswill, A. R., Bubeck Wardenburg, J., Hwang, S. W., Carroll, M. C., & Woolf, C. J. (2013). Nature, 501, 52–57.
Tsai, S. Y., Segovia, J. A., Chang, T. H., Morris, I. R., Berton, M. T., Tessier, P. A., Tardif, M. R., Cesaro, A., & Bose, S. (2014). PLoS Pathogens, 10, e1003848.
Grimm, M. J., Vethanayagam, R. R., Almyroudis, N. G., Dennis, C. G., Khan, A. N., D’Auria, A. C., Singel, K. L., Davidson, B. A., Knight, P. R., Blackwell, T. S., Hohl, T. M., Mansour, M. K., Vyas, J. M., Rohm, M., Urban, C. F., Kelkka, T., Holmdahl, R., & Segal, B. H. (2013). Journal of Immunology, 190, 4175–4184.
Magalhaes, E. S., Mourao-Sa, D. S., Vieira-de-Abreu, A., Figueiredo, R. T., Pires, A. L., Farias-Filho, F. A., Fonseca, B. P., Viola, J. P., Metz, C., Martins, M. A., Castro-Faria-Neto, H. C., Bozza, P. T., & Bozza, M. T. (2007). European Journal of Immunology, 37, 1097–1106.
Rim, H. K., Cho, W., Sung, S. H., & Lee, K. T. (2012). Journal of Pharmacology and Experimental Therapeutics, 342, 654–664.
Viackova, D., Pekarova, M., Crhak, T., Bucsaiova, M., Matiasovic, J., Lojek, A., & Kubala, L. (2011). Immunobiology, 216, 457–465.
El-Mahmoudy, A., Shimizu, Y., Shiina, T., Matsuyama, H., Nikami, H., & Takewaki, T. (2005). Acta Diabetologica, 42, 23–30.
Momi, S., Monopoli, A., Alberti, P. F., Falcinelli, E., Corazzi, T., Conti, V., Miglietta, D., Ongini, E., Minuz, P., & Gresele, P. (2012). Cardiovascular Research, 94, 428–438.
Chang, S. Y., Kim, D. B., Ryu, G. R., Ko, S. H., Jeong, I. K., Ahn, Y. B., Jo, Y. H., & Kim, M. J. (2013). Journal of Cellular Biochemistry, 114, 844–853.
Chan, E. D., & Riches, D. W. (2001). American Journal of Physiology. Cell Physiology, 280, C441–C450.
Zhang, Y., Leung, D. Y., Richers, B. N., Liu, Y., Remigio, L. K., Riches, D. W., & Goleva, E. (2012). Journal of Immunology, 188, 2127–2135.
Morimoto, Y., Kikuchi, K., Ito, T., Tokuda, M., Matsuyama, T., Noma, S., Hashiguchi, T., Torii, M., Maruyama, I., & Kawahara, K. (2009). Biochemical and Biophysical Research Communications, 389, 90–94.
Kwon, O. K., Ahn, K. S., Park, J. W., Jang, H. Y., Joung, H., Lee, H. K., & Oh, S. R. (2012). Inflammation, 35, 535–544.
Santos, S. D., Cahu, T. B., Firmino, G. O., de Castro, C. C., Carvalho, L. B., Jr., Bezerra, R. S., & Filho, J. L. (2012). Journal of Food Science, 77, H141–H146.
Murata, T., Kohno, S., Ito, C., Itoigawa, M., Sugiura, A., Hikita, K., & Kaneda, N. (2013). Journal of Pharmacology and Pharmacotherapeutics, 65, 1204–1213.
Nairz, M., Schleicher, U., Schroll, A., Sonnweber, T., Theurl, I., Ludwiczek, S., Talasz, H., Brandacher, G., Moser, P. L., Muckenthaler, M. U., Fang, F. C., Bogdan, C., & Weiss, G. (2013). The Journal of Experimental Medicine, 210, 855–873.
Conflict of Interest
The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.H., Kim, M.E. & Lee, J.S. Inhibitory Effects of Extract from G. lanceolata on LPS-Induced Production of Nitric Oxide and IL-1β via Down-regulation of MAPK in Macrophages. Appl Biochem Biotechnol 175, 657–665 (2015). https://doi.org/10.1007/s12010-014-1301-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1301-8