Abstract
Valporic acid (VPA) has been implicated to have anti-inflammatory and anti-oxidant activities in several ischemic/reperfusion (I/R) injury models. This study intended to evaluate whether VPA could affect the inflammatory/anti-inflammatory cytokines balance and severity of renal I/R injury in rat. I/R injury was induced in two groups of animals, vehicle normal saline and VPA-treated (IP injection, 150 mg/kg) rats, by 45 min occlusion of both left and right renal arteries followed by 3, 24 and 120 h reperfusion in separate groups. After each time point, kidneys and blood samples were collected for cytokine genes (TNF-α, IL-1β, IL-10 and TGF-β) expression analysis and histological examinations in the kidney tissues. Serum creatinine levels were measured for evaluation of renal function. We observed significantly downregulated mRNA expressions for IL-1β and TNF-α in blood and tissue samples 24 and 120 h post I/R injury in VPA-treated animals compared to control groups (P < 0.0001). On the other hand, mRNA expression levels for IL-10 and TGF-β were significantly increased in the blood samples from VPA-treated animals at two time points after I/R injury (P < 0.0001) and at 120 h in tissue samples (P < 0.001). Histopathology analysis showed downgraded ischemic changes in VPA group compared to sham control. Also, decreased serum creatinine levels were observed in VPA-treated animals particularly 120 h post I/R injury (P < 0.0001) that was correlated with less pathological changes in this group. Our results indicate that VPA can attenuate pro-inflammatory responses and augment the anti-inflammatory condition in favor of faster renal recovery from ischemic changes and improved renal function after renal I/R injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ischemia/reperfusion (I/R) injury is one of the major causes of acute renal failure with high mortality and morbidity. Renal IR injury is an unavoidable episode during renal transplantation procedure, shock and decreased or entire blockage of blood flow to the kidney in various types of operations [1, 2].
Renal vasoconstriction, tubular damage, acute tubular necrosis and glomerular injury are caused by renal IR injury [3]. Moreover, extensive interstitial infiltration predominantly by macrophages, neutrophils and fewer by lymphocytes is a characteristic pathologic change in I/R injury [4]. Infiltration of leukocytes following blood reperfusion plays a crucial role in development and progression of renal damage, as they are the main source of reactive oxygen species, proteolytic enzymes and inflammatory cytokines [5].
It has been shown that various cytokines and their receptors contribute in kidney tissue injury through mediating the inflammatory responses and disruption of cellular homeostasis [6]. For instance, tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1) expressions induce recruitment of neutrophil into the renal tissue. These proinflammatory cytokines also induce apoptosis and production of reactive oxygen species which ultimately lead to the destruction of kidney parenchyma [6, 7].
In this context, administration of anti-inflammatory agents could be useful to attenuate renal I/R-induced injury and leukocytes infiltration. Interleukin-10 (IL-10) and transforming growth factor β (TGF-β) as the potent anti-inflammatory cytokines restrain cytotoxic pathways and inflammatory condition during acute renal injury [8]. Current therapeutic approaches to prevent renal dysfunction subsequent to I/R injury and to accelerate recovery from pathologic damages are limited and mortality is still high. Hence, the need for developing and more promising therapeutic agents to reduce inflammation and to maintain homeostasis following I/R injury deserves further investigations.
Valproic acid (VPA) as an inhibitor of histone deacetylase (HDAC) and a potent anti-epileptic drug has been shown to have protective effects via anti-inflammatory, anti-apoptotic and anti-fibrotic properties in several tissues in animal studies [9,10,11]. Van Beneden et al. study showed that premedication of kidney injury by using VPA downregulates the profibrotic and pro-inflammatory genes and inhibits the onset of glomerulosclerosis and development of proteinuria [10].
In this regard, protective effect of VPA in the rat model of renal I/R injury has been evaluated in some studies but its exact mechanism in relation to decreased renal dysfunction and rapid recovery from I/R injury is still under debate. For this reason, we intended to examine the anti-inflammatory effect of VPA in renal I/R injury by quantification of the mRNA expression levels for both inflammatory and anti-inflammatory cytokines, evaluation of renal dysfunction as well as histopathological changes in VPA-treated animals compared to vehicle controls.
MATERIALS AND METHODS
Experimental Groups
Forty-eight male Wistar rats weighing 180–200 g were obtained from the animal facility of Hamedan University of Medical Sciences. Animals were housed in standard cages under a 12-h light/dark cycle at 20–22 °C and fed with enough food and water. Animals were randomly divided into six groups (n = 8 in each group); Three groups as sham-operated rats that received vehicle normal saline solution 30 min prior to ischemia/reperfusion injury and the other three groups as VPA-treated rats that received intraperitoneally (IP) injection of 150 mg/kg VPA (Orfiril® 100 mg/ml Injectionslosung, Hamburg, Germany) before renal ischemia. Thereafter, animals in both groups were anesthetized by IP injection of ketamine (40–100 mg/kg) and xylazin (5–13 mg/kg) and then subjected to an abdominal incision to perform I/R injury for both kidneys. The left and right renal arteries occlusion was performed for 45 min under above-mentioned anesthesia conditions. In the next step, both sham-operated and VPA-treated rats were subdivided in three groups based on the time-intervals after reperfusion so that 45 min of renal ischemia in each group was followed by 3, 24 and 120 h of reperfusion separately. After each time point, animals were scarified and then blood samples collection as well as kidney tissues harvesting were performed for gene expression analysis and histopathological evaluations. All animal experiments were approved (no. 9312126503) by the institutional animal care and use committee of research deputy, Hamadan University of Medical Sciences.
Relative Quantification of mRNA Levels for Cytokines by qRT-PCR
Total RNA was extracted from 50 mg kidney tissue and 200 μL of blood samples using Trizol solution (Invitrogen, USA). After assessment of the quality and quantity RNA samples, 1 μg of total RNA was used for cDNA synthesis in a final volume of 20 μl as per manufacturer’s instructions (RocketScript™ Reverse Transcriptase, Bioneer, South Korea). In the next step, qRT-PCR reaction mixture consisted of 3 μL cDNA, 5 μL H2O, 10 μl SYBR green master mix (AccuPower® GreenStar™ qPCR PreMix, Bioneer, South Korea) and 10 pmol of each specific primer [12] was carried out in a duplicate manner. Thermal cycling condition was defined as follows: Initial denaturation for 2 min at 94 °C, and continued with 40 cycles of a two-step amplification, 20 s at 94 °C and 60 s at 64 °C. However, GAPDH mRNA expression served as an endogenous control for normalization of target mRNA expressions and 2−ΔΔCT analysis method was used for relative expression analysis.
Histopathological Examinations
The kidney specimens were fixed in 10% formalin and embedded in paraffin. Three μm cross-sections were used for hematoxylin and eosin staining (H&E) to evaluate histopathological changes such as ischemic changes, tissue necrosis, and regenerative changes. These changes were reported per high power field (×400) of light microscopy.
Renal Functional Biomarker Analysis
The serum creatinine level is known as an index of renal function in clinical practice. Serum creatinine levels in all samples were determined by enzymatic method based on manufacturer’s instructions (COBAS-C311, Roche, Switzerland).
Statistical Analysis
The significant differences between all data were determined by one-way analysis of variance (ANOVA) and Tukey’s post hoc tests. Pearson correlation test was used for correlation analyses.
P < 0.05 was considered as statistically significant differences. All data were shown as mean ± standard error of the mean (SEM).
RESULTS
Analysis of mRNA Expressions of Inflammatory and Anti-inflammatory Cytokines
We observed significantly downregulated mRNA expressions for IL-1β and TNF-α in peripheral blood samples 24 and 120 h post I/R injury in VPA-treated animals compared to control groups (P < 0.0001, Fig. 1). Similarly, downregulations of both cytokines at two time points after I/R injury were observed in kidney tissue samples of VPA-treated rats (P < 0.0001 and P = 0.001 respectively, Fig. 1). However, downregulation of IL-1β was more obvious in the blood samples compared to kidney tissues.
Analysis of mRNA expression levels for inflammatory and anti-inflammatory cytokines. a, b The expression of TNF-α and IL-1β were significantly decreased compared to vehicle controls in both tissue and blood samples 24 and 120 h of reperfusion. c Significantly increased levels of mRNA expression for TGF-β at both time points in tissue and blood samples in compare with vehicle control. d Increased levels for IL-10 mRNA expression 120 h post I/R injury but decreased level in tissue sample 24 h of reperfusion. Data were normalized to the expression level of GAPDH. **P < 0.01 and ***P < 0.001.
On the other hand, mRNA expression levels for TGF-β as an anti-inflammatory cytokine were significantly increased in both blood and kidney tissue samples from VPA-treated animals so that it was higher in the kidney after 24 h (P = 0.002) and 120 h (P < 0.0001) and also its expression was increased in the blood 1.5 folds after 24 h, and 3 folds after 120 h of reperfusion (P < 0.0001, Fig. 1).
IL-10 mRNA expression level was significantly increased in the kidney 120 h after I/R injury (P < 0.0001), although its expression was decreased at 24 h time point (P < 0.001). The mRNA expression levels of IL-10 in the blood samples were 2.5 and 3 folds higher at 24 and 120 h of reperfusion respectively in VPA treated rats compared to vehicle controls (P < 0.0001, Fig. 1). Unfortunately, we missed the molecular data for 3 h time point just because of mislabeling of preserved samples in the freezer. For this reason, herein we presented the molecular analysis for the other two time points (24 and 120 h).
Pearson correlation analysis between cytokines mRNA expressions revealed a positive correlation between IL-1β and TNF-α in the blood at 24 h (r = 0.89, P = 0.007) and 120 h (r = 0.96, P = 0.0001) respectively. In addition, IL-1β showed a direct correlation with TNF-α in tissue samples 24 h post I/R injury (r = 0.82, P = 0.02). Also, remarkable correlation between IL-10 and TGF-β cytokines was observed only at 24 h of reperfusion in the blood samples (r = 0.95, P = 0.0007, Fig. 2).
Correlation between cytokine mRNA expressions in the blood and tissue samples 24 h/120 h post I/R injury. Significantly direct correlations were found between IL-1β and TNF-α in tissue (a) and blood (b) at 24 h of reperfusion, in the blood at 120 h (c) and between IL-10 and TGF-β cytokines only at 24 h of reperfusion in the blood samples (d).
Histopathological Analyses
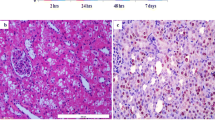
The histological findings were determined at 3, 24 and 120 h after I/R injury in both groups of the VPA-treated and vehicle animals. Totally, the severity of ischemic changes was shown to be reduced from renal cortex to medulla and even, the most changes were observed in proximal rather than distal tubules. We observed the most ischemic damages without regenerative changes 3 h post I/R injury in both groups of the animals as follows: (1) detachment of epithelial cell from basement membrane, (2) peritubular accumulation of neutrophils in the interstitial tissue and a few neutrophil in glomeruli as an early change, (3) irregularity of tubular cells, (4) peritubular capillary congestion as well as glomerular congestion, (5) tubular cell necrosis, (6) cell and nuclei sloughing inside the tubule lumen and (7) dilation of the tubular lumen and interstitial edema. These changes were almost similar in VPA-treated and vehicle animals (Fig. 1a, b). After 24 h of reperfusion, regenerative changes including tubular dilation, vesicular chromatin with nucleoli, and larger nucleus along with cytoplasmic contraction were observed in both groups which were more remarkable at 5 days post I/R injury. The main differences were found between two groups after 5 days of reperfusion so that VPA-treated animals showed significantly reduced ischemic changes and cell necrosis without interstitial edema, regular distribution of nuclear in the proximal tubules and normal representation of tubules compared to vehicle animals (Fig. 1c, d). While, we observed ischemic changes, mild lymphocytic infiltration in glomeruli in the absence of neutrophil infiltration, mild cell necrosis as well as cell swelling in control groups compared to VPA-treated group (Fig. 3e, f).
Histopathology analysis of kidney tissues stained with hematoxylin and eosin. a Vehicle/sham-operated control (saline control) after 3 h. b Valproic acid (VPA) treated rats after 3 h. c Vehicle control after 24 h. d VPA after 24 h. e Vehicle control after 120 h. f VPA after 120 h. Ischemic changes such as nuclear disappearance, cell edema and tissue necrosis in the absence of marked leukocyte infiltrates (a few neutrophil infiltration) were observed in both groups 3 h of reperfusion. Ischemic changes and tissue necrosis declined in vehicle control after 120 h compared to 3 and 24 h time points in the same group, but cell edema and regenerative changes were still observed. The severity of tissue injury in VPA group after 24 and 120 h were decreased compared to control groups but there were no remarkable changes in VPA group after 3 h. Regular arrangement of cells, large number of nuclear and no cell edema were seen in VPA after 120 h group.
Serum Creatinine Levels Measurement
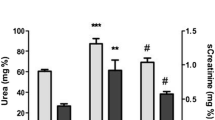
Measuring serum creatinine levels 3, 24 and 120 h after reperfusion revealed improved renal function as shown by decreased levels of creatinine in VPA-treated animals versus sham-operated controls at two time points. Although, these differences were only statistically significant 120 h post I/R injury between both groups of the study (P < 0.0001, Fig. 4).
DISCUSSION
Renal ischemia/reperfusion injury induces leukocyte infiltration into the renal tissue and subsequently inflammatory responses along with cell death which in turn, contribute to tubular injury and renal dysfunction [13]. Therefore, therapeutic intervention by using anti-inflammatory and anti-apoptotic drugs may prevent or downgrade tubular destruction.
The current study has evaluated the anti-inflammatory effect of VPA as a potential therapeutic agent for amelioration of renal I/R injury. VPA which is implicated as an anticonvulsant drug has also been documented to have anti-inflammatory, anti-oxidant and anti-apoptotic properties in various models of I/R injury [14, 15]. Our results in this study demonstrated that pre-medication with VPA in a rat model of renal I/R injury induces the mRNA expressions of IL-10 and TGF-β cytokines as well as downregulation in IL-1β and TNF-α cytokines genes transcriptions compared to vehicle animals. We observed downregulation of pro-inflammatory cytokines (IL-1 and TNF-α) concurrent with upregulation of anti-inflammatory cytokines (IL-10 and TGF-β) at mRNA levels 24 and 120 h post I/R injury in both peripheral blood and tissue samples. Also, we observed less histopathological changes along with improved renal function in VPA treated animals compared to vehicle group.
Following renal I/R injury, signal transduction through NFκB and P38 mitogen activated protein kinase (MAPK) pathways induces TNF-α production which in turn, leads to decrease in glomerular filtration rate (GFR), renal cell apoptosis, upregulation of the other inflammatory genes and facilitation of cellular infiltration into the ischemic renal tissues [6]. Administration of VPA inhibits LPS-induced secretion of TNF-α and nitric oxide production from activated microglia in the midbrain of rat which is also associated with low number of microglia cells [16]. Also, Ichiyama et al. investigated the effects of VPA on immune system and stated that VPA significantly suppressed LPS-induced production of TNF-α and IL-6 from human monocytic leukemia cells via suppression of NF-κB transcriptional activity. However, the exact underlying mechanism of VPA-mediated inhibition of NF-κB pathway remained to be determined [17]. Alternatively, a study by Li et al. showed that the expression of endothelial cell adhesion molecules, E-selectin and vascular cell adhesion molecule-1 (VCAM-1) are significantly diminished through VPA administration in the microvasculature of mice kidney and liver which it was correlated with impaired neutrophils adhesion/transmigration subsequent to hemorrhagic shock/resuscitation. Moreover, the reduced histone acetylation in the kidneys and nuclear translocation of NF-κB in glomerular endothelial cells after hemorrhagic shock/resuscitation were restored by VPA treatment [18].
In line with these findings, we observed a significantly decreased level of mRNA expression for TNF-α in renal tissue and peripheral blood samples of VPA-treated animals compared to control group. Similarly, Van Beneden et al. study showed that the administration of VPA prior to kidney injury (Adriamycin-induced nephropathy) in mice, leads to the downregulation of pro-fibrotic and pro-inflammatory genes such as TNF-α and VCAM-1, deposition of collagen, and infiltration of macrophages into the renal tissue that supports our results in the current study [10].
Another major player in renal I/R injury is IL-1β which implements the same signaling pathway (NF-kB) for TNF-α to induce the inflammation and tissue injury as well as delayed recovery of renal function [19, 20]. A recent study by Speir et al. in the rat model of renal I/R injury revealed that VPA administration attenuated renal injury via downregulation of IL-1β and IL-6 cytokines and induction of BCL2 as an anti-apoptotic molecule [2]. Consistently, we found significantly lower expression of TNF-α and IL-1β concomitant with high expression of IL-10 and TGF-β cytokines in VPA-treated animals that remarkably were accompanied with less histopathological changes and improved renal function as judged by serum creatinine levels.
Protective effects of VPA have been well documented in various rat models of I/R injury, but in other organs like cerebral tissues [15], retina [21] and intestinal tissues [22]. These studies clearly demonstrated an anti-inflammatory, anti-oxidative and anti-apoptotic properties for VPA as confirmed by decrease in TNF-α, IL-1β, IL-6, nitric oxide, iNOS and prostaglandin E2 in VPA-treated animals compared to control groups [15, 21, 22]. It is believed that VPA could inhibit NF-κB activity by decreasing the levels of p50 subunit and NF-κB DNA binding activity which in turn, regulates the expression of NF-κB-dependent genes like IL-1β and TNF-α [23].
In support of these findings, our results showed a protective effect of VPA in renal I/R injury through attenuation of inflammatory conditions as well as induction of anti-inflammatory mediators in favor of improved renal function and less tubular changes in VPA-treated animals. Moreover, a direct correlation between IL-1β and TNF-α mRNA expression levels and IL-10 with TGF-β as well, at two time points post I/R injury could be indicative for potent anti-inflammatory effect of VPA as an alternative immunomodulatory agent. This indicates that VPA could be used as an anti-inflammatory agent in clinical applications particularly renal transplantation and other type of operations with unavoidable ischemic/reperfusion condition.
Interleukin-10 (IL-10) as an anti-inflammatory cytokine has been shown recently to suppress inflammatory and cytotoxic pathways in lung and heart ischemia-reperfusion injury models [24, 25]. Deng et al. reported that IL-10 protects renal dysfunction in ischemic renal tissue and cisplatin-induced kidney injury via downregulation of TNF-α, intercellular adhesion molecule-1 (ICAM-1) and NOS-II [8]. Schneider et al. also showed that VPA induces IL-10 production by concanavalin A stimulated splenocytes in an animal model of autism [26]. On the other hand, Kim et al. study showed the decreased levels of IL-10 and IL-6 in VPA-treated animal post intestinal I/R injury [22]. While, we observed significantly high levels of IL-10 and TGF-β mRNA expressions 24 and 120 h post I/R injury in VPA-treated animals compared to control groups.
Given the contribution of dendritic cells (DCs) in the early inflammatory responses subsequent to acute I/R injury, impact of VPA on inflammatory DCs in this condition has been evaluated and impaired stimulatory mode of DC through interference with NF-κB and the IFN regulatory factors (IRF-3, IRF-8) pathways has been well documented. In this sense, low surface expression of co-stimulatory molecules and adhesion molecules as well as decreased mRNA expression levels for IL-6, IL-10, IL-12, IFN, TNF-α and nitric oxide synthases were observed in VPA-treated animals [27, 28].
Another anti-inflammatory cytokine (TGF-β) which has been examined in renal and myocardial I/R injury models may have protective effects through (a) suppression of TNF-α, (b) decrease in free radicals generation, (c) inhibition of neutrophil diapedesis and (d) stimulation of extracellular matrix synthesis during renal recovery [29,30,31]. In a study by Causey et al. VPA administration was shown to be protective in a swine model of hemorrhage coupled with ischemia–reperfusion injury as judged by decrease in pathologic endothelial cell function, apoptosis and impaired angiogenesis, via modulation of TGF-β and VEGF genes which are the central players for these processes [32]. Consistently, we found less histopathological changes as well as improved renal function (significantly decreased serum creatinine levels) in relation to upregulated expressions of IL-10 and TGF-β cytokines in VPA treated animals versus control groups. However, change in serum creatinine levels was more obvious 120 h post I/R between two groups which is also in line with Spier et al. study that showed similar decrement 24 h after I/R injury in VPA treated versus control animals [2].
Additionally, it has been shown that HDAC inhibitors like VPA enhance the FOXP3+ regulatory T cells functions in a TGF-β-dependent manner. This may highlight the clinical implication of VPA administration in order to ameliorate I/R injury in the setting of solid organ transplantation [33,34,35]. Ischemic/reperfusion injury is one of the major conclusive factors in relation to allograft function as well as in early and late allograft outcomes [36]. According to the critical and destructive role of inflammatory cytokines (TNF-α and IL-1β) in this inevitable step during organ transplantation, inhibition of those cytokines besides induction of anti-inflammatory cytokines (e.g. TGF-β and IL-10) possibly by usage of VPA, would be of great importance to improve allograft function and outcomes [37].
In conclusion, our results indicate that VPA can attenuate pro-inflammatory responses and enhance the anti-inflammatory condition after renal I/R injury. In addition, these favorable changes in cytokine profile were accompanied with faster renal recovery from ischemic changes and improved renal function in VPA-treated animals. These beneficial effects of VPA treatment on renal function along with its immunomodulatory potential would be more useful in the clinical settings particularly in renal transplantation. Finally, further studies are required to confirm these preclinical data on VPA treatment and to translate it in the clinical transplantation as a well-known therapeutic approach with unavoidable I/R injury.
References
Ling, H., H. Chen, M. Wei, X. Meng, Y. Yu, and K. Xie. 2016. The effect of autophagy on inflammation cytokines in renal ischemia/reperfusion injury. Inflammation 39 (1): 347–356.
Speir, R.W., J.D. Stallings, J.M. Andrews, M.S. Gelnett, T.C. Brand, and S.K. Salgar. 2015. Effects of valproic acid and dexamethasone administration on early bio-markers and gene expression profile in acute kidney ischemia-reperfusion injury in the rat. PloS One 10 (5): e0126622.
Bonventre, J.V. 1993. Mechanisms of ischemic acute renal failure. Kidney international. 43 (5): 1160–1178.
Rabb, H., C.C. Mendiola, S.R. Saba, J.R. Dietz, C. Smith, J.V. Bonventre, et al. 1995. Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochemical and biophysical research communications. 211 (1): 67–73.
Solez, K., E.C. Kramer, J.A. Fox, and R.H. Heptinstall. 1974. Medullary plasma flow and intravascular leukocyte accumulation in acute renal failure. Kidney international. 6 (1): 24–37.
Donnahoo, K.K., B.D. SHAMES, A.H. HARKEN, and D.R. MELDRUM. 1999. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. The Journal of urology. 162 (1): 196–203.
Sancak, E.B., H. Turkön, S. Çukur, S. Erimsah, A. Akbas, M.T. Gulpinar, et al. 2016. Major ozonated autohemotherapy preconditioning ameliorates kidney ischemia-reperfusion injury. Inflammation 39 (1): 209–217.
Deng, J., Y. Kohda, H. Chiao, Y. Wang, X. Hu, S.M. Hewitt, et al. 2001. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney international. 60 (6): 2118–2128.
Kim, H.J., M. Rowe, M. Ren, J.-S. Hong, P.-S. Chen, and D.-M. Chuang. 2007. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. Journal of Pharmacology and Experimental Therapeutics. 321 (3): 892–901.
Van Beneden, K., C. Geers, M. Pauwels, I. Mannaerts, D. Verbeelen, L.A. van Grunsven, et al. 2011. Valproic acid attenuates proteinuria and kidney injury. Journal of the American Society of Nephrology. 22 (10): 1863–1875.
Göttlicher, M., S. Minucci, P. Zhu, O.H. Krämer, A. Schimpf, S. Giavara, et al. 2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO journal. 20 (24): 6969–6978.
Peinnequin, A., C. Mouret, O. Birot, A. Alonso, J. Mathieu, D. Clarençon, et al. 2004. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC immunology. 5 (1): 3.
Pulskens, W.P., G.J. Teske, L.M. Butter, J.J. Roelofs, T. Van Der Poll, S. Florquin, et al. 2008. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PloS One 3 (10): e3596.
M-h, Ji, Li G-m, M. Jia, S.-h. Zhu, D.-p. Gao, Fan Y-x, et al. 2013. Valproic acid attenuates lipopolysaccharide-induced acute lung injury in mice. Inflammation 36 (6): 1453–1459.
Suda, S., K.-I. Katsura, T. Kanamaru, M. Saito, and Y. Katayama. 2013. Valproic acid attenuates ischemia-reperfusion injury in the rat brain through inhibition of oxidative stress and inflammation. European journal of pharmacology. 707 (1): 26–31.
Peng, G.-S., G. Li, N.-S. Tzeng, P.-S. Chen, D.-M. Chuang, Y.-D. Hsu, et al. 2005. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Molecular Brain Research. 134 (1): 162–169.
Ichiyama, T., K. Okada, J.M. Lipton, T. Matsubara, T. Hayashi, and S. Furukawa. 2000. Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain research. 857 (1): 246–251.
Li, R., A. Aslan, R. Yan, R.M. Jongman, J. Moser, P.J. Zwiers, et al. 2015. Histone deacetylase inhibition and IκB kinase/nuclear factor-κB blockade ameliorate microvascular proinflammatory responses associated with hemorrhagic shock/resuscitation in mice. Critical care medicine. 43 (12): e567–ee80.
Rhodus, N.L., B. Cheng, S. Myers, W. Bowles, V. Ho, and F. Ondrey. 2005. A comparison of the pro-inflammatory, NF-κB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clinical Immunology. 114 (3): 278–283.
Haq, M., J. Norman, S.R. Saba, G. Ramirez, and H. Rabb. 1998. Role of IL-1 in renal ischemic reperfusion injury. Journal of the American Society of Nephrology. 9 (4): 614–619.
Zhang, Z., X. Qin, X. Zhao, N. Tong, Y. Gong, W. Zhang, et al. 2012. Valproic acid regulates antioxidant enzymes and prevents ischemia/reperfusion injury in the rat retina. Current eye research. 37 (5): 429–437.
Kim, K., Y. Li, G. Jin, W. Chong, B. Liu, J. Lu, et al. 2012. Effect of valproic acid on acute lung injury in a rodent model of intestinal ischemia reperfusion. Resuscitation 83 (2): 243–248.
Rao, J.S., R.P. Bazinet, S.I. Rapoport, and H.J. Lee. 2007. Chronic treatment of rats with sodium valproate downregulates frontal cortex NF-κB DNA binding activity and COX-2 mRNA1. Bipolar disorders. 9 (5): 513–520.
Eppinger, M.J., P.A. Ward, S.F. Bolling, and G.M. Deeb. 1996. Regulatory effects of interleukin-10 on lung ischemia-reperfusion injury. The Journal of thoracic and cardiovascular surgery. 112 (5): 1301–1306.
Hayward, R., T.O. Nossuli, R. Scalia, and A.M. Lefer. 1997. Cardioprotective effect of interleukin-10 in murine myocardial ischemia-reperfusion. European journal of pharmacology. 334 (2): 157–163.
Schneider, T., A. Roman, A. Basta-Kaim, M. Kubera, B. Budziszewska, K. Schneider, et al. 2008. Gender-specific behavioral and immunological alterations in an animal model of autism induced by prenatal exposure to valproic acid. Psychoneuroendocrinology 33 (6): 728–740.
Dong, X., S. Swaminathan, L. Bachman, A. Croatt, K.A. Nath, and M. Griffin. 2007. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia–reperfusion injury. Kidney international. 71 (7): 619–628.
Nencioni, A., J. Beck, D. Werth, F. Grünebach, F. Patrone, A. Ballestrero, et al. 2007. Histone deacetylase inhibitors affect dendritic cell differentiation and immunogenicity. Clinical Cancer Research. 13 (13): 3933–3941.
Chen, H., D. Li, T. Saldeen, and J.L. Mehta. 2003. TGF-β1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. American Journal of Physiology-Heart and Circulatory Physiology. 284 (5): H1612–H16H7.
Lefer, A.M., X.L. Ma, A.S. Weyrich, and R. Scalia. 1993. Mechanism of the cardioprotective effect of transforming growth factor beta 1 in feline myocardial ischemia and reperfusion. Proceedings of the National Academy of Sciences. 90 (3): 1018–1022.
Basile, D.P., D.R. Martin, and M.R. Hammerman. 1998. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-β in repair. American Journal of Physiology-Renal Physiology. 275 (6): F894–F903.
Causey, M.W., S. Salgar, N. Singh, M. Martin, and J.D. Stallings. 2012. Valproic acid reversed pathologic endothelial cell gene expression profile associated with ischemia–reperfusion injury in a swine hemorrhagic shock model. Journal of Vascular Surgery 55 (4): 1096–1103 e51.
Gandolfo, M.T., H.R. Jang, S.M. Bagnasco, G.-J. Ko, P. Agreda, S.R. Satpute, et al. 2009. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney international. 76 (7): 717–729.
Andersson, J., D.Q. Tran, M. Pesu, T.S. Davidson, H. Ramsey, J.J. O'Shea, et al. 2008. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-β–dependent manner. The Journal of experimental medicine. 205 (9): 1975–1981.
Wang, L., E.F. de Zoeten, M.I. Greene, and W.W. Hancock. 2009. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature Reviews Drug Discovery. 8 (12): 969–981.
Gueler, F., W. Gwinner, A. Schwarz, and H. Haller. 2004. Long-term effects of acute ischemia and reperfusion injury. Kidney international. 66 (2): 523–527.
Sankaran, D., A. Asderakis, S. Ashraf, I.S. Roberts, C.D. Short, P.A. Dyer, et al. 1999. Cytokine gene polymorphisms predict acute graft rejection following renal transplantation. Kidney international. 56 (1): 281–288.
Acknowledgments
This study is financially supported by the research affair of Hamadan University of Medical Sciences. (Grant No.: 9312126503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Amirzargar, M.A., Yaghubi, F., Hosseinipanah, M. et al. Anti-inflammatory Effects of Valproic Acid in a Rat Model of Renal Ischemia/Reperfusion Injury: Alteration in Cytokine Profile. Inflammation 40, 1310–1318 (2017). https://doi.org/10.1007/s10753-017-0574-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0574-9