Abstract
Ischemia-reperfusion (IR) has been reported to be associated with augmented reactive oxygen radicals and cytokines. Currently, we aimed to examine the influence of fluoxetine, which is already used as a preoperative anxiolytic, in the context of IR induced by occlusion of infrarenal abdominal aorta (60 min of ischemia) and its effects on renal oxidative status, inflammation, renal function, and cellular integrity in reperfusion (120 min post-ischemia). Male rats were randomly assigned as control, IR, and pretreated groups. The pretreated group animals received fluoxetine (20 mg/kg, i.p.) once daily for 3 days. Renal tissue oxidative stress, myeloperoxidase activity, proinflammatory cytokines (tumor necrosis factor-α, interleukin-1β, interleukin-6), histology, and function were assessed. As an anti-inflammatory cytokine, interleukin-10 was also assessed. IR led to a significant increase in lipid hydroperoxide, malondialdehyde, and pro-oxidant antioxidant balance and decrease in superoxide dismutase activity and ferric reducing/antioxidant power level (p < 0.05), but fluoxetine was able to restore these parameters. High concentrations of tumor necrosis factor-α, interleukin-1β, interleukin-6, and myeloperoxidase activity caused by IR were significantly decreased in kidney tissue with fluoxetine. In addition, interleukin-10 levels were high in fluoxetine pretreated group. IR resulted in disrupted cellular integrity, infiltration of tissue with leukocytes, and decreased serum creatinine-urea levels (p < 0.05). Fluoxetine significantly restored impaired redox balance and inflammation parameters of rats subjected to IR to baseline values. This beneficial effect of fluoxetine on redox balance might be addressed to an improvement in renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aortic ischemia reperfusion is an important factor in development of postoperative kidney injury after abdominal aortic surgery [13]. Prolonged ischemia and subsequent reperfusion period is known to alter local homeostatic reactions. This results in the death of cells due to apoptosis and necrosis [1]. Moreover, both reactive oxygen species (ROS) and inflammatory mediators have been implicated as a corner stone of reperfusion injury. It is known that excessive ROS generation and/or reperfusion-related inflammatory mediators (TNF-α, IL-1β, IL-6, IL-10, etc.) [2] attenuate in antioxidant defense in ischemia-reperfusion (IR) injury [30]. Antioxidants that effectively remove ROS have been found to be effective against IR injury [25]. Hence, antioxidant and anti-inflammatory therapies take important place. In this respect, compounds with antioxidant property, such as fluoxetine, can be a good candidate for the prevention and treatment of oxidative stress-mediated renal injury.
Fluoxetine (N-methyl-3-[4-(trifluoromethyl) phenoxy] benzenepropanamine) is known as a selective serotonin reuptake inhibitor (SSRI) and a non-tricyclic antidepressant and commonly used for treatment of anxiety. The protective effects of fluoxetine against different types of stress sources have also been proven [11, 14, 33]. Researches on fluoxetine, which is already used as preoperative anxiolytic, showed a better safety profile, lesser side effects, and faster elimination compared to the older conventional tricyclic antidepressants [12].
Non-serotonergic effects of fluoxetine have also been reported. Fluoxetine is protective against monocrotaline- [9] and chronic hypoxia-induced [19] pulmonary hypertension in the adult rat and mice by suppressing proliferation of pulmonary arterial muscle cells. Moreover, we have previously showed that acute central fluoxetine administration increases normoxic ventilation and also augments the stimulatory effect of hypercapnia on respiratory neuronal network [27].
It is known that, regardless of chronic or acute fluoxetine administration, fluoxetine reduces some effects of stress on the immune system [23] and shows anti-inflammatory [26] and antioxidant effects [32]. It has been also suggested that fluoxetine is able to prevent mice spleen from melanoma-induced oxidative changes via its antioxidant activity, thus strengthening an antioxidant cell defense [12]. In the same concept, we have previously demonstrated the antioxidant effect of fluoxetine in aortic ischemia reperfusion-induced lung injury [8].
To the end point of our knowledge, no study has as yet worked on the in vivo antioxidant- and anti-inflammatory-modulating effects of fluoxetine on IR-induced kidney injury. In the present study, we tested the hypothesis that short-term pre-administration of fluoxetine may have a potential effect in a rat model of abdominal aortic surgery-induced IR injury by suppressing both oxidant and proinflammatory responses including cytokines.
Materials and methods
Chemicals
All chemicals used in this study were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and were of analytical grade or of the highest grade available. Deionized water was used in all analytical procedures. All reagents were stored at +4 °C. The reagents were equilibrated at room temperature before use.
Animals
All experiments in this study were approved and reviewed by the Animal Research Committee of the University of Istanbul (2012/48). Care and handling of the animals were in accordance with the Helsinki Declaration. Animals were housed in individual cages in a temperature-controlled room (23 ± 1 °C) and a light-dark cycle-controlled environment (12 h) with free access to food and water. Experiments were performed on 21 Sprague-Dawley rats with body weight of 300 ± 25 g.
Surgical preparation
The rats were anesthetized with an intraperitoneal injection of 60 mg/kg pentobarbital sodium. After tracheotomy, the animals were allowed to breathe spontaneously. Body temperature was maintained at 37 ± 0.5 °C during the entire experiment. The skin was aseptically prepared, and a midline laparotomy was performed. Ten milliliters of warm saline was instilled into the peritoneal cavity to prevent fluid loss. The abdominal aorta was exposed by gently deflecting the intestine loops to the left. After fine isolation of the infrarenal segment, an atraumatic microvascular clamp (vascu-statts II, midi straight 1001-532; Scanlan Int., St Paul, MN, USA) was placed on the aorta for 60 min. The abdomen was then closed, and the operation field was covered with a humidified gauze compress throughout the entire experiment to prevent drying of the surgical field. The microvascular clamp on the infrarenal abdominal aorta (IAA) was then removed, and reperfusion period was performed as 120 min [8]. Before inducing ischemia, each animal received 50 U/kg (total volume 500 μL) heparin (Nevparin; Mustafa Nevzat Drug Company, Istanbul, Turkey) in saline intravenously via tail vein injection. Five minutes after heparin administration, the ischemia protocol was applied. Aortic occlusion and reperfusion was confirmed by the loss and reappearance of satisfactory pulsation in the distal aorta. The experiment was terminated by infusion of 1 mL of 3 M potassium chloride. Finally, blood was withdrawn via abdominal aorta and the kidney was removed and weighed.
Biochemical evaluation
Blood samples were collected in Vacutainer tubes and immediately transported to the laboratory on ice. On arrival at the laboratory, approximately 1 mL of blood samples were separated for assessment of kidney function, and then, plasma was obtained by centrifugation (+4 °C, 3000 rpm, 10 min). The tissues were weighed and washed in 0.9 % NaCl. Plasma and a piece of tissue sample were stored at –80 °C until assayed for levels of oxidative stress and inflammation markers, and the remaining part of the kidneys was stored in formalin for histological evaluation. Protein contents in tissue were determined spectrophotometrically using a Folin kit (Sigma Diagnostics, St. Louis, MO, USA).
Assessment of kidney function
Plasma levels of creatinine and urea were determined by using an automatic biochemistry analyzer (Roche/Hitachi P 800 Modular Analytics System, USA).
Preparation of tissue samples
About 190–200 mg of each kidney tissue sample was weighed and diluted 20 % w/v in 20 mM ice-cold Tris-HCl, pH 7.4, and homogenized with a Bosch Scintilla SA (Switzerland). The homogenate was centrifuged at 5000×g for 10 min, and biochemical parameters were performed in the supernatant fraction.
Measurement of malondialdehyde levels
Lipoperoxidation was ascertained by the formation of malondialdehyde (MDA), which was estimated by the modified thiobarbituric acid method, described by Buege and Aust [5]. Thiobarbituric acid-reactive substance (TBARS) concentration was calculated using 1.56 × 10−5 M−1 cm−1 as moles per liter extinction coefficient.
Measurement of tissue lipid hydroperoxide levels
Tissue lipid hydroperoxide (LOOH) levels were determined spectrophotometrically according to the method of ferrous oxidation with xylenol orange version 2 (FOX2) [22]. Hydroperoxides oxidize ferrous to ferric ions selectively in dilute acid, and the resultant ferric ions can be determined by using ferric-sensitive dyes as an indirect measure of hydroperoxide concentration. Xylenol orange binds ferric ions with high selectivity to produce a colored (blue-purple) complex with an extinction coefficient of 1.5 × 104 M−1 × cm−1. Ninety-microliter aliquots of homogenate were transferred into microcentrifuge reaction vials. Triphenylphosphine (TPP) (10 mM in methanol; 10 mL) was added to vials to remove hydroperoxides. Methanol alone (10 mL) was added to the remaining vials. The LOOH content in the plasma samples is determined as a function of the absorbance difference of samples with and without elimination of LOOHs by TPP. The samples were vortexed and subsequently incubated at room temperature for 30 min. FOX2 reagent (900 mL) was added, and the samples were vortexed. After incubation at room temperature for 30 min, the samples were centrifuged at 15,000×g at 20 °C for 10 min, the supernatant was carefully decanted into a cuvette, and absorbance was read at 560 nm.
Measurement of tissue pro-oxidant-antioxidant balance levels
The pro-oxidant-antioxidant balance (PAB) assay was performed according to the method of Alamdari et al. with minor modifications [3]. The standard solutions were prepared by mixing varying proportions (0–100 %) of 1 mM H2O2 with 3 mM uric acid (in 10 mM NaOH). Sixty milligrams of 3,3′,5,5′-tetramethylbenzidine (TMB) powder was dissolved in 10 mL DMSO. To prepare the TMB cation, 400 μL of TMB/DMSO was added to 20 mL of acetate buffer [0.05 M buffer, pH 4.5]. Then, 70 μL of fresh chloramine T (100 mM) solution was added to this solution, mixed well, and incubated for 2 h at room temperature in the dark. Following this incubation, 25 U of peroxidase enzyme solution was added to the 20 mL TMB cation, dispensed in 1 mL, and placed at −20 °C. To prepare the TMB solution, 200 μL of TMB/DMSO was added to 10 mL of acetate buffer [0.05 M buffer, pH 5.8]. The working solution was prepared by mixing 1 mL of the TMB cation with 10 mL of the TMB solution and incubating for 2 min at room temperature in the dark. Working solutions were used immediately. Ten microliters of each sample, standard or blank (distilled water), were mixed with 200 μL of the working solution in each well of a 96-well plate, which was then incubated at 37 °C for 12 min in the dark. Following the incubation time, 100 μL of 2 N HCl was added to each well, and the absorbance was measured using an ELISA plate reader at 450 nm with a reference wavelength of 620 or 570 nm. A standard curve was determined using the values obtained from the standard samples. The values of the PAB are expressed in arbitrary Hamidi-Koliakos (HK) units, which represent the percentage of hydrogen peroxide in the standard solution.
Measurement of tissue superoxide dismutase (Cu,Zn SOD) levels
The renal tissue Cu,Zn-SOD activities were determined by the method of Sun et al. [28] by inhibition of nitroblue tetrazolium (NBT) reduction with xanthine/xanthine oxidase used as a superoxide generator. One unit of Cu,Zn SOD was defined as the amount of protein that inhibits the rate of NBT reduction by 50 %.
Measurement of tissue ferric reducing antioxidant power levels
The ferric reducing antioxidant power (FRAP) assay was performed according to the protocol of Benzie and Strain with minor modifications [4]. To prepare the FRAP solution, 10 mL of acetate buffer (300 mmol/L, pH 3.6) was mixed with 1 mL of 20 mmol/L FeCl3 dissolved in distilled water and 1 mL of 10 mmol/L TPTZ dissolved in 40 mmol/L HCl. Next, 50 μL of homogenate was added to 1.5 mL of a freshly prepared FRAP solution and measured during 4 min at 593 nm. Different concentrations of uric acid (3–0.09 mmol/L) were used to obtain a calibration curve on each day of the experiment. Uric acid is the primary contributor to the antioxidant capacity of serum and was thus used as a standard.
Measurement of myeloperoxidase activity
Myeloperoxidase (MPO) activity, a sensitive index of tissue polymorphonuclear leukocyte sequestration, was measured by using the peroxidase-catalyzed, H2O2-dependent oxidation of tetramethylbenzidine as a measure of enzymatic activity [20]. A piece of renal tissue samples was homogenized and centrifuged for 30 min at 13,000×g at 4 °C. The supernatant was assayed for MPO content spectrophotometrically by measuring the change in optical density at 460 nm over time. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide/min at 37 °C and was expressed in milliunits/gram of wet tissue.
Measurement of proinflammatory and anti-inflammatory cytokines
Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) as proinflammatory cytokines, and interleukin-10 (IL-10) as anti-inflammatory cytokine in tissue homogenates were measured with commercial ELISA kits (Cayman Chemical Company, Ann Arbor, MI, USA), following the protocol provided by the manufacturer. All samples were measured in duplicate.
Histological evaluation
The tissue pieces taken from the kidneys from time-control, IR, and Flx + IR rats were fixed in Bouin’s solution for the preparation of histological slides and subsequently embedded in paraffin after known routine procedures. The paraffin blocks were cut into 4-μm-thick slices and stained with hematoxylin and eosin (HE) and were examined under Olympus CX-31 light microscope and photographed with an Olympus LC20 digital camera. For the kidney sections, general structure of glomerulus and tubules, the renal corpuscles with pathologic lesions, and microhemorrhages were focused for each slide. Representative photomicrographs were taken from each slide for comparison among the groups. The histological analysis was performed by a person blinded to the groups to which the kidneys belonged. Kidney injury was graded according to Park et al. as follows: 0, normal; 1, mild (<10 %); 2, moderate (10–25 %); 3, moderate to severe (25–50 %); 4, severe (50–75 %); and 5, very severe (>75 %). A mean value of scores was calculated for each animal [24].
Experimental protocol
The rats were divided into three groups (seven rats for each): (1) a time-control (as sham) group; animals underwent midline laparotomy and dissection of IAA without occlusion; (2) an IR group; animals were subjected to aortic ischemia reperfusion as indicated above; and (3) an IR-pretreated group with fluoxetine (Flx + IR). The rats in the Flx + IR group received Flx (20 mg/kg, i.p.) once daily for 3 days before ischemic surgery. Thirty minutes after the last injection, the IR procedure was performed [8]. We have decided to use 20 mg kg−1 day−1, i.p. dose which produces no neuroprotective effect and has been widely used by other studies [11, 32].
Statistical analysis
Values are reported as means ± SEM. Statistical analysis was performed with GraphPad Prism version 5.0 for Windows (GraphPad Software v5.0, San Diego, CA, USA). One-way ANOVA analysis was used for comparisons; post hoc analyses were used with Tukey’s post hoc test when p < 0.05. For all analyses, p < 0.05 was considered significant.
Results
Effects of fluoxetine on renal function parameters
At the end of the protocol, serum urea and creatinine levels were higher in IR group compared to the control group (p < 0.001 and p < 0.01 vs. control, respectively). Serum urea and creatinine levels were significantly lower in fluoxetine pretreated group compared to IR group (p < 0.05) (Fig. 1).
Effects of fluoxetine on oxidative stress markers
Concentrations of MDA and LOOH as lipid peroxidation markers were increased in the IR group (0.9 ± 0.1 mmol/g protein, 0.4 ± 0.0 mmol H2O2/g protein, respectively) compared to control (0.8 ± 0.0 mmol/g protein, 0.2 ± 0.0 mmol H2O2/g protein, respectively) (p < 0.01). Kidney tissue MDA and LOOH concentrations were similar to control in Flx + IR group (0.8 ± 0.0 mmol/g protein, 0.2 ± 0.0 mmol H2O2/g protein, respectively) (p > 0.05). MDA concentrations were significantly different between IR and Flx + IR groups (p < 0.05). Additionally, LOOH concentrations were decreased in Flx + IR compared to IR group (p < 0.05).
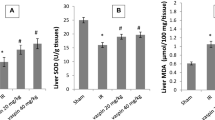
Cu,Zn-SOD activity was significantly decreased in the IR group (12.5 ± 1.4 U/g protein) compared to control (23.1 ± 3.2 U/g protein) (p < 0.05), but Cu,Zn-SOD activity was similar in fluoxetine pretreated group (23.3 ± 2.8 U/g protein) and control group (p > 0.05).
Additionally, PAB levels were significantly higher in IR group (22.4 ± 1.5 HK unit) when compared to those in control group (15.0 ± 1.6 HK unit) (p < 0.01) while it was decreased in Flx + IR group (15.5 ± 1.0 HK unit) (p < 0.01 vs. IR group).
FRAP levels of IR group (0.7 ± 0.1 mmol uric acid/g protein) were significantly higher compared to those of the control group (1.3 ± 0.1 mmol uric acid/g protein) (p < 0.01), but it was found to be restored in Flx + IR group (1.2 ± 0.2 mmol uric acid/g protein) (p < 0.05 vs. IR group) (Table 1).
Effect of fluoxetine on MPO activity and cytokine levels
Figure 2 shows MPO activity and cytokine levels. As an evident marker of inflammation, the MPO activities of tissues were determined. Low levels of MPO activities were measured in control group (17.5 ± 0.2 μU/g tissue). Post hoc comparisons using Tukey’s test indicated that IR produces a significant increase (42.1 ± 0.6 μU/g tissue) (p < 0.001 vs. control) in MPO activity of the kidney. This change was significantly attenuated by fluoxetine (16.7 ± 0.3 μU/g tissue) (p < 0.001 vs. IR).
The levels of TNF-α (4.1 ± 0.1 ng/mg protein), IL-6 (129.5 ± 1.1 ng/mg protein), and IL-1β (3.2 ± 0.3 pg/mg protein) were significantly increased (p < 0.001) in IR group compared to control group (1.1 ± 0.0 ng/mg protein, 29.4 ± 1.1 ng/mg protein, 0.9 ± 0.0 pg/mg protein, respectively). Pretreatment with fluoxetine increased TNF-α (1.8 ± 0.1 ng/mg protein), IL-6 (70.0 ± 0.8 ng/mg protein), and IL-1β (1.4 ± 0.1 pg/mg protein) levels (p < 0.001 vs. IR). The tissue levels of IL-10 were decreased in IR-induced group (1.9 ± 0.1 ng/mg protein) (p < 0.01 vs. control) whereas increased and found even higher than control (2.7 ± 0.1 ng/mg protein) (p < 0.05) in fluoxetine pretreatment group (3.3 ± 0.2 ng/mg protein) (p < 0.001 vs. IR) (Fig. 2).
Effects of fluoxetine on histological evaluation
Time-control group shows normal architecture of renal tissue (Fig. 3a). IR produced a significant increase in the total histological injury, indicating significant corpuscular injury (G) (p < 0.001 vs. control) and cellular hemorrhage (black arrow) (p < 0.05 vs. control) (Figs. 3b and 4a–b) when compared to slides obtained from control group. Total histological injury was significantly decreased in the rats receiving fluoxetine (Fig. 3c). However, fluoxetine treatment resulted in marked attenuation of corpuscular degeneration (p < 0.001 vs. IR), but could not prevent cellular hemorrhage induced by ischemia/reperfusion and this was not significant (p > 0.05 vs. control) (Figs. 3c and 4a–b).
Discussion
In the present study, we have found that IR was associated with significant increase in tissue LOOH, MDA, and PAB and a significant decrease in tissue Cu,Zn-SOD and FRAP levels. Besides the oxidative stress-related biomarkers in tissue, proinflammatory cytokines including TNF-α, IL-6, and IL-1β were excessively increased and resulted in loss of cellular integrity. These disturbances were associated with reduced renal function in terms of increased plasma urea and creatinine levels. Pre-administration of fluoxetine was able to decrease oxidative stress and inflammation and improve cellular structure and thereby improve renal function.
It was previously reported that IR injury causes both glomerular and tubular dysfunction [7]. IR injury is a series of biochemical reactions in which unbalanced ROS scavenging and ROS generation are playing an important role leading to tissue injury [2]. Mitochondrion is the primer source of ROS, and excess ROS injure the mitochondria themselves, impair cellular function, and promote cell death [10]. It has been suggested that antioxidants can decrease cellular and tissue damage by decreasing intracellular ROS levels and overwhelming oxidative stress [7, 25]. Our results also indicate that fluoxetine reduced renal lipid peroxidation in renal tissue after IR as showed by decreased tissue LOOH levels. Even if there was no statistical significance, our results regarding MDA levels are consistent with those of the LOOH levels due to decreased mean values of MDA. In the same way, Patel et al. have previously showed that administration of a SOD mimetic reduces ischemia-related injury to peritubular cells, thereby reducing renal dysfunction [25]. Moreover, they reported that this was without the adverse cardiovascular effects observed when using other nitroxyl radical scavenging agents. It was also showed that both selective iNOS inhibitor and Cu,Zn-SOD administration improve renal function due to scavenging of ROS and thereby preventing lipid peroxidation and oxidative damage to DNA [21]. However, the literature lacks information about the influence of fluoxetine on markers of free radical damage and the antioxidant defense in kidney.
We found that antioxidant enzyme activity (Cu, Zn-SOD) and indices of tissue injury (LOOH) were ameliorated in fluoxetine treatment groups. The generation of ROS and/or the decline of antioxidant defenses lead to oxidative stress, which plays an important role in the IR injury and acute kidney injury (AKI) development [7]. Under normal conditions, antioxidant enzymes such as Cu, Zn-SOD, which converts superoxide to hydrogen peroxide, can scavenge ROS. We have evaluated tissue antioxidant statues using FRAP assay in kidney tissue [25]. According to our results, plasma total FRAP was lower in the IR group. This decrease in FRAP was highly correlated PAB levels. These findings are in line with those of other investigators [1] and strongly suggest an ischemia-related risk of uncontrolled oxidative stress in people subjected to abdominal surgery.
The accumulation of ROS and reduction in antioxidant enzyme activities lead to damage in cellular compartments such as lipids and proteins, resulting in an increase of LOOH and PAB levels. Several investigations have demonstrated the beneficial effects of antioxidants, which prevent ROS generation, and ROS-scavenging molecules in IR injury [7, 25]. Our observation on the amelioration of Cu,Zn-SOD enzyme activity and lipid peroxidation suggests that fluoxetine administration decreases the oxidative stress by increasing Cu,Zn-SOD activity, which is an indication of a strengthening of cellular defenses.
IR injury pathophysiology still remains obscure; however, ROS released by inflammatory cells around the IR injured areas are suggested to play a key role [2, 30]. The increase in ROS triggers the expression of a number of proinflammatory genes, and this phenomenon is called post-ischemic inflammation. Post-ischemic inflammation is a common step for every type of cell after an ischemic insult. Activated infiltrating immune cells and injured cells are responsible for producing various inflammatory cytokines and mediators. IL-1β is one of the expressed cytokines in the ischemic tissue within 30 min after ischemia-reperfusion [15]. TNF-α is another essential cytokine related to ischemic injury. TNF-α is expressed in ischemic tissue within 1 h after ischemia-reperfusion [18]. TNF-α shows toxic effects by promoting cell death and the expression of MHC class II and intercellular adhesion molecule-1 (ICAM-1) in cells such as astrocytes, resulting in leukocyte infiltration. Likewise, it seems that both cytokines worked at anti-inflammatory process in our model. Moreover, IL-6 is a cytokine that acts as both a proinflammatory and an anti-inflammatory cytokine [29]. Our result concerning IL-6 shows that IL-6 is a member of proinflammatory cascade. IL-10 is known as an anti-inflammatory cytokine. Our results also support this phenomenon [16].
Besides the production of oxidative mediators and proinflammatory cytokines, acute inflammation is defined as increased MPO activity [6]. Our study is in agreement with previous articles showing that IR-induced organ injury accompanies changes in tissue neutrophil infiltration, which is relevant to the increase in MPO activity [31]. As clearly shown in the fluoxetine pretreated group, the attenuation of PMN infiltration into damaged tissue ensues with reduction of MPO activity. Moreover, MPO activity was also significantly decreased by fluoxetine when compared to IR. This clearly shows the amelioration of tissue damage by fluoxetine. It has been suggested that fluoxetine and other tricyclic antidepressants have protective antioxidant effects on oxidative stress induced by different types of stress sources [8, 20, 25]. Although many studies like ours indicated that fluoxetine has antioxidant effects, which part of the molecular structure of fluoxetine increases the endogenous antioxidants is still unknown.
From our survey of the literature, another proposed mechanism appears that fluoxetine could block the production of superoxide anions from activated leukocytes and inhibit iNOS via NFκB apart from scavenging peroxynitrite [17]. Based on these observations, it is likely that superoxide scavenging may be a therapeutic target of IR injury.
As renal dysfunction is a common complication following ischemia, studies investigating the beneficial effects of various drugs with respect to IR injury have mainly focused on the kidney. However, the type of drug that should be used for treatment to yield the best renal outcome remains controversial today and the antioxidant and/or anti-inflammatory properties of those drugs are still subjects of debate.
Conclusion
Based upon the findings obtained as a result of current study, in reperfusion injury, fluoxetine treatment significantly improves impaired redox homeostasis and inflammatory process caused by IR-induced kidney injury in rats. Our study clearly demonstrated that using fluoxetine not only reduced renal oxidative stress and inflammation following IR but also prevented renal tissue injury. We propose that the mechanisms underlying this protective effect of fluoxetine for improving kidney function involves the reduction of oxidative stress and subsequent lipid peroxidation and the inhibition of inflammatory response. Nevertheless, further experimental studies with fluoxetine are required to specifically define the precise mechanism of antioxidant and also anti-inflammatory effects of fluoxetine on a molecular basis.
References
Aivatidi C, Vourliotakis G, Georgopoulos S, Sigala F, Bastounis E, Papalambros E (2011) Oxidative stress during abdominal aortic aneurysm repair biomarkers and antioxidant’s protective effect: a review. Eur Rev Med Pharmacol Sci 153:245–252
Aksu U, Demirci C, Ince C (2011) The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol 174:119–128
Alamdari DH1, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F, Kazemi-Bajestani SM, Paletas K, Pegiou T, Koliakos G (2008) Prooxidant-antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem 41:375–380
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Butterfield TA, Best TM, Merrick MA (2006) The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train 41:457–465
Chatterjee PK (2007) Novel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol 376(1–2):1–43
Guner I, Yaman MO, Aksu U, Uzun D, Erman H, Inceli M, Gelisgen R, Yelmen N, Uzun H, Sahin G (2014) The effect of fluoxetine on ischemia-reperfusion following aortic surgery in a rat model. J Surg Res 189(1):96–105. doi:10.1016/j.jss.2014.02.033
Han DD, Wang Y, Zhang XH, Liu JR, Wang HL (2012) Fluoxetine protects against monocrotaline-induced pulmonary arterial remodeling by inhibition of hypoxia-inducible factor-1α and vascular endothelial growth factor. Can J Physiol Pharmacol 90:445
Hüttemann M, Helling S, Sanderson TH, Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J, Ramzan R, Vogt S, Grossman LI, Doan JW, Marcus K, Lee I (2012) Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta 1817(4):598–609
Kim H, Li H, Yoo KY, Lee BH, Hwang IK, Won MH (2007) Effects of fluoxetine on ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol 204(2):748–758
Kirkova M, Tzvetanova E, Vircheva S, Zamfirova R, Grygier B, Kubera M (2010) Antioxidant activity of fluoxetine: studies in mice melanoma model. Cell Biochem Funct 28(6):497–502
Koch A, Pernow M, Barthuber C, Mersmann J, Zacharowski K, Grotemeyer D (2012) Systemic inflammation after aortic cross clamping is influenced by Toll-like receptor 2 preconditioning and deficiency. J Surg Res 178(2):833–841
Kolla N, Wei Z, Richardson JS, Li XM (2005) Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J Psychiatry Neurosci 30:196
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97
Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF (2012) IL-35 is a novel responsive anti-inflammatory cytokine—a new system of categorizing anti-inflammatory cytokines. PLoS One 7(3):e33628. doi:10.1371/journal.pone.0033628
Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK (2009) Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res 87(4):1037–1045
Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25:1481–1488
Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, Eddahibi S (2003) Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. Am J Respir Crit Care Med 168:487
Naito Y, Yoshikawa T, Matsuyama K, Yagi N, Arai M, Nakamura Y, Kaneko T, Yoshida N, Kondo M (1998) Neutrophils, lipid peroxidation, and nitric oxide in gastric reperfusion injury in rats. Free Radic Biol Med 24(3):494–502
Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS (2001) Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281(5):F948–F957
Nourooz-Zadeh J (1999) Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 300:58–62
Núñez MJ, Balboa J, Rodrigo E, Brenlla J, González-Peteiro M, Freire-Garabal M (2006) Effects of fluoxetine on cellular immune response in stressed mice. Neurosci Lett 396:247–251
Park Y, Hirose R, Dang K, Xu F, Behrends M, Tan V, Roberts JP, Niemann CU (2008) Increased severity of renal ischemia-reperfusion injury with venous clamping compared to arterial clamping in a rat model. Surgery 143(2):243–251
Patel NS, Chatterjee PK, Chatterjee BE, Cuzzocrea S, Serraino I, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C (2002) TEMPONE reduces renal dysfunction and injury mediated by oxidative stress of the rat kidney. Free Radic Biol Med 33(11):1575–1589
Roumestan C, Michel A, Bichon F, Portet F, Detoc M, Henriquet C, Jaffuel D, Mathieu M (2007) Anti-inflammatory properties of desipramine and fluoxetine. Respiratory Research 3:8:35
Sahin G, Guner I, Yelmen N, Yaman O, Mengi M, Simsek G, Sipahi S (2011) Alterations of central hypercapnic respiratory response induced by acute central administration of serotonin re-uptake inhibitor, fluoxetine. Chin J Physiol 54(5):356–366
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF (1997) Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176(2):439–444. doi:10.1086/514062
Vinas JL, Sola A, Hotter G (2006) Mitochondrial NOS upregulation during renal I/R causes apoptosis in a peroxynitrite-dependent manner. Kidney Int 69(8):1403–1409
Wu Y, Wan J, Zhen WZ, Chen LF, Zhan J, Ke JJ, Zhang ZZ, Wang YL (2014) The effect of butorphanol postconditioning on myocardial ischaemia reperfusion injury in rats. Interact Cardiovasc Thorac Surg 18(3):308–312
Zafir A, Ara A, Banu N (2009) In vivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry 33:220–228
Zou C, Ding X, Flaherty JH, Dong B (2013) Clinical efficacy and safety of fluoxetine in generalized anxiety disorder in Chinese patients. Neuropsychiatr Dis Treat 9:1661-1670. Epub 2013 Nov 1. Review
Conflict of interest
All authors have disclosed potential conflicts of interest and all authors have read the journal’s policy on conflicts of interest and have none to declare. AS is a Ph.D. student at Istanbul University, Cerrahpasa Medical School, and no financial benefit was provided by Asfarma, International Pharmaceutical Marketing Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aksu, U., Guner, I., Yaman, O.M. et al. Fluoxetine ameliorates imbalance of redox homeostasis and inflammation in an acute kidney injury model. J Physiol Biochem 70, 925–934 (2014). https://doi.org/10.1007/s13105-014-0361-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-014-0361-0