Abstract
The present study was designed to investigate the effects of pilose antler peptide (PAP) on lipopolysaccharide (LPS)-induced lung injury. BalB/c mice intraperitoneally received PAP (10 and 20 mg/kg) or dexamethasone (2 mg/kg) 1 h prior to intratracheal instillation of LPS. PAP significantly decreased lung wet-to-dry weight (W/D) ratio and lung myeloperoxidase (MPO) activity and restored LPS-induced lung histopathological changes. PAP also increased super oxide dismutase (SOD) level and inhibited malondialdehyde (MDA) content and levels of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in bronchoalveolar lavage fluid (BALF) in LPS-stimulated mice. Furthermore, we demonstrated that PAP inhibited Rho/NF-κB pathway in LPS-induced mice. Our experimental results indicated that the protective mechanism of PAP might be attributed partly to the inhibition of Rho/NF-κB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The pathophysiology of acute lung injury (ALI) has been expounded for many years in intensive care medicine, but the mortality of ALI is still up to 40% with an age-adjusted incidence of 86.2 per 100.000 person-years [1]. ALI is characterized by the pulmonary edema, neutrophil infiltration, uncontrolled oxidative stress, and inflammatory process. A considerable amount of literature has described that the injury of the alveolar epithelium and capillary endothelium may increase alveolar barrier permeability, which results in airspace edema and inflammation [2]. Despite extensive studies about the relevant pathogenesis reported to date, there is no effective treatment for ALI. Therefore, it is necessary to explore the effective medication and innovative therapies.
Deer antlers, namely “lu rong” in China, “nokyong” in Korea, or “tokujo” in Japan, were widely used folk medicines in Asia. These soft growing tissues were applied in traditional Chinese medicine for strengthening the kidney, nursing the blood, treating neurosis, and prolonging life [3, 4]. Deer antlers have been reported to exert a variety of properties, such as anti-inflammatory, anti-stress, and anti-aging effects in previous research. Pilose antler peptide (PAP: MW: 7200; amino acid residue: 68) is isolated from the deer antlers and has shown beneficial effects on chronic inflammatory and oxidative damages [5]. However, the research on anti-inflammatory role of PAP in lung injury remains limited. In view of the anti-inflammatory activities of PAP, we hypothesized that it was advantageous to treat acute lung injury.
Recent clinical studies have shown that patients with inflammation may present several neurological disorders with the spillover of the inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [6]. A large and growing body of literature has investigated that lipopolysaccharide (LPS), an important component of the Gram-negative bacterial cell wall, can induce inflammatory responses and lead to the disturbance in immune system function [7]. Intravenous infusion of LPS induced ALI and cause changes in lung physiologic processes, which was similar to those in humans [8]. Thus, in this study, we chose this model to determine whether PAP could prevent ALI induced by LPS.
Rho is a small GTP-binding protein, and its target protein, Rho-associated coiled-coil-forming protein kinase (ROCK), is a calcium-sensitizing signaling pathway which is implicated in the cytoskeletal contractile response through its myosin ATPase activity. It was suggested that RhoA plays a critical role in the regulation of the inflammation response [9]. Several studies have revealed that inhibition of RhoA/Rho-kinase pathway has been shown to improve endothelial barrier integrity and is protective in models of inflammation [10]. Fasudil is a Rho-kinase inhibitor that has been clinically developed to treat pulmonary hypertension by ROCK inhibition [11]. Consequently, we chose Fasudil to investigate the associated signal pathway of BB on LPS-induced ALI. NF-κB is the classic pro-inflammatory transcription factor containing p50/p65 and IκBα. Many studies have found that NF-κB could activate and regulate the transcription and the expressions of downstream pro-inflammatory cytokines. Hence, the purpose of the present study was to evaluate the therapeutic effect of PAP on ALI induced by LPS and its anti-inflammatory activity through Rho/NF-κB signaling pathway.
MATERIALS AND METHODS
Reagents
PAP was provided by Changchun University of Chinese Medicine (Changchun, China). Dexamethasone (Dex) was provided by Xian Sheng Drug Store (Nanjing, China). Lipopolysaccharides (LPS, Escherichia coli serotype 0111:B4, No. L-2630) were supplied by Sigma-Aldrich (St. Louis, MO, USA). TNF-α, IL-6, and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were supplied by Nanjing Key GEN Biotech. Co., Ltd. (Nanjing, China). MDA, MPO, and SOD kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). Rho, ROCK-I, ROCK-II, p-IκBα, IκBα, NF-κBp65, and p-NF-κBp65 antibodies were produced by Cell Signaling Technology (Danvers, USA).
Animals
One hundred twenty male BALB/c mice (18–22 g) were obtained from Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China). The animals were maintained in an animal room with standard conditions such as water and normal diet. The mice were maintained in a 12-h light/dark cycle. All experimental procedures for the Care and Use of Laboratory Animals, and animal handling followed the dictates of the National Animal Welfare Law of China.
Experimental Protocol
Mice were divided into the five groups randomly, each of which consists ten mice: (1) control group (phosphate-buffered saline [PBS]), (2) LPS group, (3) LPS + dexamethasone group (LPS + Dex, 2 mg/kg), (4) LPS + PAP group (PAP, 10 mg/kg), and (5) LPS + PAP group (PAP, 20 mg/kg).
As to the pre-treatment regimen, the animals were intraperitoneally received PBS or drugs 1 h prior to intratracheal instillation of 20 μg LPS. The LPS was dissolved in 50 μl PBS to induce acute lung injury. Meanwhile, the mice in the control group were intratracheally given 50 μl PBS.
Collection of Bronchoalveolar Lavage Fluid
The animals were sacrificed 6 h after receiving the drugs or intratracheal instillation. The lungs were lavaged three times by 500 μl of autoclaved PBS (pH 7.2). Bronchoalveolar lavage fluid (BALF) samples were centrifuged at 1500 rpm for 10 min at 4 °C; the supernatants were stored in −80 °C for another analysis.
Measurement of Wet-to-Dry Ratio of the Lungs
The right lungs were removed at the end of the experiment. The trachea and esophagus were separated from the right middle lobe by blunt dissection, and the wet weight of the latter was determined. Subsequently, the lung were incubated at 60 °C for 48 h to remove all moisture, then the dry lungs were weighted, and the lung W/D ratios were calculated.
Pulmonary Histopathology
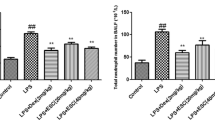
In order to evaluate tissue inflammation, the left lungs of the mice were excised at 6 h after LPS administration. The lungs were fixed in 4% neutral-buffered formalin for 24 h, section and then embedded in paraffin wax, and cut into 4-μm sections. Hematoxylin-Eosin (H&E) stains were performed using standard protocol. After staining, pathological changes in the lung tissues were observed under a light microscope. Lung inflammatory cell count based on a 5-point scoring system was performed to estimate the severity of leukocyte infiltration. The scoring system was 0: no cells, 1: a few cells, 2: a ring of cells with 1 cell layer deep, 3: a ring of cells with 2–4 cell layers deep; and 4: a ring of cells with more than 4 cell layers deep.
Measurement of MPO, SOD, and MDA Activities
The activities of MPO, SOD, and MDA in bronchoalveolar lavage fluid (BALF) in LPS-stimulated mice were measured by corresponding commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
Cytokine Measurements
The levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in BALF in LPS-stimulated mice were determined on the basis of enzyme immunoassays by ELISA kit in accordance with the instruction of the manufacturers.
Western Blot Analysis
The lung tissues in each group were chopped into small pieces and extracted by RIPA buffer (RIPA with 0.1% phenylmethylsulfonyl fluoride) on ice. Then, the samples were centrifuged at 12,000 rpm for 10 min at 4 °C. A BCA protein assay kit was chosen to measure the concentration of total protein of lung tissues and A549 cell lysates. The samples of lung tissues and A549 cell lysates were isolated through SDS-polyacrylamide gel electrophoresis and then transferred on the PVDF membranes. After incubated with the specific antibodies (1:1000) overnight at 4 °C, the membranes were exposure into the secondary antibodies for 2 h. The blots were washed for three times by TBST and analyzed for quantification.
Statistical Analysis
All of the data in this experiment was presented as mean ± standard error. Divergence between groups was compared by one-way ANOVA with Tukey multiple comparison test. P < 0.05 was considered as significant.
RESULTS
Effect of PAP on Wet-to-Dry Weight Ratio in Lung Tissues
In order to determine the level of pulmonary edema, we calculated the wet-to-dry (W/D) weight ratio of the lung tissues. As shown in Fig. 1, after injecting LPS, the W/D weight ratio of the lung tissues was significantly increased compared to the control group. However, PAP notably decreased the W/D weight ratio of mice than that in model group. This result suggested that treatment with PAP markedly attenuated the W/D weight ratio in lung tissues of mice induced by LPS.
Effect of PAP on Pathological Changes in Lung Tissues
We put H&E staining to use for disclosing the pathological changes on pulmonary. As shown in Fig. 2, after giving LPS, the histopathological observations showed obvious neutrophil sequestration or infiltration in lung tissues than that of the control group. Nevertheless, the group of PAP and Dex expressed the different degrees of protective effect on LPS-induced mice model.
Effect of PAP on MPO in Lung Tissue, MDA, and SOD Activities in BALF
As shown in Fig. 3, compared with the control group, the production of MPO and MDA was notably increased after induced by LPS. However, administration of PAP (10 and 20 mg/kg) to mice before or after giving LPS remarkably downregulated MPO and MDA level in BALF as compared with the model group. Figure 3 suggested that the level of SOD was decreased following LPS treatment and PAP (10 and 20 mg/kg) could elevate the SOD activity in BALF.
Effect of PAP on TNF-α, IL-6, and IL-1β Levels in BALF
As shown in Fig. 4, the injection of LPS resulted in an obviously increased TNF-α, IL-6, and IL-1β levels in BALF compared with the control group, while the level of TNF-α, IL-6, and IL-1β was decreased correspondingly after PAP (10 and 20 mg/kg) treatment. This result suggested that PAP could significantly downregulate the expression of inflammatory factors in LPS-induced acute lung injury mice model.
Western Blot
As shown in Fig. 5, for the sake of exploring the mechanism of LPS-induced acute lung injury in mice and cells, we determined the Rho/NF-κB signaling pathway-related proteins through Western blot analysis. The content of phosphorylations of NF-κB and IκBα and the expression of Rho, ROCK-I, and ROCK-II were upregulated compared with those of control group. According to our expectation, after treatment with PAP (10 and 20 mg/kg) and Dex, the level of those protein was significantly decreased. This result suggested that PAP showed the anti-inflammation activity in treat with LPS-induced acute lung injury according to Rho/NF-κB signaling pathway.
DISCUSSION
Acute lung injury (ALI) is the damnification of alveolar epithelial cells and capillary endothelial cell caused by some wound agent, which bring about diffuse interstitial lung and alveolar edema [12], and it may also cause the acute hypoxic respiratory insufficiency. What is more, the lung volume reduction, lung compliance, and ventilation/blood imbalance are important pathophysiological characteristics during acute lung injury [13]. The main clinical expressions are progressive hypoxia and respiratory distress [14].
Lipopolysaccharides (LPS) are the key component of cell wall on Gram-negative bacillus. Previous studies have reported that LPS possesses similar pathological characteristics of ALI and it is the most appropriate reagent for the model of ALI [15]. In this study, we detected the treatment or pre-treatment effect of PAP on LPS-induced acute lung injury in mice. During our study, PAP significantly decreased the lung wet-to-dry (W/D) weight ratio, lung myeloperoxidase (MPO) activity, molondialdehyde (MDA) content, and several inflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in bronchoalveolar lavage fluid (BALF) in LPS-stimulated mice and LPS-induced cellular supernatant. Besides, PAP also increased the super oxide dismutase (SOD) level. Furthermore, according to pathological section, PAP obviously relieved the acute lung injury.
MPO is an important lysosomal which contains iron. MPO represents the function of neutrophil granulocyte, which reveals the active condition of polymorphonuclear neutrophil (PMN) [16]. One of the biological effects of MPO is that it is involved in the inflammatory response and cause oxidative reaction which form plenty of oxide. It may bring about damage to cells and tissues [17]. In this study, after induced by LPS, the MPO activity on lung tissues was significantly increased. However, after treatment with PAP, the MPO activity on lung tissues was decreased. Previous studies have reported that lipid peroxidation reaction in organisms will produce molondialdehyde (MDA) [18], which lead to crosslinking and polymerization of protein or nucleic acid, and has plenty of cytotoxicity. Our experiment showed that after given with PAP, the content of MDA was notably depressed. SOD is a kind of active agent which can eliminate the harmful substance through the process of metabolism. What is more, it was regarded as the primary substance to eliminate the free radical [19]. Our research indicated that PAP could increase the level of SOD compared with that of the model group.
Acute lung injury has an acknowledged risk of acute inflammatory process which released some inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [20]. A considerable amount of literature has been published on these inflammatory cytokines. TNF-α is produced by mononuclear macrophage, which has the function of immunoregulation and inflammation regulation. IL-1β is regarded as a kind of pro-inflammatory cytokine, which participates in destruction or edema of tissue. IL-6 possesses various immune regulation functions which can enhance the immune function [21, 22]. In this study, the injection of LPS resulted in an obviously increased TNF-α, IL-6, and IL-1β levels in BALF compared with the control group, while the level of TNF-α, IL-6, and IL-1β was decreased correspondingly after PAP treatment. These results indicated that PAP might alleviate the acute lung injury induced by LPS.
Finally, we chose Western blot to discuss the mechanism of PAP on LPS-induced acute lung injury in vivo and in vitro. The present studies have shown that numerous external stimuli signal could lead to the activation of NF-κB signaling pathway including LPS [23, 24]. The main function of IκB protein is to prevent the NF-κB protein to enter the nucleus and combine with the DNA, which keeps the NF-κB protein stay in the cytoplasm. Therefore, the research of IκB protein seems so important when exploring the mechanism of NF-κB signaling pathway [25, 26]. ROCK is a part of serine/threonine protein kinase family, which contains two isomers called ROCK-I and ROCK-II. It is the downstream molecule of Rho [27]. A number of studies have found that ROCK signaling pathway has a close relationship with inflammation [28]. Hence, the Western blot analysis indicated that the protective mechanism of PAP might be attributed partly to the inhibition of Rho/NF-κB pathway.
In conclusion, our study indicated that PAP could alert protective effects on LPS-induced acute lung injury, which might be attributed to the inhibition of Rho/NF-κB pathway. Although, our current research is limited. We provided a potential drug for acute lung injury. Further clinical research of PAP is necessary before clinical application.
References
Matthay, M.A., L.B. Ware, and G.A. Zimmerman. 2012. The acute respiratory distress syndrome. New England Journal of Medicine 332(14): 27–37.
Elicker, B.M., et al. 2016. Imaging of acute lung injury. Radiologic Clinics of North America.
Kim, Y.K., K.S. Kim, K.H. Chung, J.G. Kim, K.S. Kim, Y.C. Lee, Y.C. Chang, and C.H. Kim. 2003. Inhibitory effects of deer antler aqua-acupuncture, the pilose antler of Cervus korean TEMMINCK var. mantchuricus Swinhoe, on type II collagen-induced arthritis in rats. International Immunopharmacology 3: 1001–1010.
Lin, J.-H., L.-X. Deng, Z.-Y. Wu, L. Chen, and L. Zhang. 2011. Pilose antler polypeptides promote chondrocyte proliferation via the tyrosine kinase signaling pathway. Journal of Occupational Medicine Toxicology 6: 27.
Takikawa, K., N. Kokubu, M. Kajihara, M. Dohi, and N. Tahara. 1972. Studies of experimental Whiplash Injury (III): changes in enzyme activities of cervical cords and effect of Pantui extracts, Pantocrin as a remedy. Folia Pharmacologica Japonica 68: 489–493.
Zhu, L., et al. 2015. Salidroside attenuates lipopolysaccharide(LPS) induced serum cytokines and depressive-like behavior in mice. Neuroscience Letters 606: 1–6.
Lou, T., et al. 2015. Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation 38(3): 1–8.
Ghosh, S., et al. 1993. Endotoxin-induced organ injury. Critical Care Medicine 21(2 Suppl): 19–24.
Segain, J.P., et al. 2003. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology 124(5): 1180–1187.
Gibson, C.L., et al. 2014. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. Journal of Neurochemistry 129(5): 816–826.
Ishikura, K., et al. 2006. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circulation Journal 70(2): 174–178.
Zhou, F., et al. 2016. Liraglutide attenuates lipopolysaccharide-induced acute lung injury in mice. European Journal of Pharmacology 791: 735–740.
Kellner, P., et al. 2016. Sevoflurane abolishes oxygenation impairment in a long-term rat model of acute lung injury. Anesthesia and Analgesia.
Li, C., et al. 2016. NFAT5 participates in seawater inhalation-induced acute lung injury via modulation of NF-kappaB activity. Molecular Medicine Reports.
Jang, Y.J., et al. 2016. Protective effect of sesquiterpene lactone parthenolide on LPS-induced acute lung injury. Archives of Pharmacal Research.
Yang, S., et al. 2016. Therapeutic effect of methyl salicylate 2-O-beta-d-lactoside on LPS-induced acute lung injury by inhibiting TAK1/NF-kappaB phosphorylation and NLRP3 expression. International Immunopharmacology 40: 219–228.
Jiang, W., et al. 2016. Protective effects of asiatic acid against spinal cord injury-induced acute lung injury in rats. Inflammation.
Diao, M., et al. 2016. Hydrogen gas inhalation attenuates seawater instillation-induced acute lung injury via the Nrf2 pathway in rabbits.
Wang, G., et al. 2016. Activation of AMPK attenuates LPS-induced acute lung injury by upregulation of PGC1alpha and SOD1. Experimental and Therapeutic Medicine 12(3): 1551–1555.
Zhao, J., et al. 2016. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. 311(5): p. L868-l880.
Matsuishi, Y., et al. 2016. Landiolol hydrochloride ameliorates acute lung injury in a rat model of early sepsis through the suppression of elevated levels of pulmonary endothelin-1. Life Sciences.
Liu, T.Y., and S.B. Chen. 2016. Sarcandra glabra combined with lycopene protect rats from lipopolysaccharide induced acute lung injury via reducing inflammatory response. Biomedicine and Pharmacotherapy 84: 34–41.
Zhe, Q., et al. 2016. Effects of Jiaotaiwan on depressive-like behavior in mice after lipopolysaccharide administration. Metabolic Brain Disease.
Mishra, R.K., et al. 2016. c-Jun is required for nuclear factor-kappaB-dependent, LPS-stimulated fos-related antigen-1 transcription in alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology 55(5): 667–674.
Courtois, G., and T.D. Gilmore. 2006. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25(51): 6831–6843.
von Bernuth, H., et al. 2005. Septicemia without sepsis: inherited disorders of nuclear factor-kappa B-mediated inflammation. Clinical Infectious Diseases 41(Suppl 7): S436–S439.
Pranatharthi, A., C. Ross, and S. Srivastava. 2016. Cancer stem cells and radioresistance: Rho/ROCK pathway plea attention. 2016: p. 5785786.
Du, L., et al. 2016. Crosstalk between inflammation and ROCK/MLCK signaling pathways in gastrointestinal disorders with intestinal hyperpermeability. 2016: p. 7374197.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, C., Long, H., Yang, C. et al. Anti-inflammatory Role of Pilose Antler Peptide in LPS-Induced Lung Injury. Inflammation 40, 904–912 (2017). https://doi.org/10.1007/s10753-017-0535-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0535-3