Abstract
Mycoplasma pneumoniae (Mp) may cause immune cell reactions as pivotal aspects of this clinically common respiratory pathogen. Our aim is to determine if Mp extract induces a cellular immune response associated with interleukin (IL)-17, leading to lung inflammation and lung injury. BALB/c mice were immunized with Mp extract intraperitoneally followed by its intratracheal administration, to mimic repeated Mp infection found in humans (repeated inoculation, RI group). Those with a single inoculation were compared as single inoculation group (SI group). Analysis of bronchoalveolar lavage fluid (BALF) demonstrated that keratinocyte-derived cytokine, tumor necrosis factor-α, and IL-6 were produced and peaked on days 0.5 or 1, followed by IL-17 on day 2. Levels of these mediators in BALF were higher in RI group than SI group (P < 0.05). Further, significantly more neutrophils were recruited to the lungs of the RI group (P < 0.05). These observations suggest that IL-17 is involved in the prolonged induction of neutrophils in mice treated with Mp extract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Mycoplasma pneumoniae (Mp) is a well-known cause of community-acquired pneumonia that can induce a cellular immune response, leading to inflammation and lung injury [1]. Even cases of pulmonary diseases caused by macrolide-resistant Mp have been successfully treated with macrolide, an agent that has an immunomodulatory effect [2], and cellular host defense reactions are enhanced by repeated Mp stimulation in animal models [3]. The clinical manifestation of Mp infection in humans varies with age, as younger children infected with Mp do not tend to develop pneumonia, whereas older children and adults do tend to develop pneumonia [4–7].
The symptoms of Mp pneumonia are more severe than expected in individuals who have been immunized with Mp vaccine [8]. Studies have demonstrated that interleukin (IL)-4 in bronchoalveolar lavage fluid (BALF) and IL-5 in blood were elevated in patients with Mp pneumonia [9, 10]. Several reports further demonstrated that corticosteroid was clinically beneficial for patients with Mp pneumonia, suggesting that anti-immune and anti-inflammatory actions of corticosteroid were effective against these cellular host defense reactions [11, 12]. Taken together, it has been speculated that cellular immune reactions play an important role in induction of lung inflammation and lung injury by Mp.
In order to mimic the effect of Mp on such immune cell reactions, we previously established a murine model of Mp pneumonia for which we employed Mp extract, rather than live Mp, in order to avoid the infectious aspect of the disease [13, 14]. Histological analysis of lung tissues of this mouse model, which had an immune cell reaction against Mp, showed lymphoplasmacytic inflammation in the peri-bronchial area, which is the same pathology as that observed in humans suffering from severe Mp pneumonia [14]. Interestingly, comprehensive analysis of inflammatory mediators in these mice demonstrated that IL-17 levels increased in BALF following Mp inoculation [13]. IL-17 concentration and neutrophil counts in BALF were elevated in parallel when mice were inoculated with live Mp [15]. Similar IL-17-linked signal activation was observed in patients suffering from Mp pneumonia who showed significantly higher levels of serum IL-17 than patients with streptococcal pneumonia [16]. Since it has been reported that IL-17 plays a role in the transition from innate immunity to adaptive immunity [17, 18], these observations led to the hypothesis that some components of the Mp extract induce IL-17, which then results in excessive inflammatory cell reactions in Mp pneumonia.
The mouse models of Mp pneumonia that we previously established [13, 14] were pretreated with alum-adjuvant, which may affect the expression of IL-17 and associated molecules. In the present study, a novel mouse model of Mp pneumonia that did not employ alum-adjuvant treatment was prepared. Furthermore, BALB/c mice were immunized with Mp extract intraperitoneally followed by its intratracheal administration, to mimic repeated Mp infection found in humans (repeated inoculation, RI group). Those with a single inoculation were compared as single inoculation group (SI group) in order to clarify whether IL-17 levels are enhanced by injection of Mp extract.
MATERIALS AND METHODS
Preparation of Mp Extract
Mp extract was prepared from cultured M. pneumoniae (ATCC 29342) in a pleuropneumonia-like organism liquid broth containing 20 % horse serum and 10 units/mL penicillin G (Nikkenkagaku, Kyoto, Japan), according to a previously published method with some modification [13]. In brief, cultured Mp was centrifuged and repeatedly washed with Hanks' balanced salt solution (HBSS) (Invitrogen, Grand Island, NY). The sediment was suspended in HBSS and sonicated. This suspension was centrifuged and the supernatant was defined as the Mp extract. The amount of Mp extract was defined in terms of protein concentration, and the Mp extract was prepared in HBSS at a concentration of 1.0 μg/μL.
Primary Culture of Lung-Derived Cells
Cells were prepared from lung tissues originating from four 12-week-old BALB/c female mice (Charles River Laboratories, Kanagawa, Japan), which were anesthetized and then sacrificed after their blood was replaced with saline. Their lungs were removed and cut into pieces, and the tissues were incubated for 2 h at room temperature in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Grand Island, NY, USA) that contained 300 U/mL collagenase type 1 (Worthington Biochemical Corporation, NJ, USA), 0.01 % gentamicin (GM), and 0.1 % amphotericin-B (AMPH-B). The suspended cells were then harvested, washed with DMEM containing 0.01 % GM, 0.1 % AMPH-B, and 10 % fetal calf serum (FCS, Invitrogen), passed five times through 40-μm nylon mesh cell strainers (BD FALCON, Franklin Lakes, NJ, USA), seeded at a concentration of 5.0 × 104 cells/well in a 96-well plate, and incubated at 37 °C in 5 % CO2. After 24 h, the culture media were replaced with DMEM containing 0.01 % GM, 0.1 % AMPH-B, 10 % FCS, and 100 U/mL polymyxin B. To assess the time-dependent release of cytokines, the cells were treated with or without Mp extract (50 μg/mL). After incubation of the cells for 24, 48, and 72 h with the Mp extract, the media were collected for measurement of IL-23 and IL-17 levels. Media were also collected after 72 h incubation with different concentrations of the Mp extract (0, 1.9, 5.6, 16.7, and 50 μg/mL) to evaluate dose-dependent stimulating effects of Mp extract. Each assay was performed in quadruplicates.
Mp Inoculation and Sampling
The protocol of Mp inoculation and sampling is shown in Fig. 1. Twelve-week-old BALB/c female mice (Charles River Laboratories, Kanagawa, Japan) were assigned to one of two groups. Mice in the SI group were intratracheally inoculated with 50 μg/50 μL of Mp extract alone on day 0. Mice in the RI group were intraperitoneally injected twice with 50 μg/250 μL of Mp extract 28 and 21 days before intratracheal injection with 50 μg/50 μL of the Mp extract (day 0). In RI group, the Mp extract was administered intraperitoneally in order to avoid damaging the trachea by repeated inoculations and to obtain reproducible results. Mice were anesthetized with pentobarbital and lavaged twice with 1 mL of HBSS through a catheter via the trachea. BALF was subsequently collected and was centrifuged at 400×g for 5 min. Total and differential cell counts were calculated for BALF (number of mice tested was indicated in each figure) collected on days 0.5, 1, 2, 3, 4, and 7. Cytokine levels were measured in BALF collected on days 0.5, 1, 2, 3, and 4 (number of mice tested was indicated in each figure). In preliminary experiments, any of cytokines tested were not detected in BALF collected on day 7. Immunohistochemical analysis of IL-23 expression in lungs (number of mice tested was indicated in each figure) was conducted on day 1 because preliminary experiments demonstrated that the percentage of IL-23-positive cells in lung tissue was higher on day 1 than on day 0.5. All animal experiments were performed in accordance with the Institutional Animal Care and Research Advisory Committee at Kyorin University.
Mp extract inoculation and sampling protocol. Mice that were SI were administered with Mp extract intratracheally on day 0. Mice that were RI were administered with Mp extract intraperitoneally on days −28 and −21 and were then inoculated intratracheally with the Mp extract on day 0. BALF was obtained on days 0.5, 1, 2, 3, 4, and 7 for analysis of cells (n = 5 per each on days 0.5, 1, 2, 3, and 4; n = 6 on day 7) and cytokines (n = 5 per each point). Lungs were obtained on day 1 for IHC analysis (n = 3 per each group).
Cytokine Measurements
The levels of IL-17, keratinocyte-derived cytokine (KC), tumor necrosis factor-α (TNF-α), IL-6, interferon-γ (IFN-γ), IL-4, and IL-12 in BALF were measured using a protein multiplex immunoassay kit (Biosource International, Camarillo, CA, USA) and a multiplex bead array (Luminex 200, Luminex, Austin, TX, USA), according to the manufacturer's instructions. The concentrations of IL-17 and IL-23 in the primary cell cultures were measured using an enzyme-linked immunosorbent assay kit (Quantikine mouse IL-17, Quantikine mouse IL-23, R&D Systems, Minneapolis, MN, USA).
IL-23 Immunohistochemistry
Mouse lungs were removed on day 1, fixed in 4 % paraformaldehyde, embedded in paraffin, and sectioned. After deparaffinization, the specimens were stained with an anti-IL-23 rabbit polyclonal antibody to IL-23p19 (Abcam, Cambridge, UK) at a dilution of 1:100, followed by staining with a peroxidase-conjugated secondary antibody at a dilution of 1:100. The number of IL-23-positive and IL-23-negative cells in ten microscope fields (330 × 430 μm) per mouse (N = 3) was counted in a blinded fashion, and the percentage of the IL-23-positive cells to the total cells was calculated.
Statistical Analysis
All data are expressed as means ± standard deviation. Statistic analysis was performed using the Statistical Package for Social Sciences (SPSS) software (SPSS, Chicago, IL, USA). The Kruskal–Wallis test was used to evaluate variance among all groups. If a significant variance was found, the Mann–Whitney test was used to determine significant differences between individual groups. P < 0.05 was considered to represent a statistically significant difference.
RESULTS
Secretion of IL-23 and IL-17 by Primary Cultured Cells
Incubation of mouse lung-derived primary cell cultures with Mp extract (50.0 μg/mL) resulted in an increase in the concentration of IL-23 and IL-17 in the culture media when compared with control (Mp extract 0 μg/mL). The IL-23 concentration peaked at 24 h, whereas the IL-17 concentration peaked at 72 h (Fig. 2a). The IL-23 concentration was significantly higher at 24 and 48 h than that at 72 h. The IL-17 concentration at 48 and 72 h was significantly higher than that at 24 h.
Effect of incubation of primary lung cultures with Mp extract on the concentration of IL-23 and IL-17 in the culture media. a The concentration of IL-23 (open bars) and IL-17 (solid bars) in primary lung culture media was measured at 24, 48, and 72 h after the addition of M. pneumonia (Mp) extract at a final protein concentration of 0 and 50.0 μg/mL. The concentration of IL-23 (b) and IL-17 (c) in primary lung culture media was measured at 72 h after addition of the indicated concentration of the Mp extract. Significant differences are indicated by the following symbols: *P < 0.05 compared with no added Mp extract (0 μg/mL); † P < 0.05 compared with the 1.9-μg/mL Mp extract; and ‡ P < 0.05 compared with the 5.6-μg/mL Mp extract. Data are expressed as means ± standard deviation. Each assay was performed in quadruplicates. N.D. indicates “not detected.”
Incubation of the lung-derived primary cultures with increasing concentrations of Mp extract (from 0 to 50 μg/mL) for 72 h resulted in a dose-dependent increase in IL-23 concentration in the media, which peaked at Mp extract concentration of 5.6 μg/mL (Fig. 2b). The IL-17 concentration in the media also significantly increased at an Mp extract concentration of no less than 5.6 μg/mL; to show a concentration dependency up to the highest Mp concentration of 50 μg/mL (Fig. 2c).
Analysis of the Cytokines in BALF
We measured the level of IL-17 and its associated cytokines in the BALF collected from mouse groups that were singly or repeatedly inoculated. IL-17 was detected on days 1 and 2 and peaked on day 2 in both the SI and the RI groups. However, on day 3, IL-17 was only detected in the RI group (P < 0.01) (Fig. 3a). KC levels were significantly higher in the RI group than in the SI group on day 1 (P < 0.05) (Fig. 3b). TNF-α levels were significantly higher in the RI group than in the SI group on days 0.5 and 1 (P < 0.01) (Fig. 3c). IL-6 levels were significantly higher in the RI group than in the SI group on days 1 (P < 0.05) and 2 (P < 0.01) (Fig. 3d). The IFN-γ levels were low in both groups, and no difference was observed between the SI and RI groups (Fig. 3e). IL-4 levels were significantly higher in the RI group than in the SI group on day 0.5 (P < 0.05) (Fig. 3f). IL-12 was not detected in either group on any day (data not shown). In preliminary experiments, these cytokines were not detected in the BALF collected on day 7.
Cytokine levels in the BALF of the mice. Cytokines in the BALF collected from SI (open bars) and RI (solid bars) mice on the indicated days following the final Mp extract inoculation were analyzed using a multiplex bead array. The concentrations of IL-17 (a), KC (b), TNF-α (c), IL-6 (d), IFN-γ (e), and IL-4 (f) are shown (n = 5 per each point). Significant differences between SI and RI groups at specific times are indicated by asterisks: *P < 0.05, **P < 0.01. Data are expressed as means ± standard deviation.
Immunohistochemical Analysis of IL-23
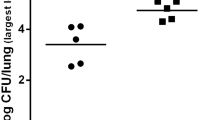
Immunohistochemical analysis of lung tissue collected on day 1 indicated the focal distribution of IL-23-positive cells in the alveolar lumen in both groups (SI, Fig. 4a; RI, Fig. 4b). These IL-23-positive cells were morphologically identified as macrophages. The percentage of these cells among the total cell counts tended to be higher in the RI group (16.0 ± 12.5 %) than in the SI group (5.9 ± 10.3 %).
Analysis of the Cells in BALF
We assayed the effect of single or repeated inoculation of Mp extract into mice on the cell types present in BALF. Total cell counts were significantly higher in the RI group than in the SI group on days 2 (P < 0.05), 3 (P < 0.01), and 4 (P < 0.01) (Fig. 5a). The number of each cell type in BALF is shown in Fig. 5b–d, and the percentages of each cell type comprising the total cell counts are shown in Fig. 5e. Neutrophil counts peaked on day 2 in both groups but were significantly higher in the RI group than in the SI group on days 2 (P < 0.05), 3 (P < 0.01), and 4 (P < 0.01) (Fig. 5b). Macrophage counts were significantly higher in the RI group than in the SI group on days 0.5 and 1 (P < 0.05) (Fig. 5c), and lymphocyte counts were significantly higher in the RI group than in the SI group on days 4 and 7 (P < 0.05) (Fig. 5d). Neutrophils were the predominant cell type in both the SI and RI groups until day 3 (Fig. 5e).
Cell populations in the BALF of mice. Cell populations in the BALF of SI (open bars) and RI groups (solid bars) on days 0.5, 1, 2, 3, 4, and 7 after the final Mp extract inoculation are shown. The vertical axis represents average cell counts (n = 5 each on days 0.5, 1, 2, 3, and 4; n = 6 on day 7). Total cell counts (a), neutrophil counts (b), macrophage counts (c), and lymphocyte counts (d) are shown. Percentages of neutrophil (black column), macrophage (gray column), and lymphocyte (white column) are shown (e). Significant differences are indicated by asterisks: *P < 0.05, **P < 0.01. Data are expressed as means ± standard deviation.
DISCUSSION
This study established a novel mouse model of Mp pneumonia without the use of alum-adjuvant treatment. We analyzed this mouse model, as well as primary cultured cells prepared from mouse lungs that were stimulated with Mp extract, in order to address our hypothesis that some components of the Mp extract induce IL-17, which is associated with excessive inflammatory cell reactions in Mp pneumonia. We used Mp extract instead of live Mp in order to avoid the infectious aspect of Mp pneumonia.
We confirmed that levels of IL-23 and IL-17 were elevated at 72 h after treatment of mouse lung-derived primary cell cultures with Mp extract (Fig. 2b, c). This is the first report to demonstrate that levels of IL-23 in the media of cultured cells increased in response to the Mp extract (50 μg/mL), followed by an increase in IL-17 levels (Fig. 2a). Although we did not define which kind of cells of lung-derived primary cells produced IL-17, several reports [19–21] have demonstrated that lymphocytes and epithelial cells are the possible sources of IL-17. Previous reports indicated that some components of Mp induced IL-8 production in A549 and BEAS-2B cells [22, 23], which are derived from human alveolar or bronchial epithelial cells. Mp extract also induced IL-6 and TNF-α in RAW 264.7 cells that were derived from murine macrophages [13].
We also demonstrated that the levels of IL-17, KC, TNF-α, IL-6, and IL-4 were higher in the BALF from the RI group of mice than that from the SI group of mice (Fig. 3). IL-17 appears to be mainly generated by Th17 cells, which differentiate from naïve T cells in response to IL-6 and are maintained by IL-23 [19, 20]. Our results suggested that Mp extract might increase the levels of IL-23-expressing cells in the lungs as assessed by immunohistochemistry (IHC) and IL-6 production in BALF from RI mice, and both cytokines led to a Th17-skewed condition. KC stimulation potently induces neutrophil recruitment to the lungs. IL-17 was reported to increase the release of KC [24], and a combination of IL-17 and TNF-α appeared to synergistically enhance KC production [25]. In the present study, the KC levels in BALF were higher in the RI mice than in the SI mice. IL-17 and TNF-α levels were also higher in the RI mice. These data appear to be consistent with a scenario in which Mp increases IL-17, together with KC and TNF-α and results in the accumulation of neutrophils in the lungs.
The total cell count in the BALF collected from RI mice was significantly higher than that in SI mice (Fig. 5a). In particular, the number of macrophages was higher in the RI mice than in the SI mice on day 0.5 and day 1 (Fig. 5c). This increase in macrophage number was followed by an increase in the number of neutrophils. The number of neutrophils was higher in the RI mice than in the SI mice at the later stages (days 2, 3, and 4) following tracheal inoculation (Fig. 5b). A previous report showed that repeated inoculation of live Mp induced neutrophilic inflammation in BALF similar to that observed in our experiment [26]. In that report, the accumulation of neutrophils in the BALF of mice repeatedly inoculated with live Mp persisted for a longer time when compared with that in mice inoculated with a single live Mp. Thus, repeated live Mp inoculation induced persistent neutrophilic inflammation similar to that observed in the RI mice in the present study.
Neutrophilic accumulation in BALF was observed in humans suffering from Mp pneumonia [9] and in mice inoculated with either live Mp [15] or with Mp extract. Few studies have assessed IL-17 levels in human BALF, although IL-17 levels in the serum of patients suffering from Mp pneumonia were higher than those suffering from streptococcal pneumonia [16]. The mechanisms of how Mp extract induced excessive infiltration of neutrophils in the lung remain not elucidated. In the mice inoculated with the Mp extract in the present study, elevation of IL-17 and associated neutrophil accumulation was observed. Previous studies of mice inoculated with live Mp reported that Mp induced an increase in the concentration of IL-17, KC, TNF-α, IL-6, IFN-γ, and IL-12 in BALF [15, 26–29]. Our current findings are generally consistent with the inflammatory reactions found in humans and mice with different experimental designs and thus suggest that lung inflammation in the RI group, similar to the lung inflammation in human Mp pneumonia [5], seemed exacerbated by repeated Mp stimulation. Some of these reports [28, 29] have also suggested that IL-12 (and the IFN-γ production that it stimulated) plays a role in the increase in neutrophilic alveolar infiltration in mice infected with live Mp, whereas we did not observe an elevation in IL-12 levels.
This study had several limitations. Firstly, it was based on the evaluation of sequential changes of inflammatory mediators and cells. It should be borne in mind that very small concentrations of pro-inflammatory cytokines and mediators may produce biologic actions in a variety of different cell types. Thus, although our result failed to detect IL-12 in BALF perhaps due to dilution effect, IL-12 may still play a role in pro-inflammatory reactions induced by Mp extract.
Secondly, the initial aim of the investigation was to elucidate the noninfectious effect of modulating neutrophilic reactions by Mp extract, in association with IL-17. Thus, we focused on the levels of IL-17 and its related mediators on days 0.5, 1, 2, and 3, which is early in the time frame at which neutrophils were recruited to the lungs of RI mice. Although we did not define which cells of lung-derived primary cells produced IL-17, several reports have demonstrated that lymphocytes and epithelial cells are the possible sources of IL-17 [19–21]. Our result also demonstrated that lymphocytes were recruited to the lung on day 7, which could be linked to the modulatory effects on immune and inflammatory reactions induced by Mp extract. It was possible that increased lymphocytes in BALF after inoculation of Mp extract were Th17 cells, but we did not analyze subpopulations of BALF lymphocytes. Further analysis of the role of IL-17 may help elucidate the role of lymphocytes in this disease.
In summary, we have established a novel mouse model of Mp pneumonia by repeated inoculations of Mp extract without the use of alum-adjuvant treatment. Our current data further indicated that repeated inoculation of the Mp extract (RI group mice) results in increased levels of inflammatory cytokines including IL-17, associated with exacerbated neutrophilic lung inflammation. Although our study did not address the mechanism by which the IL-17 was activated by the Mp extract, or which component of the Mp extract exacerbates the lung lesions, the inflammatory reactions observed upon repeated inoculation of the Mp extract might help in understanding some of the important phenomena in the cellular and molecular pathogenesis of Mp pneumonia in humans.
References
Waite, K.B., and D.F. Talkington. 2004. Mycoplasma pneumoniae and its role as a human pathogen. Clinical Microbiology Reviews 17: 697–728.
Matsuoka, M., M. Narita, N. Okazaki, H. Ohya, T. Yamazaki, K. Ouchi, I. Suzuki, T. Andoh, T. Kenri, Y. Sasaki, A. Horino, M. Shintani, Y. Arakawa, and T. Sasaki. 2004. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical obtained in Japan. Antimicrobial Agents and Chemotherapy 48: 4624–4630.
Opitz, O., K. Pietsch, S. Ehlers, and E. Jacobs. 1996–1997. Cytokine gene expression in immune mice reinfected with Mycoplasma pneumoniae: the role of T cell subsets in aggravating the inflammatory response. Immunobiology 196: 575–587.
British Thoracic Society Standards of Care Committee. 2002. BTS guidelines for the management of community acquired pneumonia in children. Thorax 57(Supple1): i1–i24.
Fernald, G.W., A.M. Collier, and W.A. Clyde Jr. 1975. Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics 55: 327–335.
Lim, W.S., S.V. Baudouin, R.C. George, A.T. Hill, C. Jamieson, I. Le Jeune, J.T. Macfarlane, R.C. Read, H.J. Roberts, M.L. Levy, M. Wani, M.A. Woodhead; Pneumonia Guidelines Committee of the BTS Standards of Care Committee. 2009. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 64(Suppl 3): iii1–55.
Steohen, G.B. Mycoplasma pneumoniae and atypical pneumonia In: Mandell, Douglas and Bennett's principle and principles and practice of infectious diseases. G.L. Mandell, J.E. Bennett, R. Dolin, editors. Philadelphia: Elsevier Inc., 2010: 2481–2489
Smith, C.B., W.T. Friedewald, and R.M. Chanock. 1967. Inactivated Mycoplasma pneumoniae vaccine. Evaluation in volunteers. The Journal of the American Medical Association 199: 353–358.
Koh, Y.Y., Y. Park, H.J. Lee, and C.K. Kim. 2001. Levels of interleukin-2, interferon-γ, and interleukin-4 in bronchoalveolar lavage fluid from patients with Mycoplasma pneumonia: implication of tendency toward increased immunoglobulin E production. Pediatrics 107: E39.
Nisar, N., R. Guleria, S. Kumar, T.C. Chawla, and N.R. Biswas. 2007. Mycoplasma pneumoniae and its role in asthma. Postgraduate Medical Journal 83: 100–104.
Lee, K.Y., H.S. Lee, J.H. Hong, M.H. Lee, J.S. Lee, D. Burgner, and B.C. Lee. 2006. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatric Pulmonology 41: 263–268.
Radisic, M., A. Torn, P. Gutierrez, H.A. Defranchi, and P. Pardo. 2000. Severe acute lung injury caused by Mycoplasma pneumoniae: potential role for steroid pulses in treatment. Clinical Infectious Diseases 31: 1507–1511.
Hirao, S., H. Wada, K. Nakagaki, T. Saraya, D. Kurai, S. Mikura, T. Yasutake, M. Higaki, T. Yokoyama, H. Ishii, K. Nakata, T. Aakashi, S. Kamiya, and H. Goto. 2011. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunology and Medical Microbiology 62: 182–189.
Saraya, T., K. Nakata, K. Nakagaki, N. Motoi, K. Iihara, Y. Fujioka, T. Oka, D. Kurai, H. Wada, H. Ishii, H. Taguchi, S. Kamiya, and H. Goto. 2011. Identification of a mechanism for lung inflammation caused by Mycoplasma pneumoniae using a novel mouse model. Results in Immunology 1: 76–87.
Wu, Q., R.J. Martin, J.G. Rino, R. Breed, R.M. Torres, and H.W. Chu. 2007. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes and Infection 9: 78–86.
Michelow, I.C., K. Katz, G.H. McCracken, and R.D. Hardy. 2007. Systemic cytokine profile in children with community-acquired pneumonia. Pediatric Pulmonology 42: 640–645.
Pridgeon, C., L. Bugeon, L. Donnelly, U. Straschil, S.J. Tudhope, P. Fenwick, J.R. Lamb, P.J. Barnes, and M.J. Dallman. 2011. Regulation of IL-17 in chronic inflammation in the human lung. Clinical Science 120: 515–524.
Vanaudenaerde, B.M., S.E. Verleden, R. De, S.I. Vos, A. Vleeschauwer, R. Willems-Widyastuti, D.E.Van Geenens, L.J. Raemdonck, E.K. Dupont, and I.Meyts Verbeken. 2011. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. American Journal of Respiratory and Critical Care Medicine 183: 977–986.
Bettelli, E., T. Korn, M. Oukka, and V.K. Kuchroo. 2008. Induction and effector functions of T(H)17 cells. Nature 453: 1051–1057.
Mills, K.H. 2008. Induction, function and regulation of IL-17-producing T cells. European Journal of Immunology 38: 2636–2649.
Shen, N., J. Wang, M. Zhao, F. Pei, and B. He. 2011. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol 23: 212–218.
Chmura, K., X. Bai, M. Nakamura, P. Kandasamy, M. McGibney, K. Kuronuma, H. Mitsuzawa, D.R. Voelker, and E.D. Chan. 2008. Induction of IL-8 by Mycoplasma pneumoniae membrane in BEAS-2B cells. American Journal of Physiology - Lung Cellular and Molecular Physiology 295: L220–L230.
Sohn, M.H., K.E. Lee, S.Y. Choi, B.C. Kwon, M.W. Chang, and K.E. Kim. 2005. Effect of Mycoplasma pneumoniae lysate on interleukin-8 gene expression in human respiratory epithelial cells. Chest 128: 322–326.
Ferretti, S., O. Bonneau, G.R. Dubois, C.E. Jones, and A. Trifilieff. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. The Journal of Immunology 170: 2106–2112.
Hartupee, J., C. Liu, M. Novotny, X. Li, and T. Hamilton. 2007. IL-17 enhances chemokine gene expression through mRNA stabilization. The Journal of Immunology 179: 4135–4141.
Chu, H.W., R. Breed, J.G. Rino, R.J. Harbeck, M.R. Sills, and R.J. Martin. 2006. Repeated respiratory Mycoplasma pneumoniae infections in mice: effect of host genetic background. Microbes and Infection 8: 1764–1772.
Fonseca-Aten, M., A.M. Ríos, A. Mejías, S. Chávez-Bueno, K. Katz, A.M. Gomez, G.H. McCracken Jr., and R.D. Hardy. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. American Journal of Physiology - Lung Cellular and Molecular Physiology 32: 201–210.
Salvatore, C.M., M. Fonseca-Aten, K. Katz-Gaynor, A.M. Gomez, A. Mejias, C. Somers, S. Chavez-Bueno, G.H. McCracken, and R.D. Hardy. 2007. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infection and Immunity 75: 236–242.
Salvatore, C.M., M. Fonseca-Aten, K. Katz-Gaynor, A.M. Gomez, and R.D. Hardy. 2008. Intranasal interleukin-12 therapy inhibits Mycoplasma pneumoniae clearance and sustains airway obstruction in murine pneumonia. Infection and Immunity 76: 732–738.
Acknowledgments
This research was partially supported by the Japanese Ministry of Education, Science, Sports and Culture Grants-in-Aid for Scientific Research of 2007, 1959091, and 2009, 21591298.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurai, D., Nakagaki, K., Wada, H. et al. Mycoplasma pneumoniae Extract Induces an IL-17-Associated Inflammatory Reaction in Murine Lung: Implication for Mycoplasmal Pneumonia. Inflammation 36, 285–293 (2013). https://doi.org/10.1007/s10753-012-9545-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-012-9545-3